Microwave-Assisted Synthesis of an Alternant Poly(fluorene–oxadiazole). Synthesis, Properties, and White Light-Emitting Devices
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
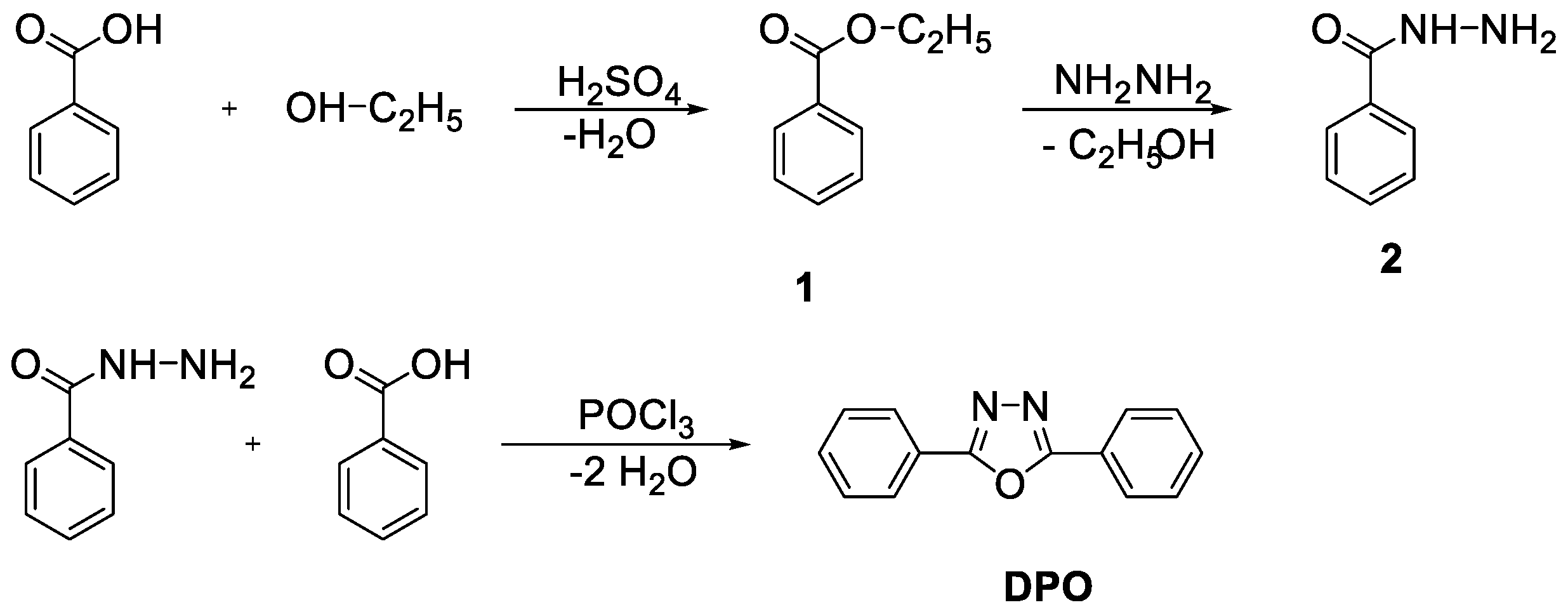
2.2.1. 2,5-diphenyl-1,3,4-oxadiazole (DPO)
2.2.2. 9,9-dihexyl-fluorene (DHF)
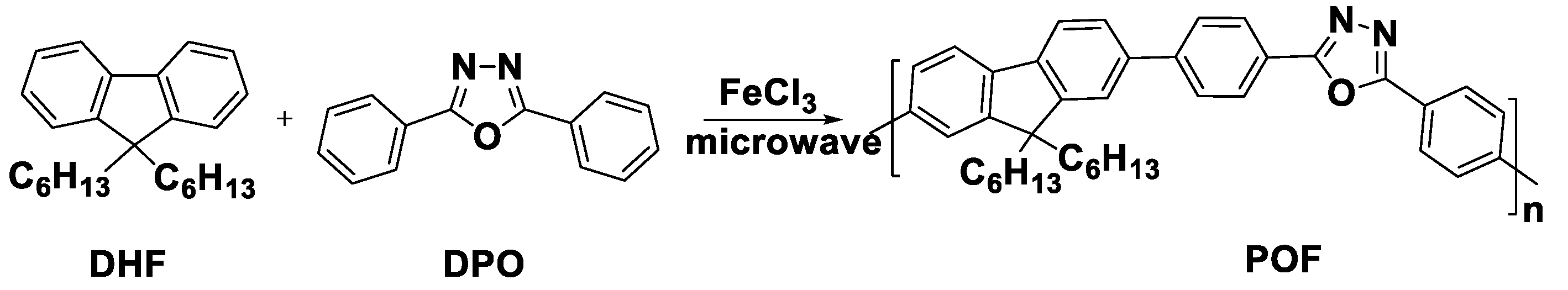
2.2.3. MW-assisted Synthesis of Poly(dihexyl fluorene-co diphenyl oxadiazole) (POF)
2.3. Equipment
Organic Light Emitting Device Preparation
3. Results and Discussions
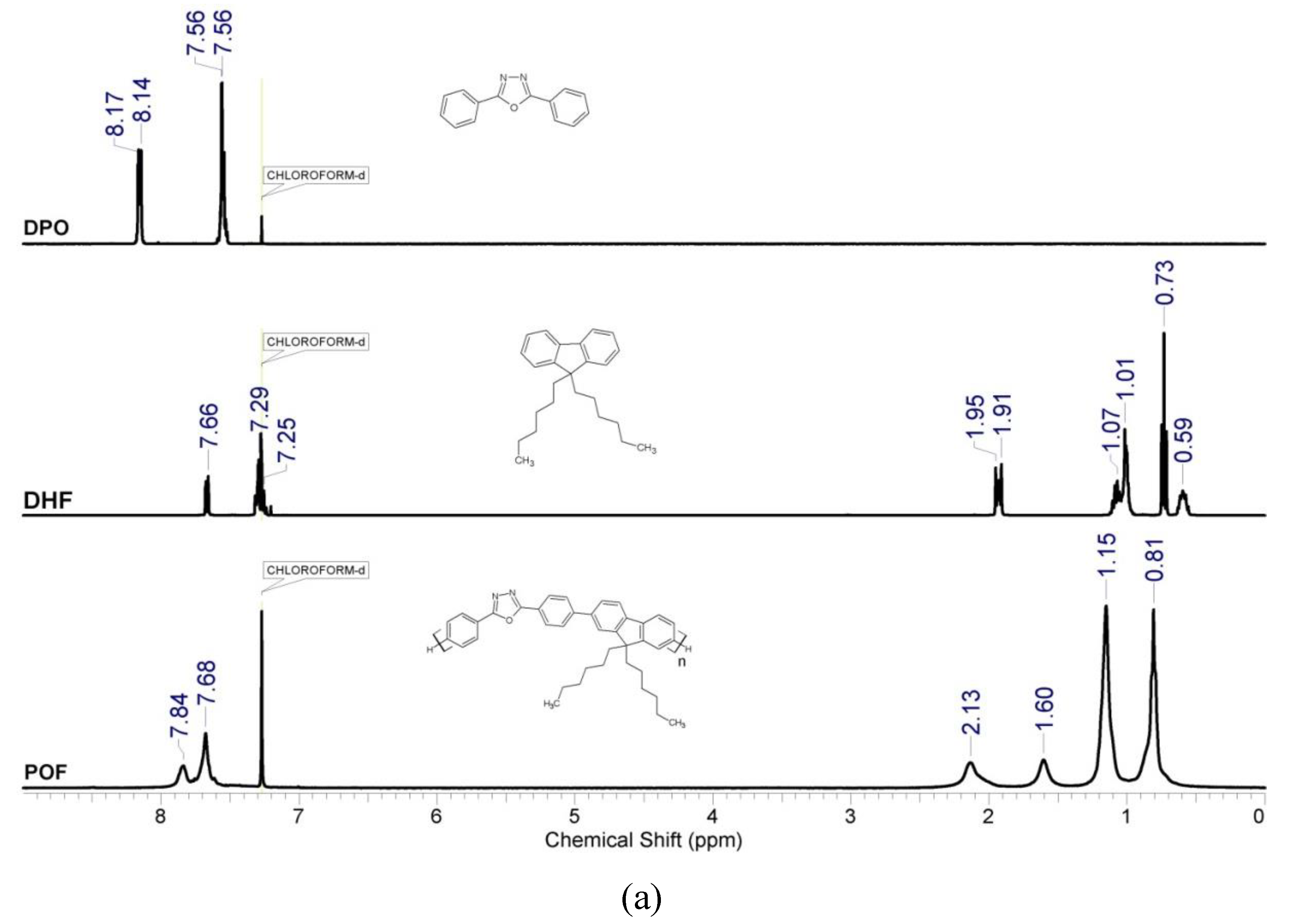
3.1. Structural Characterization
3.2. Polymer Morphology
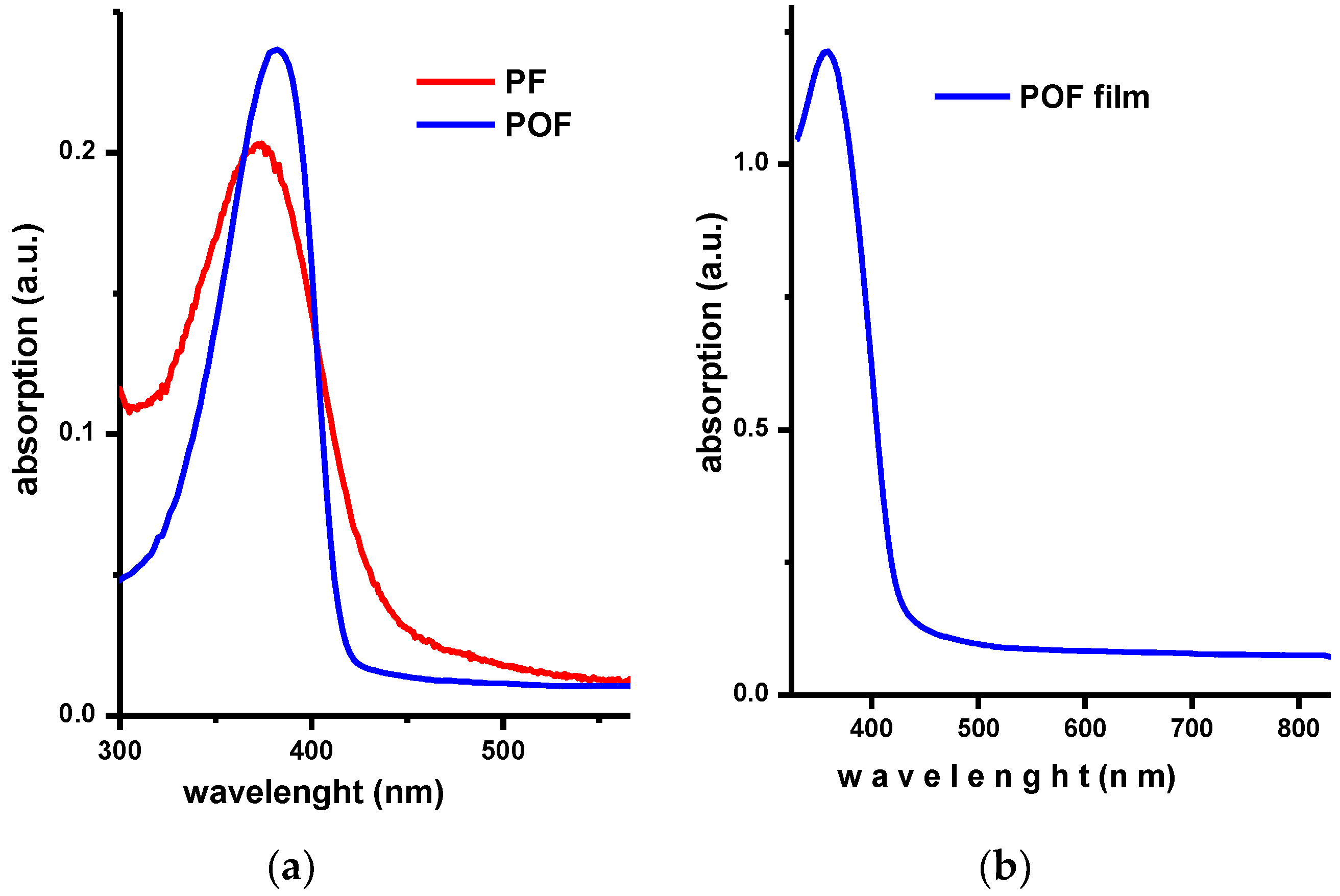
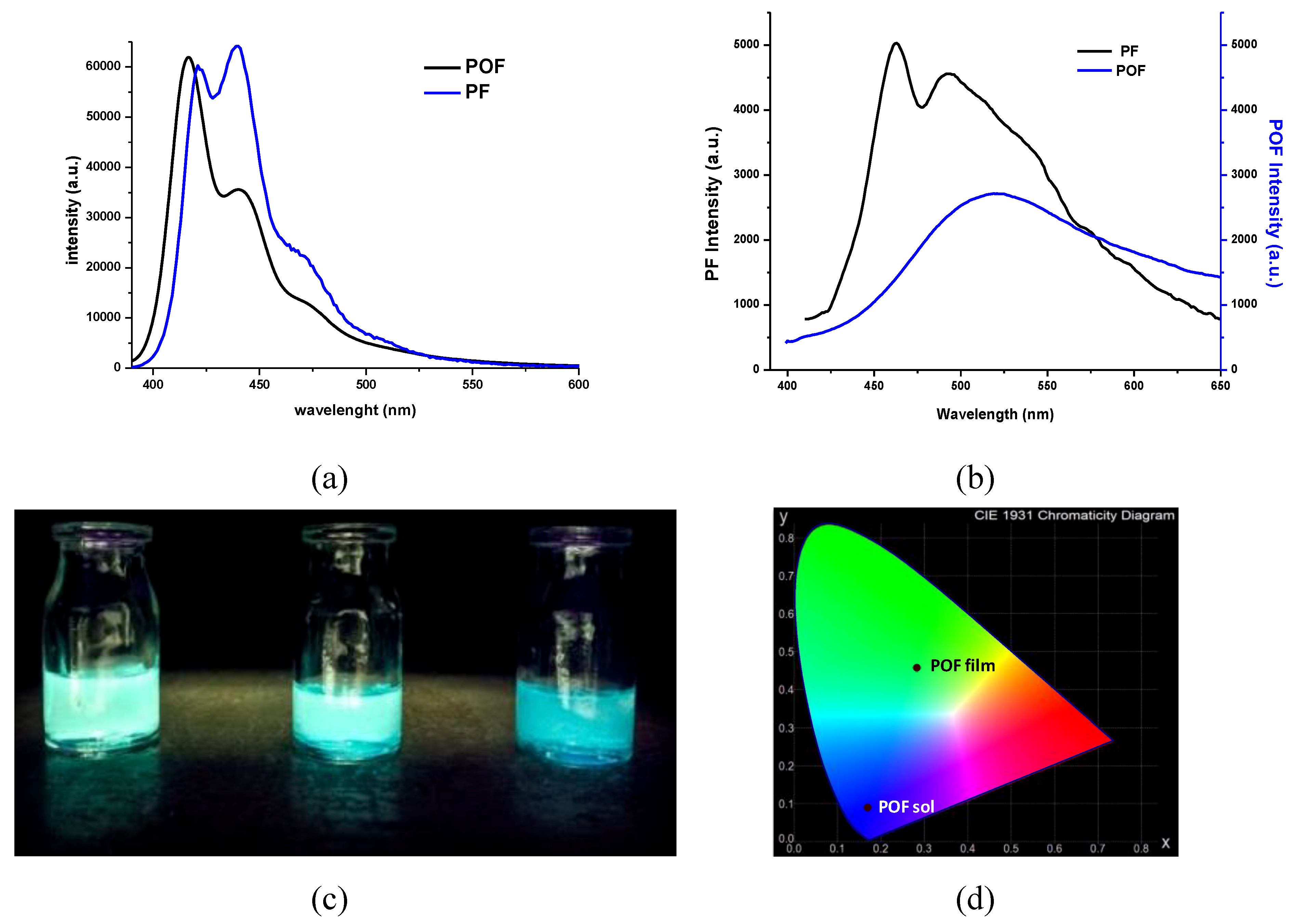
3.3. Photophysical Behavior
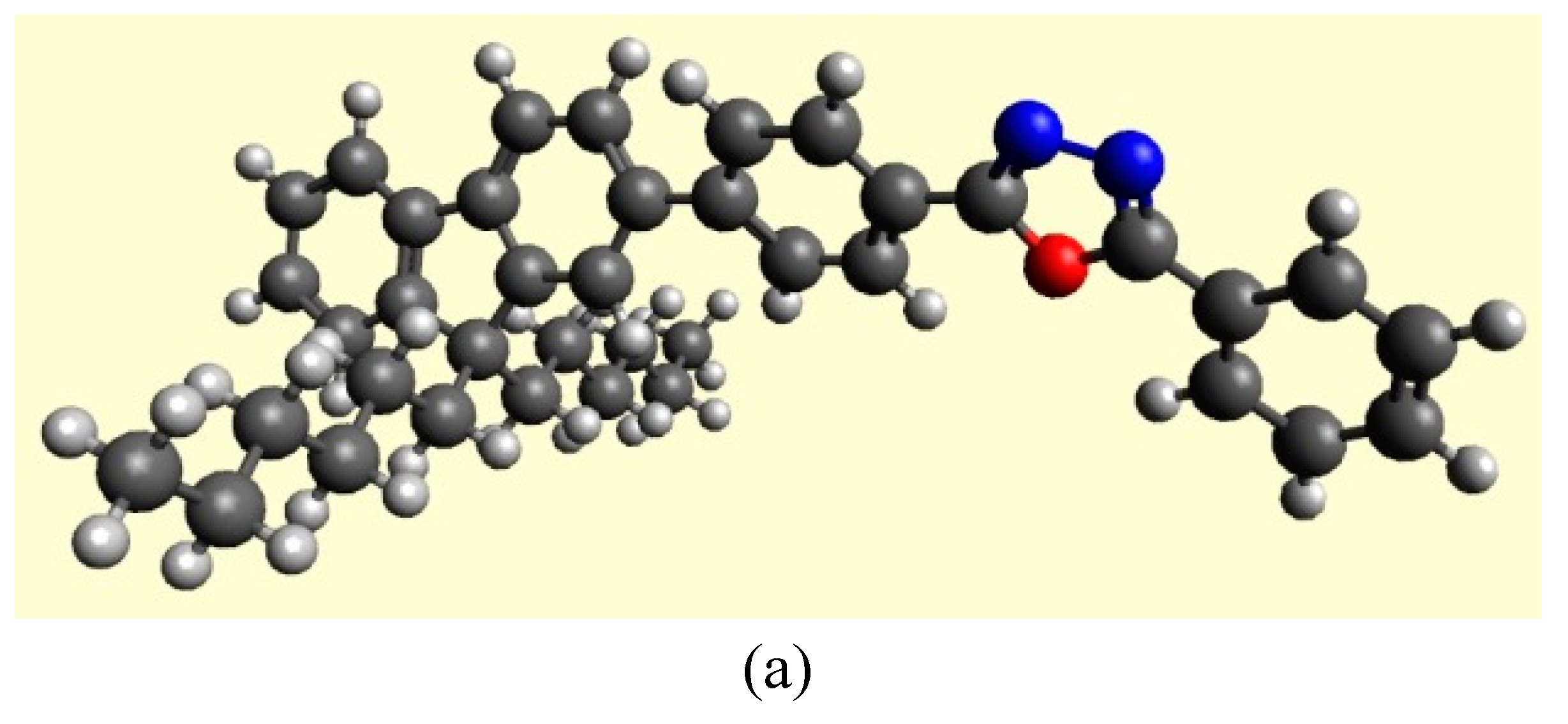
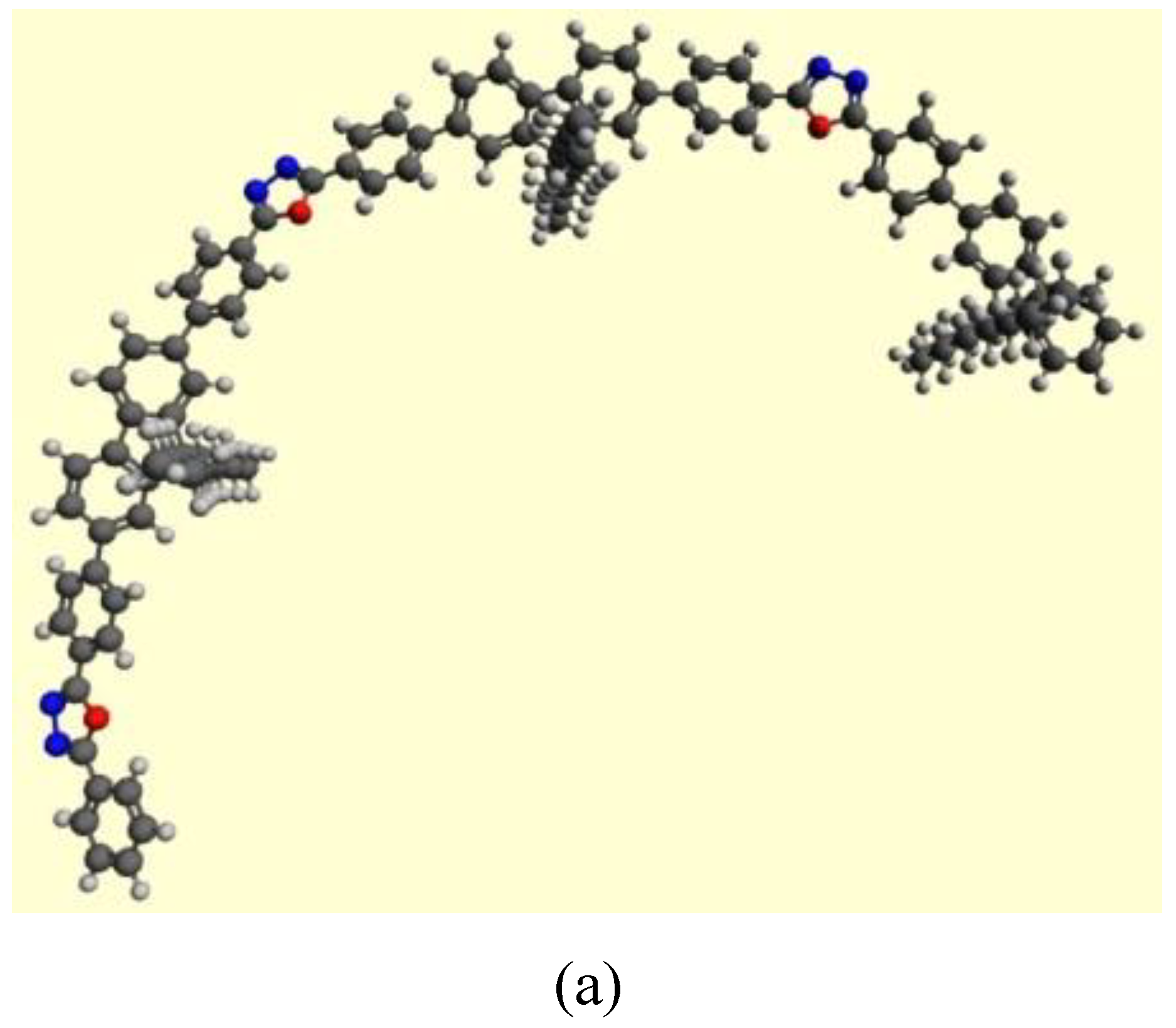
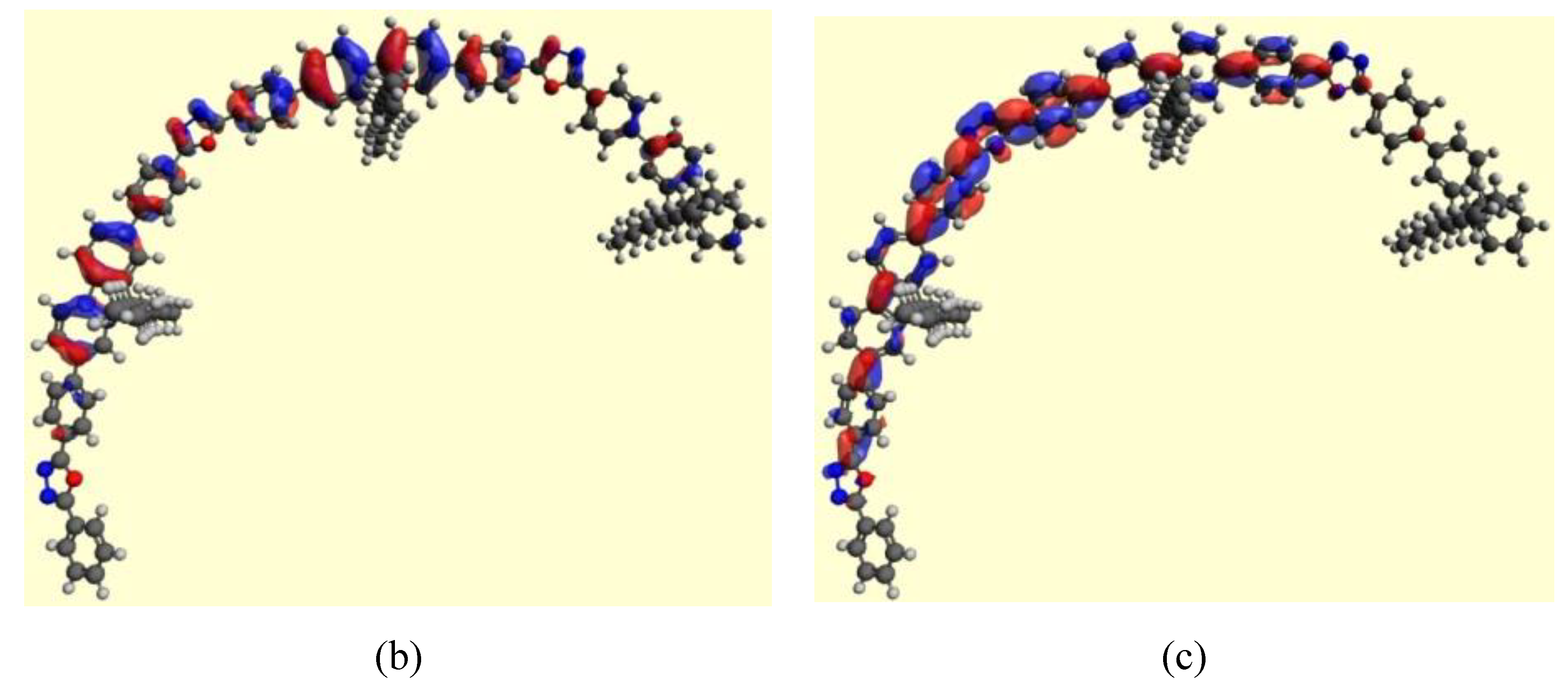
3.4. Theoretical Calculations
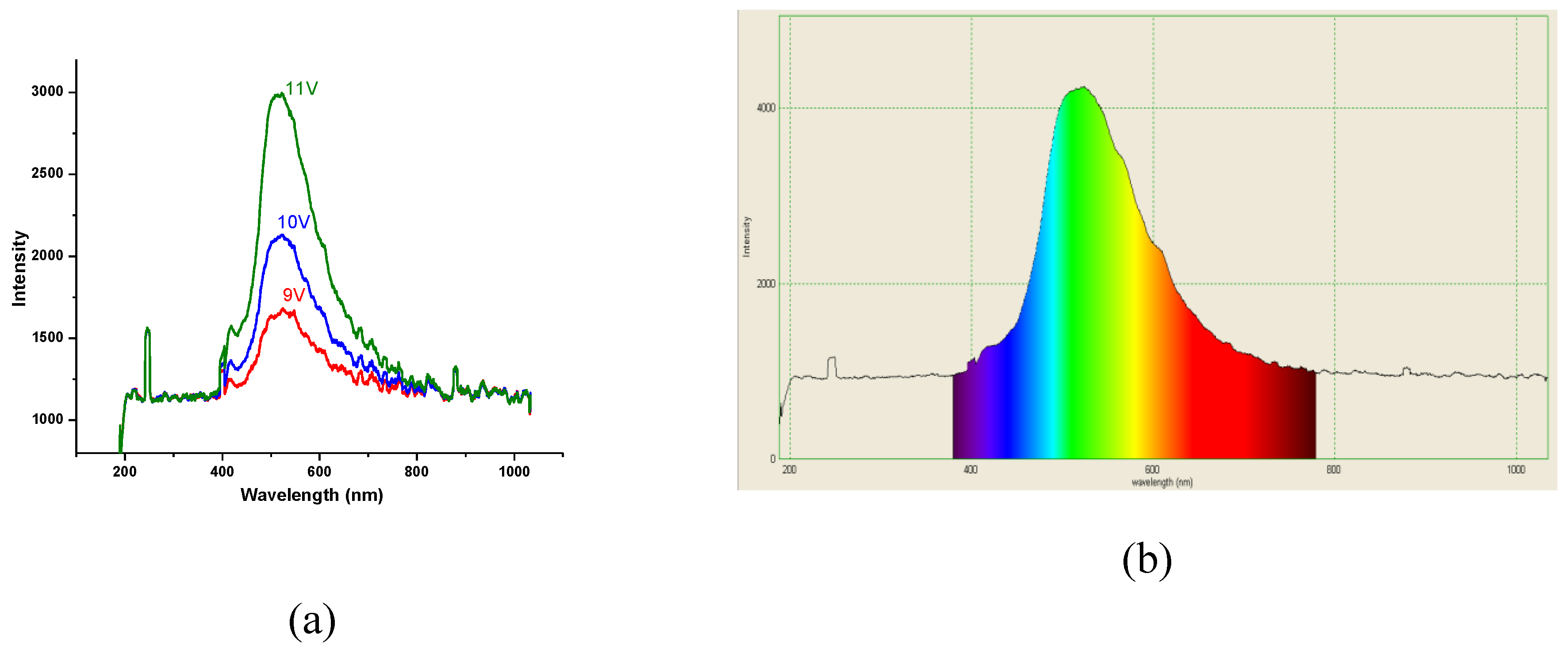
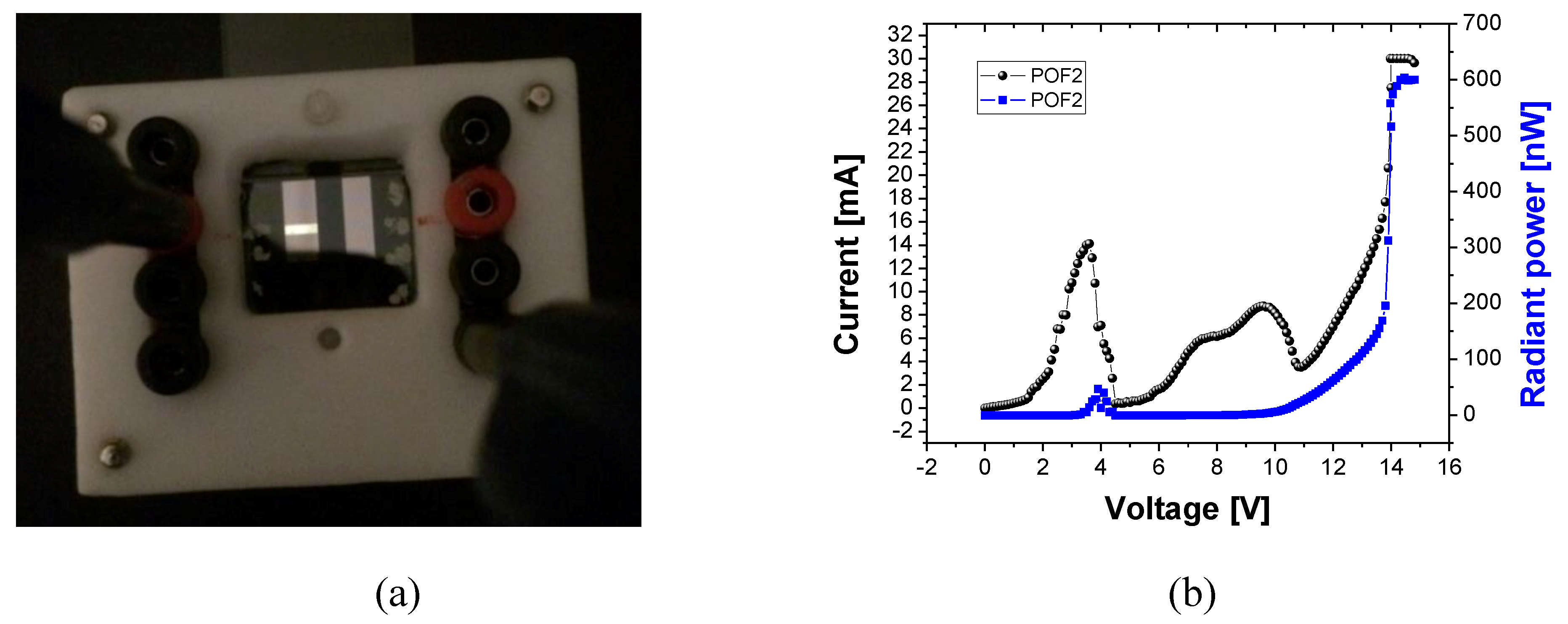
3.5. OLED Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Mitschke, U.; BaÈuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Liu, Y.F.; Feng, J.; Bi, Y.G.; Yin, D.; Sun, H.B. Recent developments in flexible organic light-emitting devices. Adv. Mater. Technol. 2019, 4, 1800371. [Google Scholar] [CrossRef]
- Wong, M.Y. Recent advances in polymer organic light-emitting diodes (pled) using non-conjugated polymers as the emitting layer and contrasting them with conjugated counterparts. J. Electron. Mater. 2017, 46, 6246–6281. [Google Scholar] [CrossRef]
- Luo, D.; Chen, Q.; Liu, B.; Qiu, Y. Emergence of flexible white organic light-emitting diodes. Polymers 2019, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sonar, P.; Chen, Z.K. Recent progress in fluorescent blue light-emitting materials. Curr. Org. Chem. 2010, 14, 2034–2069. [Google Scholar] [CrossRef]
