Optically Pumped Intensive Light Amplification from a Blue Oligomer
Abstract
1. Introduction
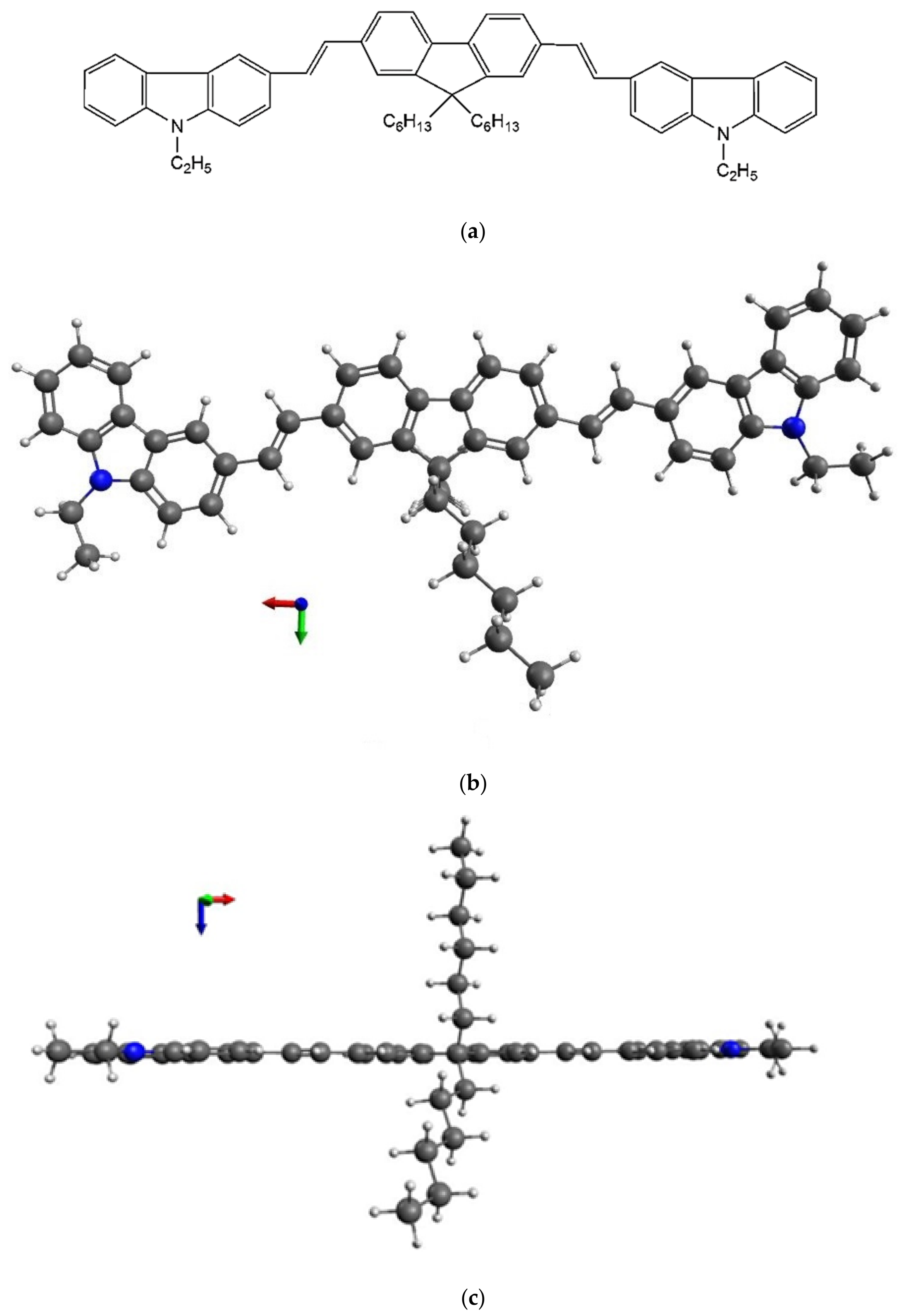
2. Materials and Methods
3. Results and Discussion
