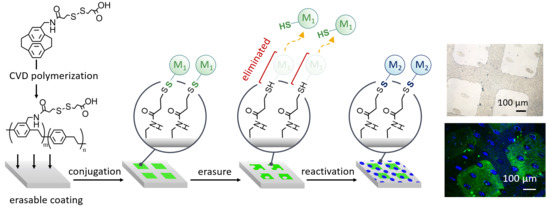
Vapor-Deposited Reactive Coating with Chemically and Topographically Erasable Properties
Abstract
:1. Introduction
2. Materials and Methods
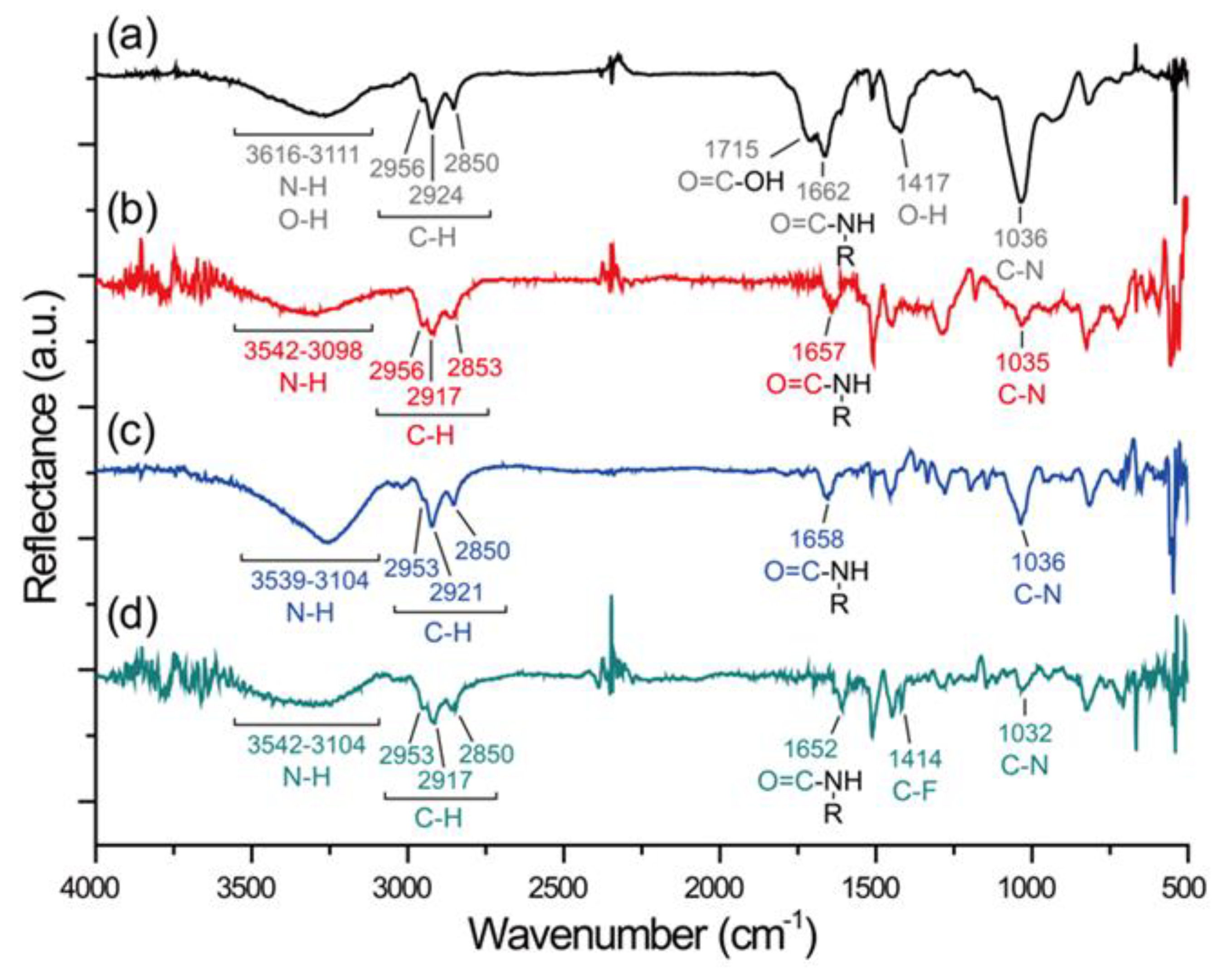
2.1. Synthesis and Chemical Vapor Deposition (CVD) Polymerization
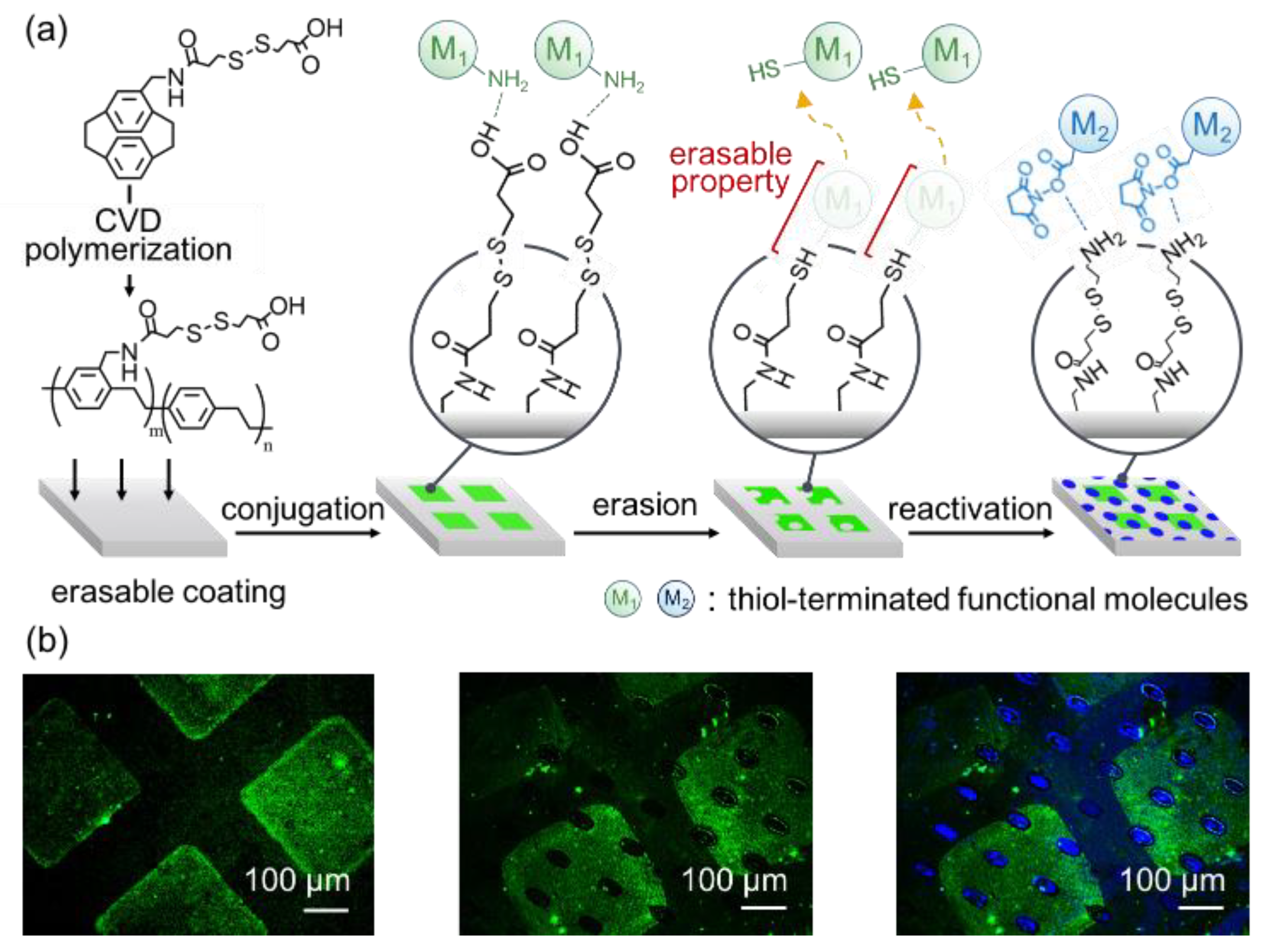
2.2. Immobilization
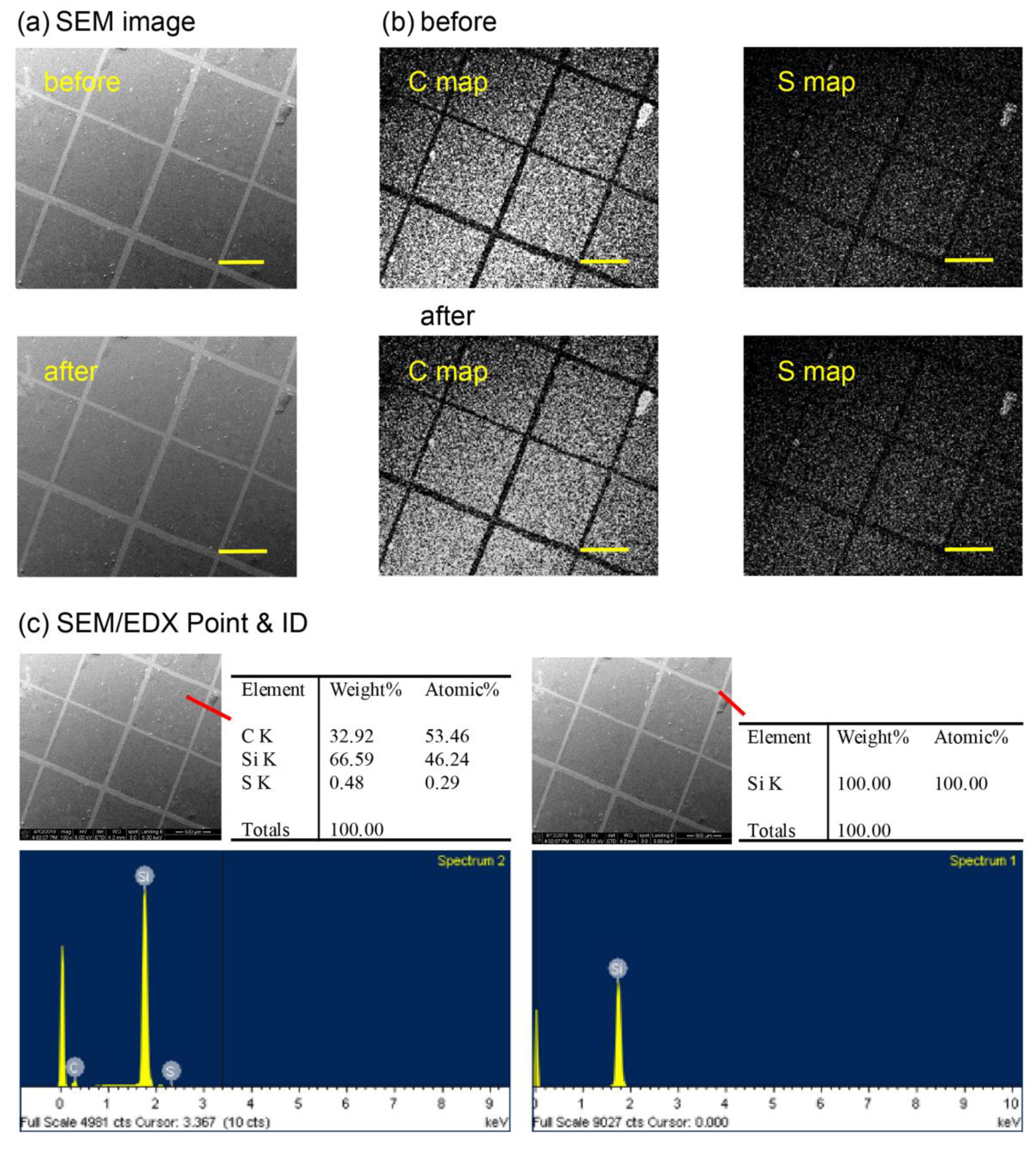
2.3. Surface Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.P.; Dubey, N.C.; Subair, R.; Choudhury, S.; Stamm, M. Enhanced hydrophilic and antifouling polyacrylonitrile membrane with polydopamine modified silica nanoparticles. Rsc Adv. 2016, 6, 4448–4457. [Google Scholar] [CrossRef]
- Höhme, C.; Filiz, V.; Abetz, C.; Georgopanos, P.; Scharnagl, N.; Abetz, V. Postfunctionalization of nanoporous block copolymer membranes via click reaction on polydopamine for liquid phase separation. Acs Appl. Nano Mater. 2018, 1, 3124–3136. [Google Scholar] [CrossRef]
- Diba, F.S.; Reynolds, N.; Thissen, H.; Wang, P.Y.; Kingshott, P. Tunable Chemical and Topographic Patterns Based on Binary Colloidal Crystals (BCCs) to Modulate MG63 Cell Growth. Adv. Funct. Mater. 2019, 29, 1904262. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Yang, R.; Asatekin, A.; Gleason, K.K.J.S.M. Design of conformal, substrate-independent surface modification for controlled protein adsorption by chemical vapor deposition (cvd). Soft Matter 2012, 8, 31–43. [Google Scholar] [CrossRef]