- Ercan, E.; Tsai, P.C.; Chen, J.Y.; Lam, J.Y.; Hsu, L.C.; Chueh, C.C.; Chen, W.C. Stretchable and ambient stable perovskite/polymer luminous hybrid Nanofibers of multicolor fiber mats and their white LED applications. ACS Appl. Mater. Inter. 2019, 11, 23605–23615. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Guo, T.; Ying, L.; Xiong, J.; Yang, W.; Cao, Y. Synthesis and properties of blue-light-emitting oligo (fluorene-co-dibenzothiophene-S, S-dioxide) s. Dye. Pigment. 2019, 166, 502–514. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.Y.; Ong, G.L.; Tan, S.H.; Tan, Z.W.; Hii, Y.H.; Wong, Y.L.; Cheah, K.S.; Yap, S.L.; Ong, T.S.; et al. Photophysical and electroluminescence characteristics of polyfluorene derivatives with triphenylamine. Polymers 2019, 11, 840. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Influence of SiO2/TiO2 nanocomposite on the optoelectronic properties of PFO/MEH-PPV-based OLED devices. Polymers 2018, 10, 800. [Google Scholar] [CrossRef]
- Yang, L.; Liao, Y.; Feng, J.K.; Ren, A.M. Theoretical studies of the modulation of polymer electronic and optical properties through the introduction of the electron-donating 3, 4-ethylenedioxythiophene or electron-accepting pyridine and 1, 3, 4-oxadiazole moieties. J. Phys. Chem. A 2005, 109, 7764–7774. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liang, J.; Zhong, W.; Guo, T.; Zhong, Z.; Peng, F.; Fan, B.; Ying, L.; Cao, Y. Improving the electroluminescence performance of blue light-emitting poly(fluorene-co-dibenzothiophene-S, S-dioxide) by tuning the intra-molecular charge transfer effects and temperature-induced orientation of the emissive layer structure. J. Mater. Chem. C 