Electrical Properties of Silver-Attached Amine Functionalized Carbon Black/Polyethylene Terephthalate Fibers Prepared by Melt-Spinning
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
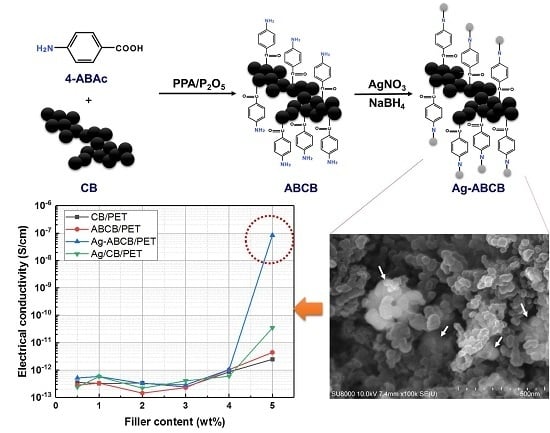
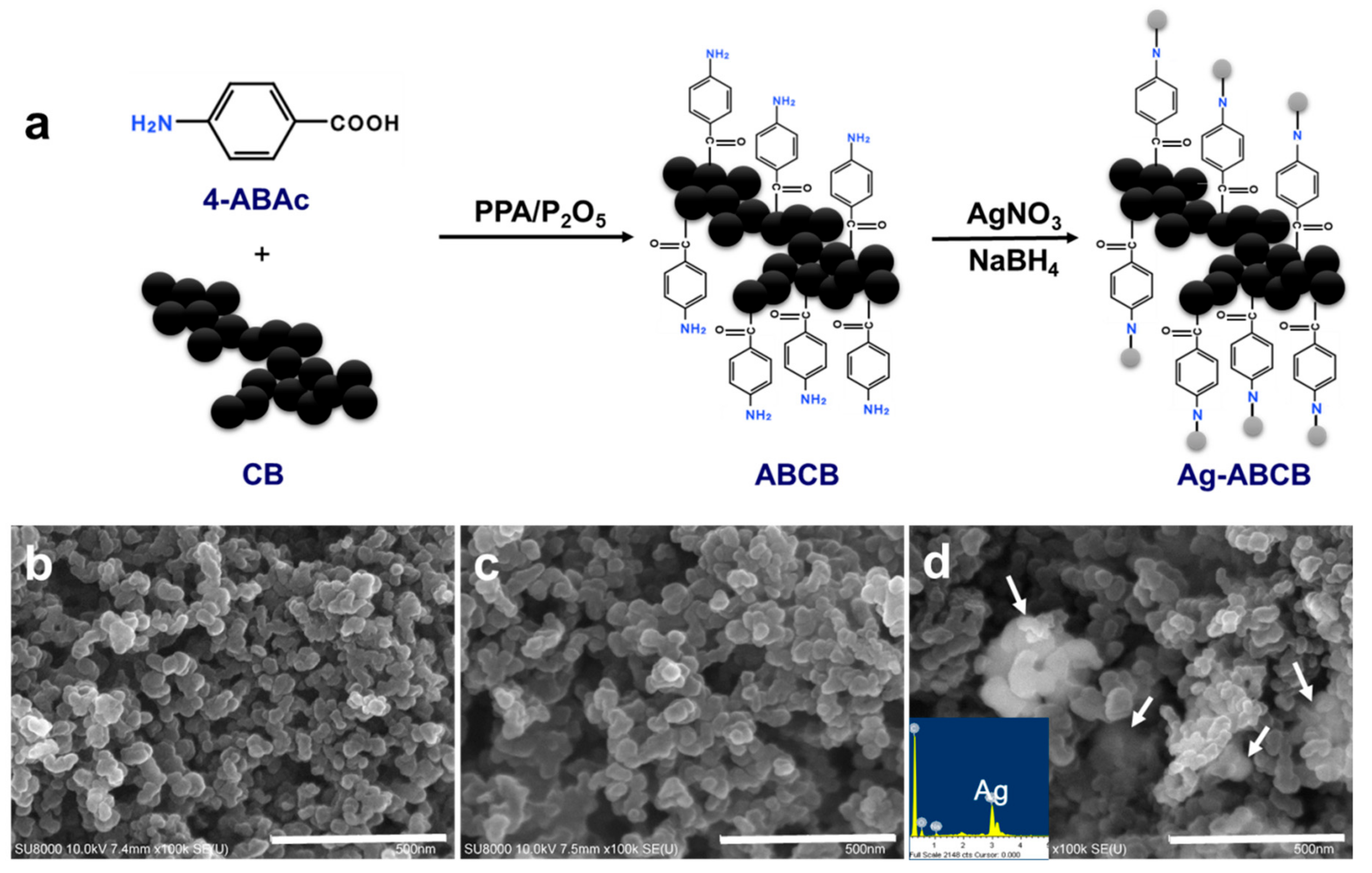
2.2.1. Synthesis of ABCB
2.2.2. Synthesis of Ag-ABCB
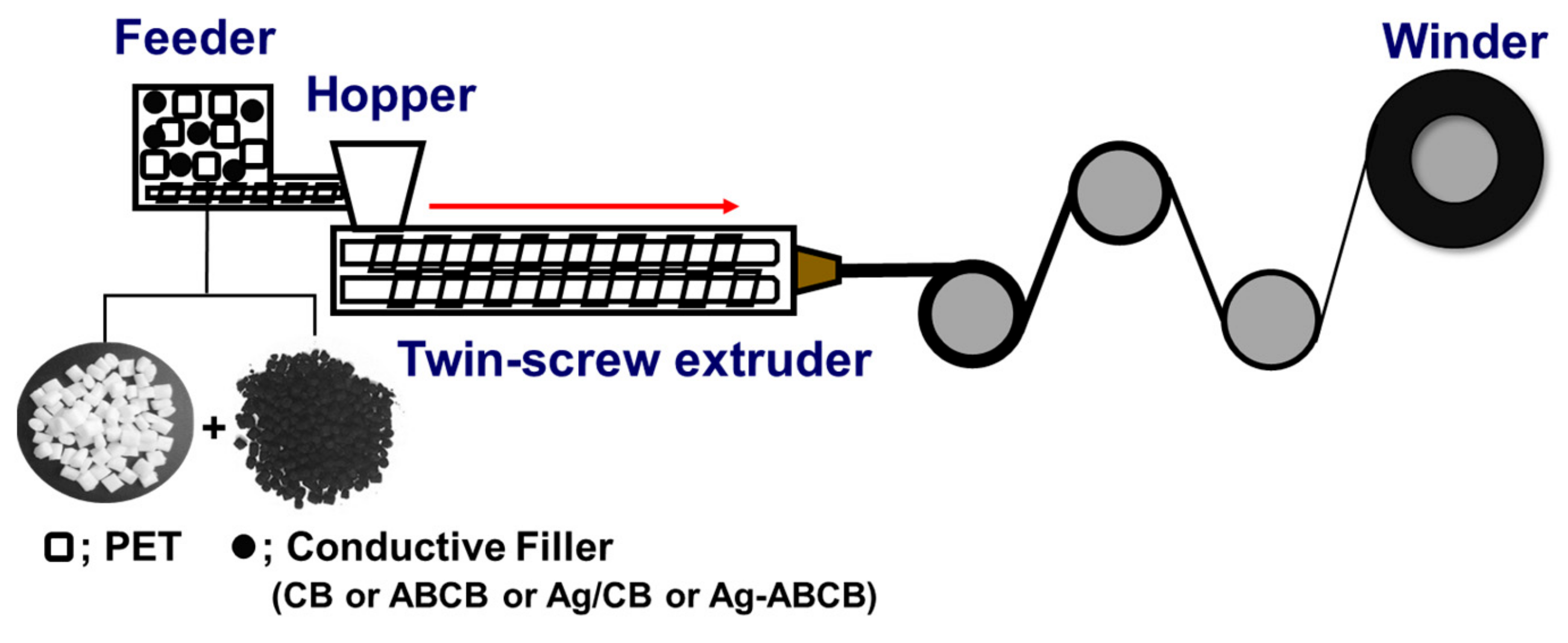
2.3. Processing
2.4. Characterizations
3. Results and Discussion
3.1. Characteristics of ABCB and Ag-ABCB Nanocomposites
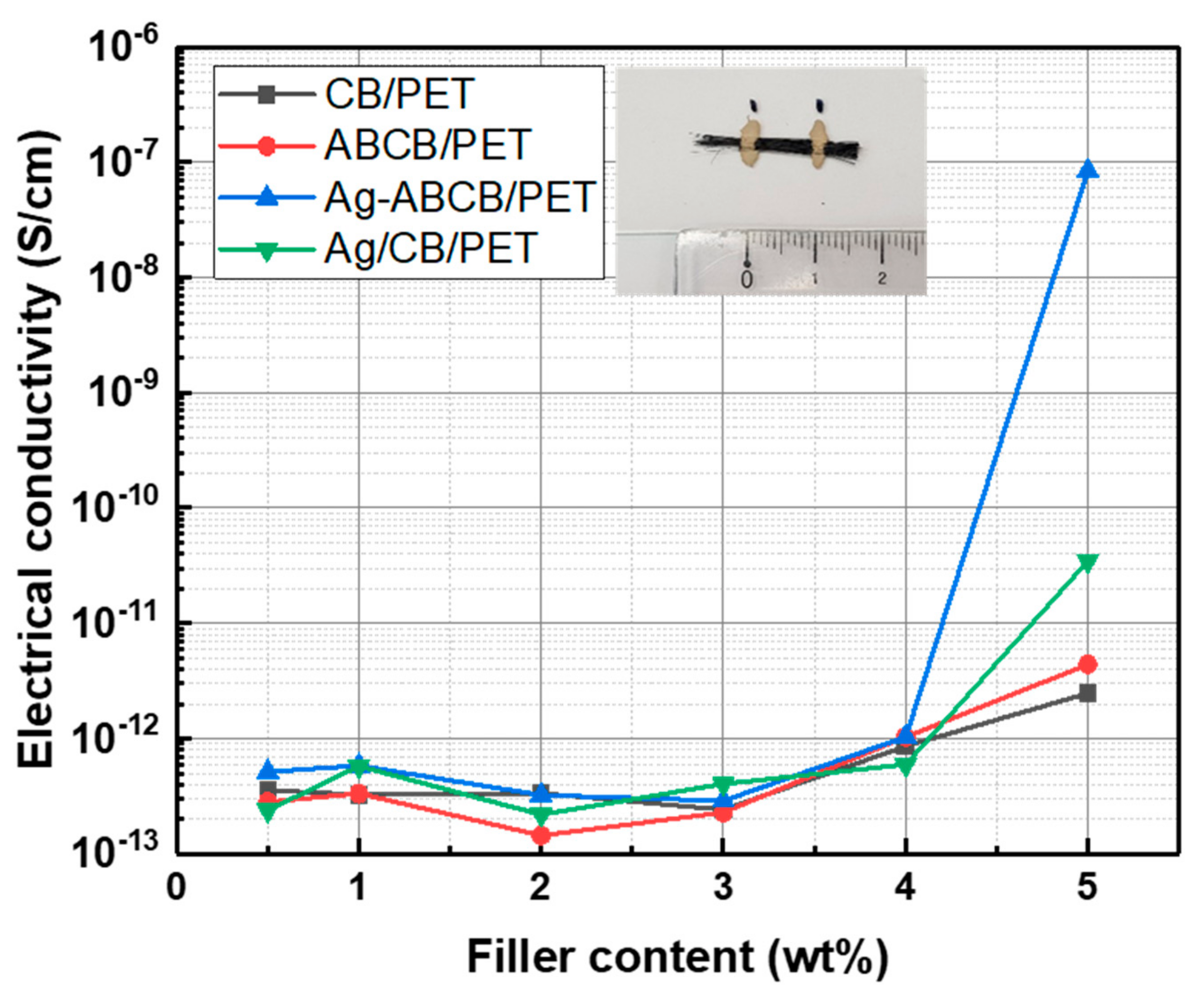
3.2. Electrical Properties of Conductive Filler/PET Fiber
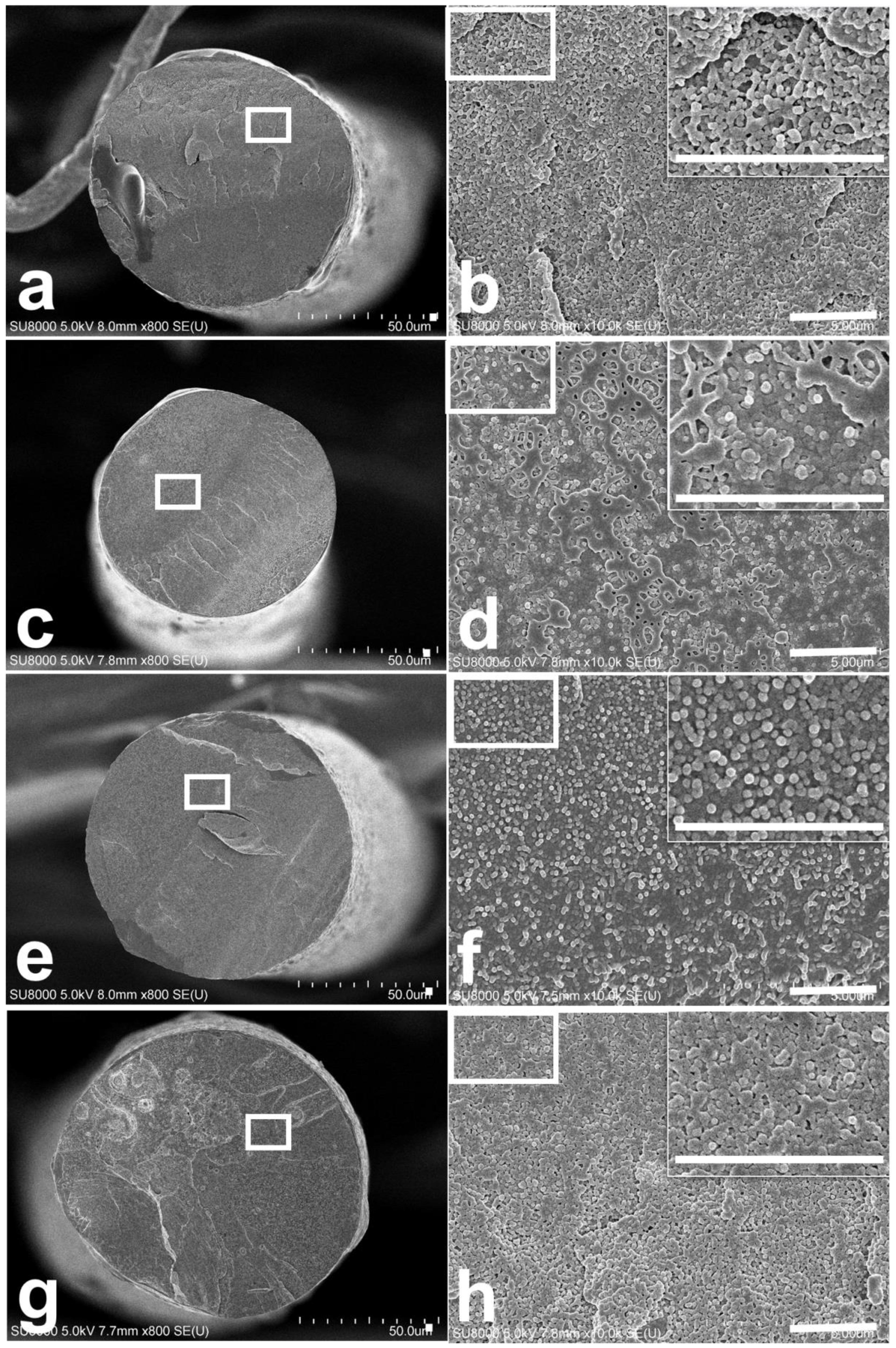
3.3. Morphologies of Conductive Filler/PET Fiber
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, C.C.; Chang, S.S.; Liang, N.Y. Preparation and Characterization of carbon black/polybutylene terephthalate/polyethylene terephthalate antistatic fiber with sheath-core structure. J. Text. I. 2015, 08, 976–984. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, M.S.; Ahn, D.; Yeo, S.Y.; Lee, S. Electrical percolation threshold of carbon black in a polymer matrix and its application to antistatic fibre. Sci. Rep. 2019, 9, 6338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Ren, H.; Zheng, L.; Wang, G.; Gao, X.; Tan, Z.; Shan, J.; Cui, L.; Li, K.; Jian, M.; Zhu, L.; et al. Transfer-medium-free nanofiber-reinforced graphene film and application in wearable transparent pressure sensors. ACS