- Alf, M.E.; Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Chelawat, H.; Ozaydin-Ince, G.; Petruczok, C.D.; Sreenivasan, R.; Tenhaeff, W.E.; Trujillo, N.J.; et al. Chemical vapor deposition of conformal, functional, and responsive polymer films. Adv. Mater. 2010, 22, 1993–2027. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, J.; Welle, A.; Li, L.; Feng, W.; Levkin, P.A. Reversible and rewritable surface functionalization and patterning via photodynamic disulfide exchange. Adv. Mater. 2015, 27, 4997–5001. [Google Scholar] [CrossRef] [PubMed]
- Gevrek, T.N.; Cosar, M.; Aydin, D.; Kaga, E.; Arslan, M.; Sanyal, R.; Sanyal, A. Facile fabrication of a modular “catch and release” hydrogel interface: Harnessing thiol–disulfide exchange for reversible protein capture and cell attachment. Acs Appl. Mater. Interfaces 2018, 10, 14399–14409. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Ozdeslik, R.N.; Sahin, G.S.; Yesilbag, G.; Mutlu, S.; Sanyal, A. Functionalization of reactive polymeric coatings via diels–alder reaction using microcontact printing. Macromol. Chem. Phys. 2012, 213, 166–172. [Google Scholar] [CrossRef]