2019, 7, 5630–5638. [Google Scholar] [CrossRef]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1, 3, 4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Chen, R.T.; Chen, S.H.; Hsieh, B.Y.; Chen, Y. Synthesis, photophysics, and electroluminescent performance of stable blue-light-emitting copoly (9, 9-diarylfluorene) s. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 2821–2834. [Google Scholar] [CrossRef]
- Chen, R.T.; Su, W.F.; Chen, Y. Highly efficient and stable blue-light-emitting copolyfluorene consisting of carbazole, oxadiazole, and charge-trapping anthracene groups. J. Polym. Sci. Part. A Polym. Chem. 2011, 49, 184–191. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, Y.J.; Park, J.W.; Kim, J.H.; Kwon, S.K. Design and synthesis of new fluorene-based blue light emitting polymer containing electron donating alkoxy groups and electron withdrawing oxadiazole. Macromol. Res. 2007, 15, 216–220. [Google Scholar] [CrossRef]
- Lee, S.J.; Gallegos, J.R.; Klein, J.; Curtis, M.D.; Kanicki, J. Poly(fluorene-oxadiazole) copolymer-based light-emitting devices on a plastic substrate. Synth. Met. 2005, 155, 1–10. [Google Scholar] [CrossRef]
- Ding, J.; Day, M.; Robertson, G.; Roovers, J. Synthesis and characterization of alternating copolymers of fluorene and oxadiazole. Macromolecules 2002, 35, 3474–3483. [Google Scholar] [CrossRef]
- Sun, M.; Zhong, C.; Li, F.; Cao, Y.; Pei, Q. A fluorene-oxadiazole copolymer for white light-emitting electrochemical cells. Macromolecules 2010, 43, 1714–1718. [Google Scholar] [CrossRef]
- Goker, S.; Hizalan, G.; Kutkan, S.; Udum, Y.A.; Cirpan, A.; Toppare, L. Incorporation of different conjugated linkers into low band gap polymers based on 5, 6-bis(octyloxy)-2, 1, 3 benzooxadiazole for tuning optoelectronic properties. J. Polym. Sci. Polym. Chem. 2016, 54, 2459–2467. [Google Scholar] [CrossRef]