Nano 2019, 13, 5541–5548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, S.Y.; Cui, N.; Zhao, P.; Tang, Q.; Tong, Y.; Liu, Y. Enhancing the intrinsic stretchability of micropatterned gold film by covalent linkage of carbon nanotubes for wearable electronics. ACS Appl. Electron. Mater. 2019. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, H.; Seo, J.; Shin, S.; Koo, J.H.; Pang, C.; Son, S.; Kim, J.H.; Jang, Y.H.; Kim, D.E.; et al. Conductive fiber-based ultrasensitive textile pressure sensor for wearable electronics. Adv. Mater. 2015, 24, 2433–2439. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, T.; Wang, J.; Liao, J.; Qui, Y.; Yang, Q.; Xue, H.; Shi, Z.; Zhao, Y.; Xiong, Z.; et al. A hybrid fibers based wearable fabric piezoelectric nanogenerator for energy harvesting application. Nano Energy 2015, 13, 298–305. [Google Scholar] [CrossRef]
- Youssefi, M.; Fanaei, E.; Shanbeh, M. Strain sensors on electroless Ni-P plated polyester woven fabrics. Faber. Polym. 2019, 20, 562–568. [Google Scholar] [CrossRef]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Waarbeek, R.F.; Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, Light, Multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 2013, 339, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hua, Z.; Yan, F.; Gang, P. Metal coating of fiber gragg grating and the temperature sensing character after metallization. Opt. Fiber Technol. 2009, 391–397. [Google Scholar] [CrossRef]