- Ross, A.; Durmaz, H.; Cheng, K.; Deng, X.; Liu, Y.; Oh, J.; Chen, Z.; Lahann, J. Selective and reversible binding of thiol-functionalized biomolecules on polymers prepared via chemical vapor deposition polymerization. Langmuir 2015, 31, 5123–5129. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.-Y.; Wu, C.-Y.; Li, Y.-J.; Chen, H.-Y. Switching the biointerface of displaceable poly-p-xylylene coatings. Acs Appl. Mater. Interfaces 2015, 7, 14431–14438. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Ramos, M.J. Theoretical insights into the mechanism for thiol/disulfide exchange. Chem.-Eur. J. 2004, 10, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gallogly, M.M.; Starke, D.W.; Mieyal, J.J. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 2009, 11, 1059–1081. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M.J.A.C.I.E. Soft lithography. Annu. Rev. Mater. Sci. 1998, 37, 550–575. [Google Scholar]
- Perl, A.; Reinhoudt, D.N.; Huskens, J. Microcontact printing: Limitations and achievements. Adv. Mater. 2009, 21, 2257–2268. [Google Scholar] [CrossRef]
- Sun, H.Y.; Fang, C.Y.; Lin, T.J.; Chen, Y.C.; Lin, C.Y.; Ho, H.Y.; Chen, M.C.; Yu, J.; Lee, D.J.; Chang, C.H.J.A.M.I. Thiol-reactive parylenes as a robust coating for biomedical materials. Advanced Materials Interfaces 2014, 1, 1400093. [Google Scholar] [CrossRef]
- Chen, S.-T.; Wu, C.-Y.; Chen, H.-Y. Enhanced growth activities of stem cell spheroids based on a durable and chemically defined surface modification coating. Acs Appl. Mater. Interfaces 2018, 10, 31882–31891. [Google Scholar] [CrossRef]