- Hamciuc, E.; Homocianu, M.; Hamciuc, C.; Carja, I.D. Synthesis and photophysical study of new blue fluorescent poly (azomethine-1, 3, 4-oxadiazole) s containing dimethylamino groups. High. Perform. Polym. 2017, 30, 339–346. [Google Scholar] [CrossRef]
- Yu, C.Y.; Shih, T.Y. Alternating copolymers containing fluorene and oxadiazole derivatives for fluorescent chemosensors. Synth. Met. 2014, 191, 12–18. [Google Scholar] [CrossRef]
- Sung, H.H.; Lin, H.C. Novel alternating fluorene-based conjugated polymers containing oxadiazole pendants with various terminal groups. Macromolecules 2004, 37, 7945–7954. [Google Scholar] [CrossRef]
- Shu, C.F.; Dodda, R.; Wu, F.Y. Highly efficient blue-light-emitting diodes from polyfluorene containing bipolar pendant groups. Macromolecules 2003, 36, 6698–6703. [Google Scholar] [CrossRef]
- Liu, J.W.; Xu, Y.N.; Qin, C.Y.; Wang, Z.N.; Wu, C.J.; Li, Y.H.; Wang, S.; Zhang, K.Y.; Huang, W. Simple fluorene oxadiazole-based Ir(iii) complexes with AIPE properties: Synthesis, explosive detection and electroluminescence studies. Dalton Trans. 2019, 48, 13305–13314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Wang, J.Y.; Wu, J.X.; Li, Y.; Zou, G.D.; Xie, G.H.; Lin, Z.Q.; Xie, L.H.; Zhang, X.W.; Zhao, J.F. Solution-processible 1, 3, 4-oxadiazole/spiro [fluorene-9, 9′-xanthene] hybrid as efficient host for green thermally activated delayed fluorescence devices. Dye. Pigment. 2019, 166, 168–173. [Google Scholar] [CrossRef]