- Pinto, J.J.; Carrión, P.; Quiñones, J.X. Electroless deposition of nickel on electrospun fibers of 2-acrylamido-2-methyl-1-propanesulfonic acid doped polyaniline. Mater. Sci. Eng. A 2004, 336, 1–5. [Google Scholar] [CrossRef]
- Gülercan, D.; Gergin, İ.; Sarac, A.S. Preparation and Electrochemical Performances of graphene oxide/PEDOT and reduced graphene oxide/PEDOT nanofibers and nanocomposites. Fiber Polym. 2018, 19, 2178–2187. [Google Scholar]
- Chen, R.X.; Li, Y.; He, J.H. Bubbfil spinning for mass-production of nanofibers. Therm. Sci. 2014, 18, 1718–1719. [Google Scholar] [CrossRef]
- Li, Z.; Luo, G.; Wei, F.; Huang, Y. Microstructure of carbon nanotubes/PET conductive composites fibers and their properties. Compos. Sci. Technol. 2006, 66, 1022–1029. [Google Scholar] [CrossRef]
- Eom, J.; Lee, J.H.; Park, S.K.; Jeong, Y.; Park, J.S.; Kim, Y.-H. Highly conductive and stretchable fiber interconnections using dry-spun carbon nanotube fibers modified with ionic liquid/poly(vinylidene fluoride) copolymer composite. Compos. Sci. Technol. 2019, 169, 1–6. [Google Scholar] [CrossRef]
- Ma, T.; Gao, H.L.; Cong, H.P.; Yao, H.B.; Wu, L.; Yu, Z.Y.; Chen, S.M.; Yu, S.H. A bioinspired interface design for improving the strength and electrical conductivity of graphene-based fibers. Adv. Mater. 2018, 30, 1706435. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Sun, H.; Gao, C. Highly Electrically conductive Ag-doped graphene fibers as stretchable conductors. Adv. Mater. 2013, 25, 3249–3253. [Google Scholar] [CrossRef]