- Paint, A.C.D.-O.; Related Coatings, M. Applications. ASTM D3359-09, Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Wu, J.-T.; Wu, C.-Y.; Fan, S.-K.; Hsieh, C.-C.; Hou, Y.-C.; Chen, H.-Y. Customizable optical and biofunctional properties of a medical lens based on chemical vapor deposition encapsulation of liquids. Chem. Mater. 2015, 27, 7028–7033. [Google Scholar] [CrossRef]
- Chang, C.H.; Yeh, S.Y.; Lee, B.H.; Hsu, C.W.; Chen, Y.C.; Chen, C.J.; Lin, T.J.; Chen, M.H.C.; Huang, C.T.; Chen, H.Y. Compatibility balanced antibacterial modification based on vapor-deposited parylene coatings for biomaterials. J. Mater. Chem. B 2014, 2, 8496–8503. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hirtz, M.; Deng, X.; Laue, T.; Fuchs, H.; Lahann, J. Substrate-independent dip-pen nanolithography based on reactive coatings. J. Am. Chem. Soc. 2010, 132, 18023–18025. [Google Scholar] [CrossRef] [PubMed]
- Kuppusami, S.; Oskouei, R.H. Parylene coatings in medical devices and implants: A review. Univers. J. Biomed. Eng. 2015, 3, 9–14. [Google Scholar]
- Johnsson, B.; Lofas, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Szajewski, R.P.; Whitesides, G.M. Rate constants and equilibrium-constants for thiol-disulfide interchange reactions involving oxidized glutathione. J. Am. Chem. Soc. 1980, 102, 2011–2026. [Google Scholar] [CrossRef]