- Homocianu, M.; Ipate, A.M.; Hamciuc, C.; Airinei, A. Photophysical Properties of some 1, 3, 4-oxadiazole derivatives containing phenolphtalein, fluorene and bisphenol A units. J. Fluoresc. 2018, 28, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Bejan, V.; Mantu, D.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of isoindolo-1, 2-diazine: A comparative study. Ultrason. Sonochem. 2012, 19, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Mallakpour, S.; Azimi, F. Microwave and ultrasound-assisted synthesis of poly (vinyl chloride)/riboflavin modified MWCNTs: Examination of thermal, mechanical and morphology properties. Ultrason. Sonochem. 2018, 41, 27–36. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Zhang, Y.; Lu, P.; Liu, L.; Ma, Y. Microwave-assisted FeCl3-mediated rapid synthesis of poly (9, 9-dihexylfluorene) with high molecular weight. Polymer 2014, 55, 5346–5349. [Google Scholar] [CrossRef]
- Negi, V.J.; Sharma, A.K.; Ram, V. Design, synthesis and pharmacological evaluation of 2, 5 di phenyl 1, 3, 4-oxadiazole derivatives as selective COX-2 inhibitors. Int. J. Pharm. Chem. 2013, 3, 24–31. [Google Scholar]
- Singh, P.; Sharma, P.K.; Sharma, J.K.; Upadhyay, A.; Kumar, N. Synthesis and evaluation of substituted diphenyl-1, 3, 4-oxadiazole derivatives for central nervous system depressant activity. Org. Med. Chem. Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry, 2nd ed.; Thieme Publishing Group: Stuttgart, Germany, 2007. [Google Scholar]
- Damaceanu, M.D.; Marin, L.; Manicke, T.; Bruma, M. Solid-state properties of mesomorphic copolymers containing oxadiazole and fluorene units. Soft Mater. 2009, 7, 164–184. [Google Scholar] [CrossRef]