- Grannam, D.M.; Garland, J.C.; Tanner, D.B. Critical behavior of the dielectric constant of a random composite near the percolation threshold. Phys. Rev. Lett. 1981, 46, 375. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthernios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotube. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Gomari, S.; Ghasemi, I.; Eafandeh, M. Functionalized graphene nanoplates/poly(ethylene oxide) nanocomposites: Correlation between crystallization behavior and mechanical performance. Fiber. Polym. 2017, 18, 2153–2160. [Google Scholar] [CrossRef]
- Baek, J.B.; Tan, L.S. Improved syntheses of poly(oxy-1,3-phenylenecarbonyl-1,4-phenylene) and related poly(ether-ketones) using polyphosphoric acid/P2O5 as polymerization medium. Polymer 2003, 44, 4135–4147. [Google Scholar] [CrossRef]
- Lee, H.J.; Han, S.W.; Kwon, Y.D.; Tan, L.S.; Baek, J.B. Functionalization of multi-walled carbon nanotubes with various 4-substituted benzoic acids in mild polyphosphoric acid/phosphorous pentoxide. Carbon 2008, 46, 1850–1859. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, S.J.; Choi, J.Y.; Kim, J.W.; Han, J.; Tan, L.S.; Baek, J.B. In situ synthesis of poly(ethylene terephthalate) (PET) in ethylene glycol containing terephthalic acid and functionalized multiwalled carbon nanotube (MWNTs) as an approach to MWNT/PET nanocomposites. Chem. Mater. 2005, 14, 5057–5064. [Google Scholar] [CrossRef]