- Keire, D.A.; Strauss, E.; Guo, W.; Noszal, B.; Rabenstein, D.L. Kinetics and equilibria of thiol disulfide interchange reactions of selected biological thiols and related molecules with oxidized glutathione. J. Org. Chem. 1992, 57, 123–127. [Google Scholar] [CrossRef]
- Cuozzo, J.W.; Kaiser, C.A. Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1999, 1, 130–135. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-C.; Ho, C.-P.; Wang, Y.-L.; Chen, P.-C.; Wang, P.-Y.; Chen, H.-Y. Vapor-Deposited Reactive Coating with Chemically and Topographically Erasable Properties. Polymers 2019, 11, 1595. https://doi.org/10.3390/polym11101595
Chiang Y-C, Ho C-P, Wang Y-L, Chen P-C, Wang P-Y, Chen H-Y. Vapor-Deposited Reactive Coating with Chemically and Topographically Erasable Properties. Polymers. 2019; 11(10):1595. https://doi.org/10.3390/polym11101595
Chicago/Turabian StyleChiang, Yu-Chih, Cuei-Ping Ho, Yin-Lin Wang, Po-Chun Chen, Peng-Yuan Wang, and Hsien-Yeh Chen. 2019. "Vapor-Deposited Reactive Coating with Chemically and Topographically Erasable Properties" Polymers 11, no. 10: 1595. https://doi.org/10.3390/polym11101595
APA StyleChiang, Y.-C., Ho, C.-P., Wang, Y.-L., Chen, P.-C., Wang, P.-Y., & Chen, H.-Y. (2019). Vapor-Deposited Reactive Coating with Chemically and Topographically Erasable Properties. Polymers, 11(10), 1595. https://doi.org/10.3390/polym11101595