- Wang, D.H.; Shen, Z.; Guo, M.; Cheng, S.Z.D.; Harris, F.W. Synthesis and properties of polyimides containing multiple alkyl side chains. Macromolecules 2007, 40, 889–900. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of biocompatible glycodynameric hydrogels joining two natural motifs by dynamic constitutional chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Giri, G.; Verploegen, E.; Mannsfeld, S.; Atahan-Evrenk, S.; Kim, D.H.; Lee, S.Y.; Becerril, H.A.; Aspuru-Guzik, A.; Toney, M.F.; Bao, Z. Tuning charge transport in solution-sheared organic semiconductors using lattice strain. Nature 2011, 480, 504–508. [Google Scholar] [CrossRef]
- Marin, L.; Perju, E.; Damaceanu, M.D. Designing thermotropic liquid crystalline polyazomethines based on fluorene and/or oxadiazole chromophores. Eur. Polym. J. 2011, 47, 1284–1299. [Google Scholar] [CrossRef]
- Liu, J.; Sun, K.; Li, Z.; Gao, J.; Su, W.; Yang, J.; Zhang, J.; Wang, P.; Zhang, Q. Banded texture of photo-aligned azobenzene-containing side-chain liquid crystalline polymers. Polymer 2004, 45, 4331–4336. [Google Scholar] [CrossRef]
- Marin, L.; Bejan, A.; Ailincai, D.; Belei, D. Poly (azomethine-phenothiazine) s with efficient emission in solid state. Eur. Polym. J. 2017, 95, 127–137. [Google Scholar] [CrossRef]
- Zabulica, A.; Perju, E.; Bruma, M.; Marin, L. Novel luminescent liquid crystalline polyazomethines. Synthesis and study of thermotropic and photoluminescent properties. Liq. Cryst. 2014, 41, 252–262. [Google Scholar] [CrossRef]
- Marin, L.; Zabulica, A.; Sava, M. Symmetric liquid crystal dimers containing a luminescent mesogen: Synthesis, mesomorphic behavior, and optical properties. Soft Mater. 2013, 11, 32–39. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lu, T.C.; Huang, C.I. Exploring the correlation between molecular conformation and UV–visible absorption spectra of two-dimensional thiophene-based conjugated polymers. Polymer 2013, 54, 6489–6499. [Google Scholar] [CrossRef]