- Baek, J.B.; Lyons, C.B.; Tan, L.S. Covalent modification of vapor-grown carbon nanofibers via direct Friedel-Crafts acylation in polyphosphoric acid. J. Mater. Chem. 2004, 14, 2052–2056. [Google Scholar] [CrossRef]
- Han, S.W.; Oh, S.J.; Tan, L.S.; Baek, J.B. Grafting of 4-(2,4,6-Trimethylphenoxy)benzoyl onto Single-Walled Carbon Nanotubes in in Poly(phosphoric acid) via Amide Function. Nanoscale Res. Lett. 2009, 4, 766–772. [Google Scholar]
- Sun, Y.; Xia, Y. Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Lim, S.K.; Lee, S.K.; Hwang, S.H.; Kim, H. Photocatalytic deposition of silver nanoparticles onto organic/inorganic composite nanofibers. Macromol. Mater. Eng. 2006, 291, 1265–1270. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar]
- Choi, H.J.; Kang, J.Y.; Jeon, I.Y.; Eo, S.M.; Tan, L.S.; Baek, J.B. Immobilization of platinum nanoparticles on ortho-diaminobenzoyl functionalized multi-walled carbon nanotube and its electrocatalytic activity. J. Nanopart. Res. 2012, 704. [Google Scholar] [CrossRef]
- Lim, T.H.; Lee, S.H.; Yeo, S.Y. Highly conductive polymer/metal carbon nanotube composite fiber prepared by the melt-spinning process. Text. Res. J. 2017, 87, 593–606. [Google Scholar] [CrossRef]