- Wong, W.W.H.; Hooper, J.F.; Holmes, A.B. Silicon analogues of polyfluorene as materials for organic electronics. Aust. J. Chem. 2009, 62, 393–401. [Google Scholar] [CrossRef]
- Zhou, J.D.; Zhang, W.Q.; Liu, L.L.; Xie, Z.Q.; Ma, Y.G. Aggregation structures of organic conjugated molecules on their optoelectronic properties. Chin. Chem. Lett. 2016, 27, 1350–1356. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Zeng, G.; Yu, W.L.; Chua, S.J.; Huang, W. Spectral and thermal spectral stability study for fluorene-based conjugated polymers. Macromolecules 2002, 35, 6907–6914. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gordon, M.S.; Schmidt, M.W. Advances in electronic structure theory: GAMESS a decade later. In Theory and Applications of Computational Chemistry: The first Forty Years; Dykstra, C.E., Frenking, G., Kim, K.S., Scuseria, G.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1167–1189. [Google Scholar]
- Wielopolski, M.; Linton, K.E.; Marszalek, M.; Gulcur, M.; Bryce, M.R.; Moser, J.E. Harvesting UV photons for solar energy conversion applications. Phys. Chem. Chem. Phys. 2014, 16, 2090–2099. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Yang, C.; Zhong, C.; Zhang, K.; Qin, J.; Ma, D. Tuning the optoelectronic properties of carbazole/oxadiazole hybrids through linkage modes: Hosts for highly efficient green electrophosphorescence. Adv. Funct. Mater. 2010, 20, 304–311. [Google Scholar] [CrossRef]
- Weinfurtner, K.H.; Fujikawa, H.; Tokito, S.; Taga, Y. Highly efficient pure blue electroluminescence from polyfluorene: Influence of the molecular weight distribution on the aggregation tendency. Appl. Phys. Lett. 2000, 76, 2502–2504. [Google Scholar] [CrossRef]
- Jessop, I.A.; Díaz, F.R.; Terraza, C.A.; Tundidor-Camba, A.; Leiva, Á.; Cattin, L.; Bèrnede, J.C. PANI branches onto donor-acceptor copolymers: Synthesis, characterization and electroluminescent properties of new 2d-materials. Polymers 2018, 10, 553. [Google Scholar] [CrossRef] [PubMed]
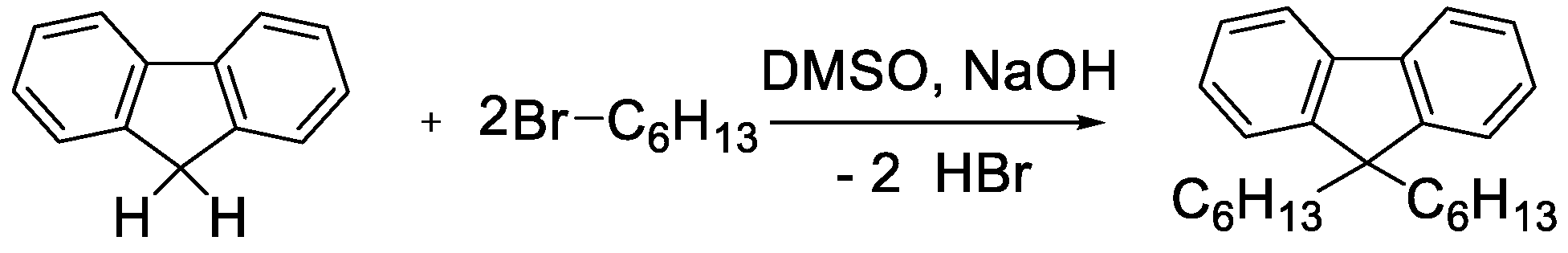
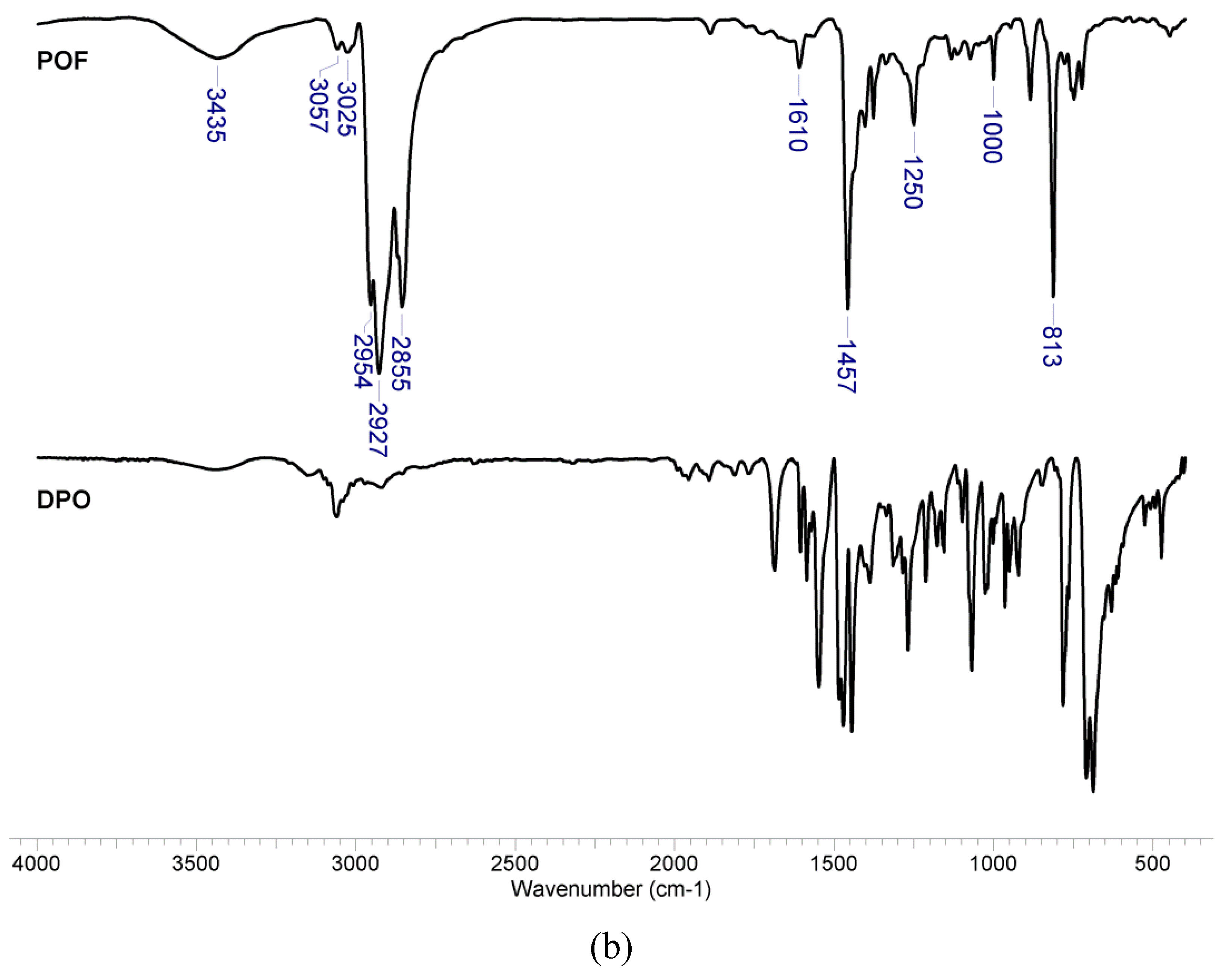
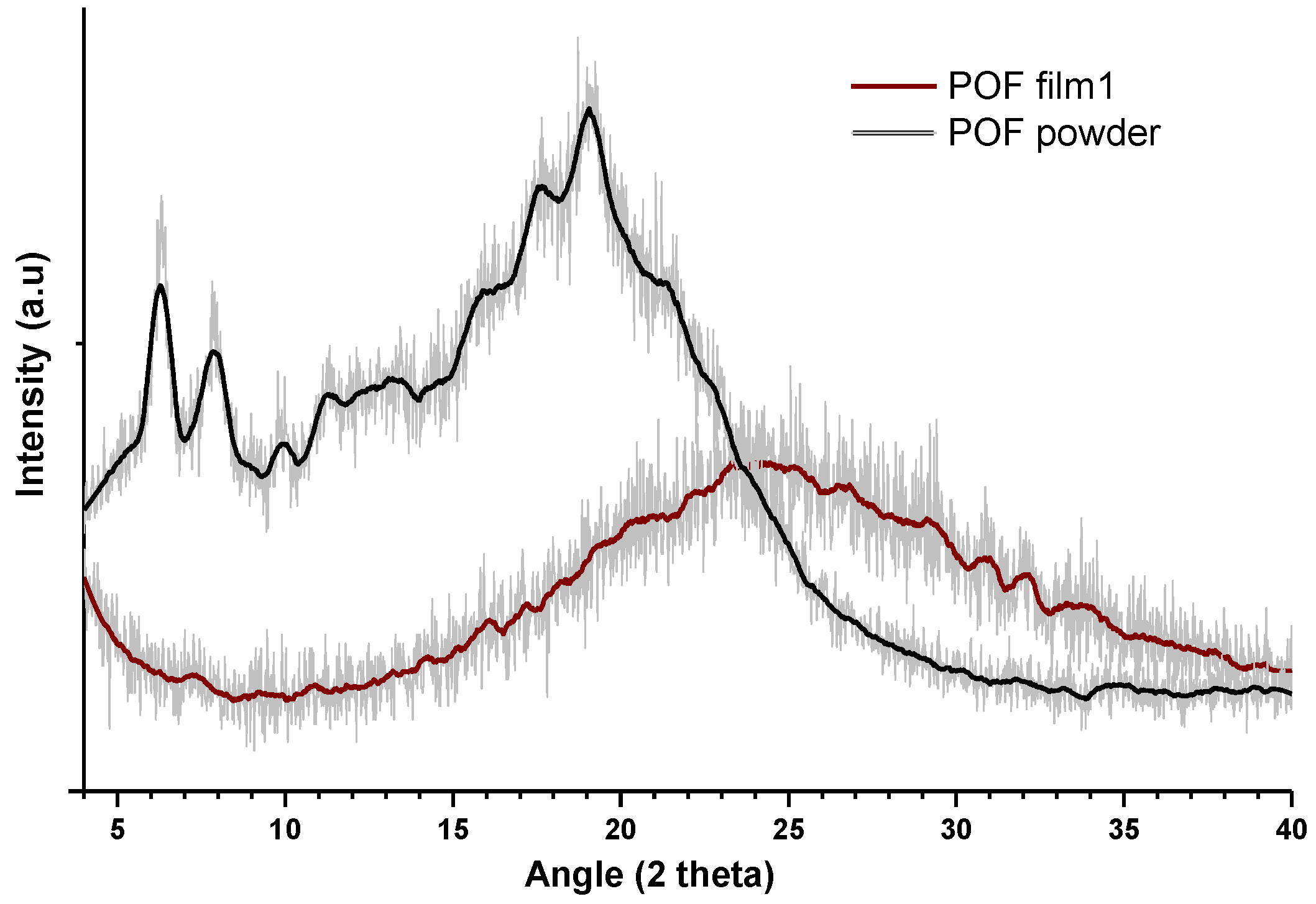
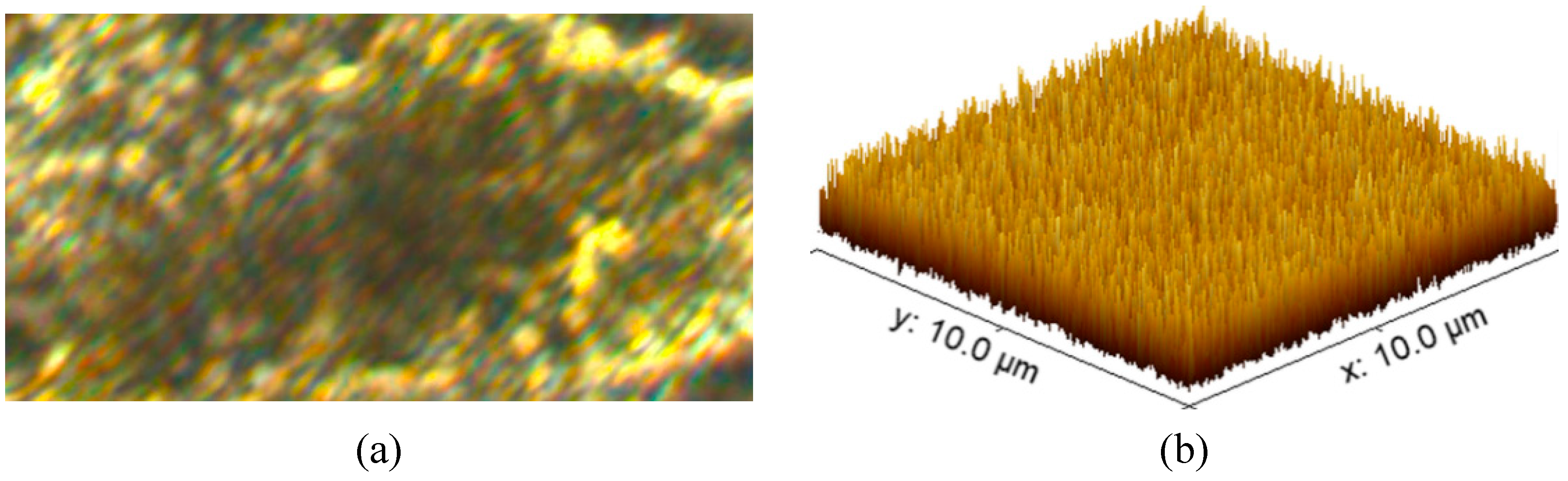
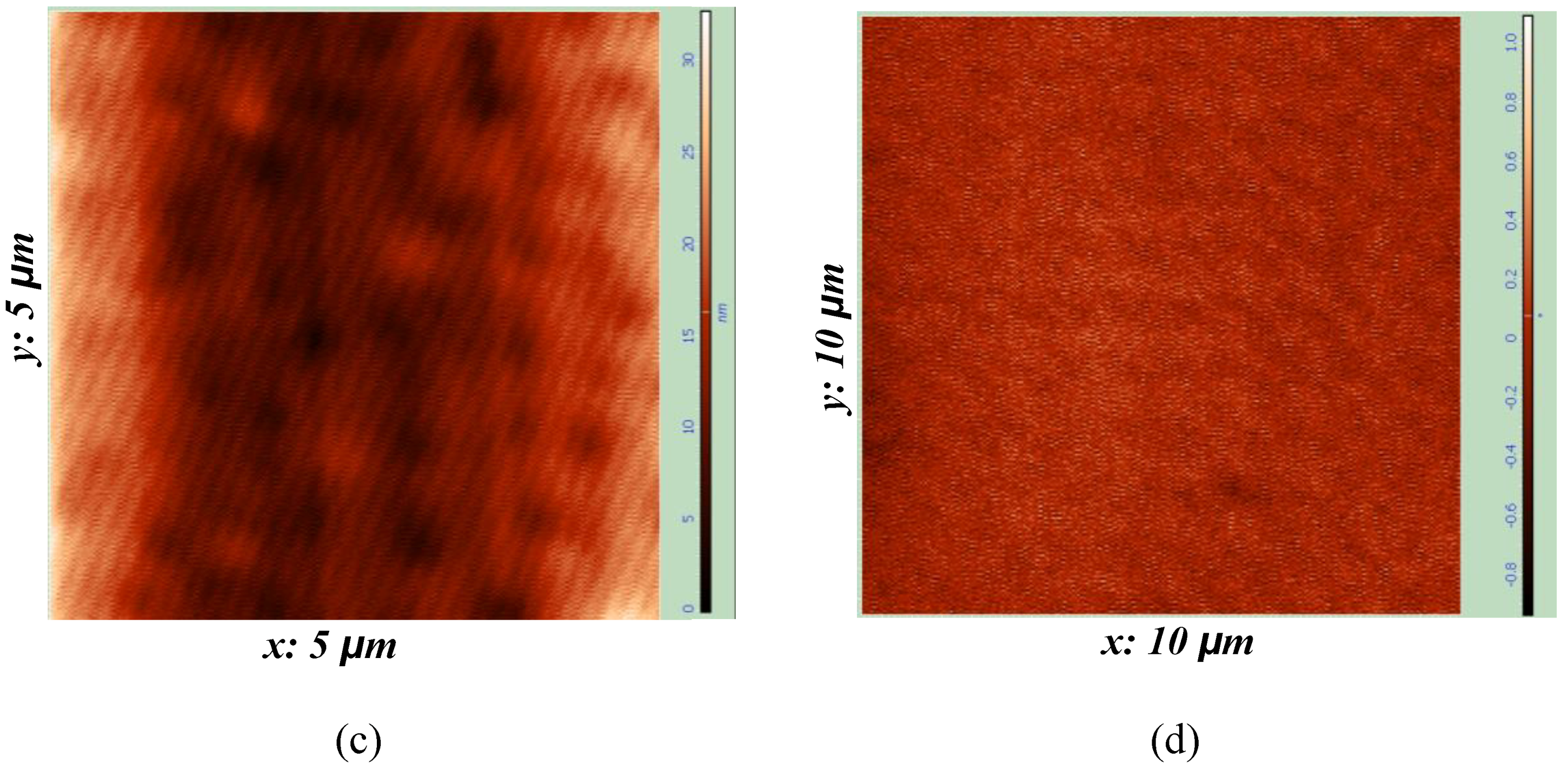

| Code | DHF: DPO (mol/mol) | η (%) | Mn (g/mol) | Mw/Mn |
|---|---|---|---|---|
| PF | 1:0 | 47 | 56 350 | 2.8 |
| POF1 | 1:3 | 5.54 | 89 000 | 3.2 |
| POF2 | 1:1 | 55.4 | 84 800 | 1.9 |
| POF3 | 3:1 | 35.15 | 80 100 | 3.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovici, D.; Diaconu, A.; Rotaru, A.; Marin, L. Microwave-Assisted Synthesis of an Alternant Poly(fluorene–oxadiazole). Synthesis, Properties, and White Light-Emitting Devices. Polymers 2019, 11, 1562. https://doi.org/10.3390/polym11101562
Popovici D, Diaconu A, Rotaru A, Marin L. Microwave-Assisted Synthesis of an Alternant Poly(fluorene–oxadiazole). Synthesis, Properties, and White Light-Emitting Devices. Polymers. 2019; 11(10):1562. https://doi.org/10.3390/polym11101562
Chicago/Turabian StylePopovici, Dumitru, Andrei Diaconu, Aurelian Rotaru, and Luminita Marin. 2019. "Microwave-Assisted Synthesis of an Alternant Poly(fluorene–oxadiazole). Synthesis, Properties, and White Light-Emitting Devices" Polymers 11, no. 10: 1562. https://doi.org/10.3390/polym11101562
APA StylePopovici, D., Diaconu, A., Rotaru, A., & Marin, L. (2019). Microwave-Assisted Synthesis of an Alternant Poly(fluorene–oxadiazole). Synthesis, Properties, and White Light-Emitting Devices. Polymers, 11(10), 1562. https://doi.org/10.3390/polym11101562