- Shin, Y.R.; Jung, S.M.; Jeon, I.Y.; Baek, J.B. The Oxidation Mechanism of Highly Ordered Pyrolytic Graphite in a Nitric Acid/Sulfuric Acid Mixture. Carbon 2013, 52, 493–498. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Baughman, R.H.; Iqbal, Z.; Cui, C.; Khayrullin, I.; Dantas, S.O.; Marti, J.; Ralchenko, V.G. Carbon structures with three-dimensional periodicity at optical wavelengths. Science 1998, 2821, 897–901. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, E.; Dai, H.; Kang, L.; Wu, H.; Fan, J.; Hu, X.; Liu, H. Photocatalytic activity of Ag-TiO2-graphene ternary nanocomposites and application in hydrogen evolution by water splitting. Int. J. Hydrog. Energy 2014, 39, 7664–7671. [Google Scholar] [CrossRef]
- Yumitori, S. Correlation of C1s chemical state intensities with the O1s intensity in the XPS analysis of anodically oxidized glass-like carbon samples. J. Mater. Sci. 2000, 35, 139–146. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Potlog, T.; Duca, D.; Dobromir, M. Temperature-dependent growth and XPS of Ag-doped ZnTe thin films deposited by close space sublimation method. Appl. Surf. Sci. 2015, 352, 33–37. [Google Scholar] [CrossRef]

| Filler1 | PET | Temperature (°C) | Screw Speed (RPM) | Feeder Speed (RPM) | Winder Speed (MPM) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone #1 | Zone #2 | Zone #3 | Zone #4 | Zone #5 | Zone #6 | |||||
| 0.5 | 99.5 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | 800 |
| 1.0 | 99.0 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | 800 |
| 2.0 | 98.0 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | 700 |
| 3.0 | 97.0 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | - |
| 4.0 | 96.0 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | - |
| 5.0 | 95.0 | 200 | 230 | 260 | 260 | 260 | 260 | 110 | 0.80 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-J.; Ahn, D.; Lee, S.; Yeo, S.Y. Electrical Properties of Silver-Attached Amine Functionalized Carbon Black/Polyethylene Terephthalate Fibers Prepared by Melt-Spinning. Polymers 2019, 11, 1611. https://doi.org/10.3390/polym11101611
Choi H-J, Ahn D, Lee S, Yeo SY. Electrical Properties of Silver-Attached Amine Functionalized Carbon Black/Polyethylene Terephthalate Fibers Prepared by Melt-Spinning. Polymers. 2019; 11(10):1611. https://doi.org/10.3390/polym11101611
Chicago/Turabian StyleChoi, Hyun-Jung, Damiro Ahn, Sohee Lee, and Sang Young Yeo. 2019. "Electrical Properties of Silver-Attached Amine Functionalized Carbon Black/Polyethylene Terephthalate Fibers Prepared by Melt-Spinning" Polymers 11, no. 10: 1611. https://doi.org/10.3390/polym11101611
APA StyleChoi, H.-J., Ahn, D., Lee, S., & Yeo, S. Y. (2019). Electrical Properties of Silver-Attached Amine Functionalized Carbon Black/Polyethylene Terephthalate Fibers Prepared by Melt-Spinning. Polymers, 11(10), 1611. https://doi.org/10.3390/polym11101611