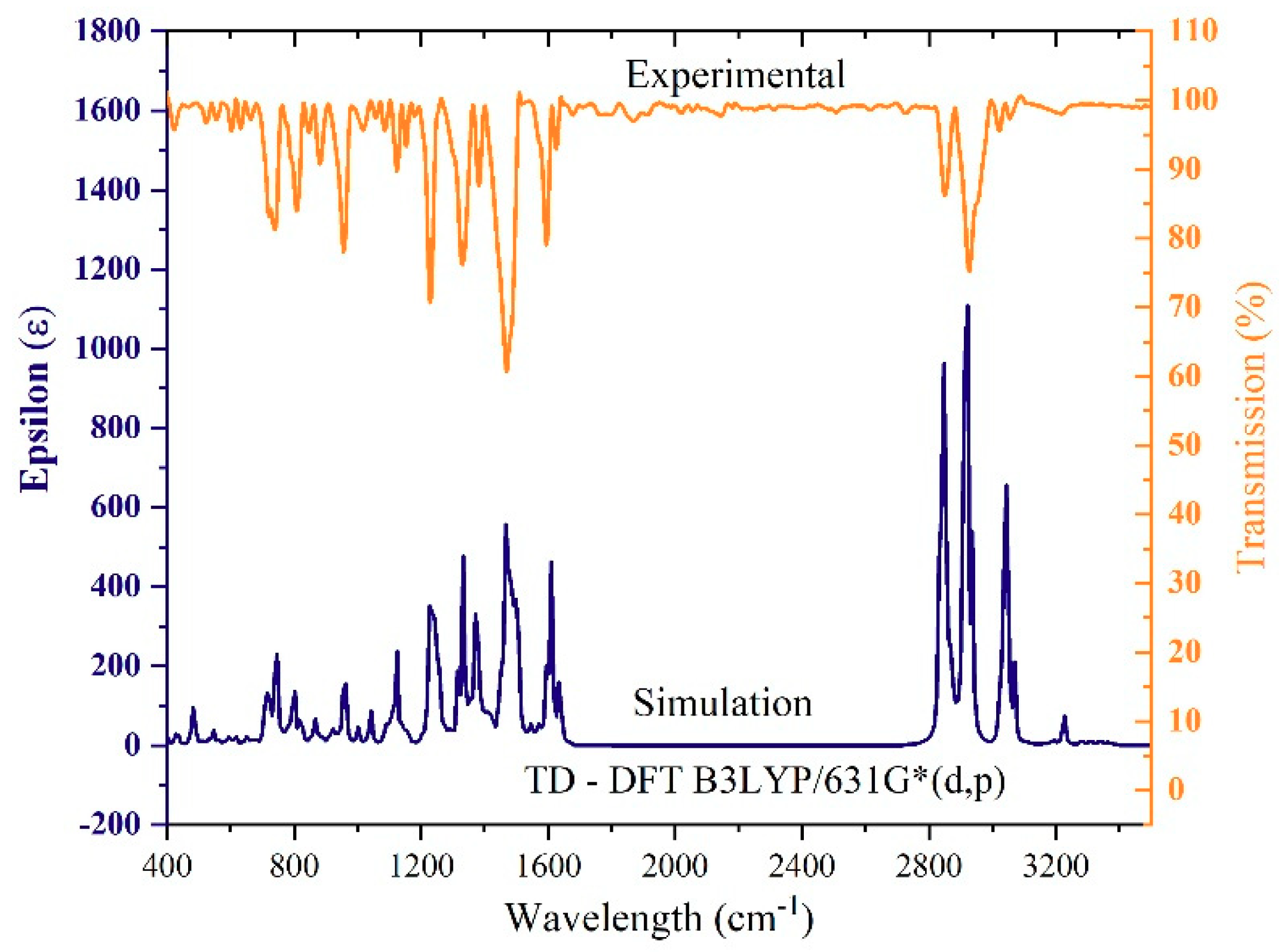
3.1. FTIR Investigation
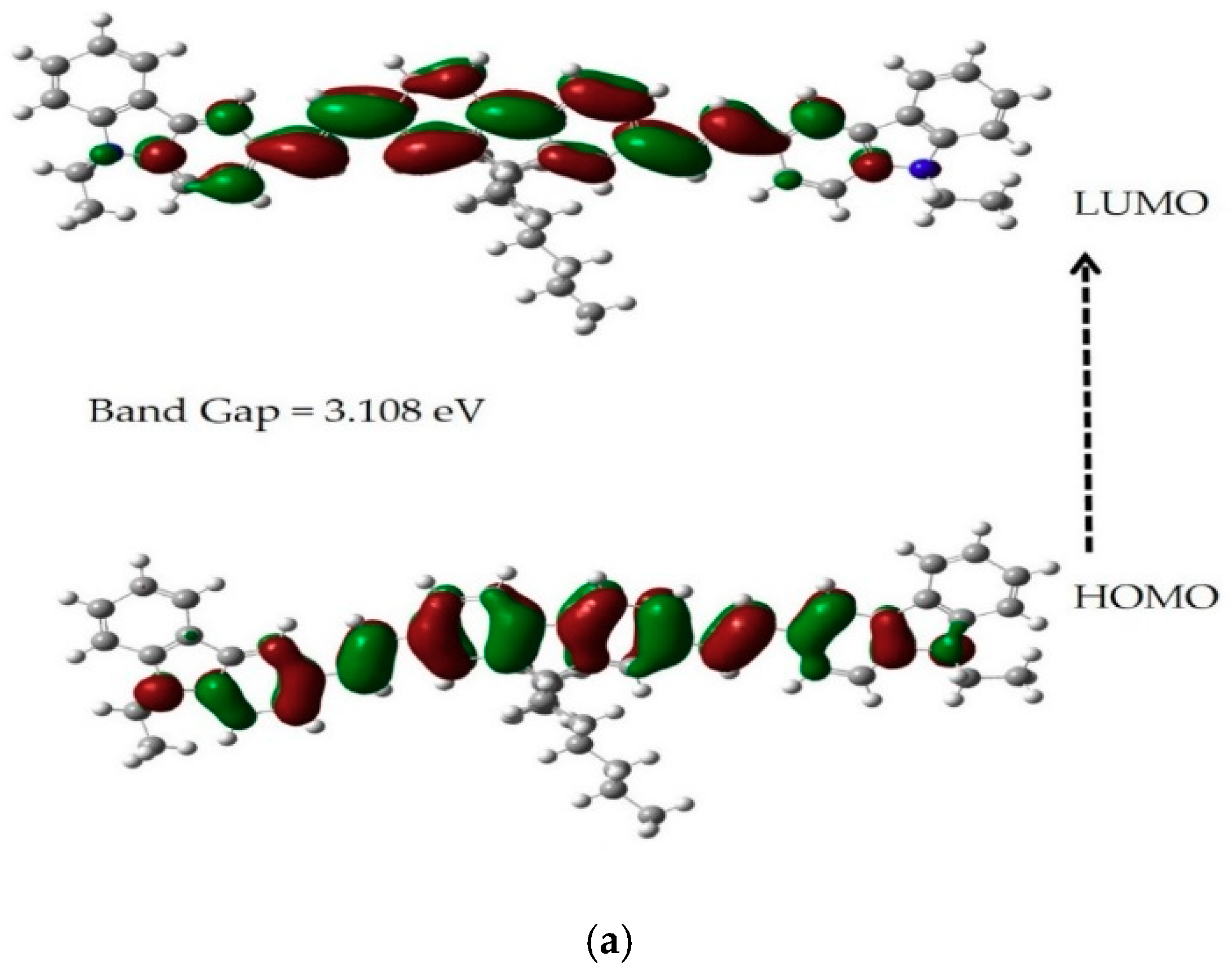
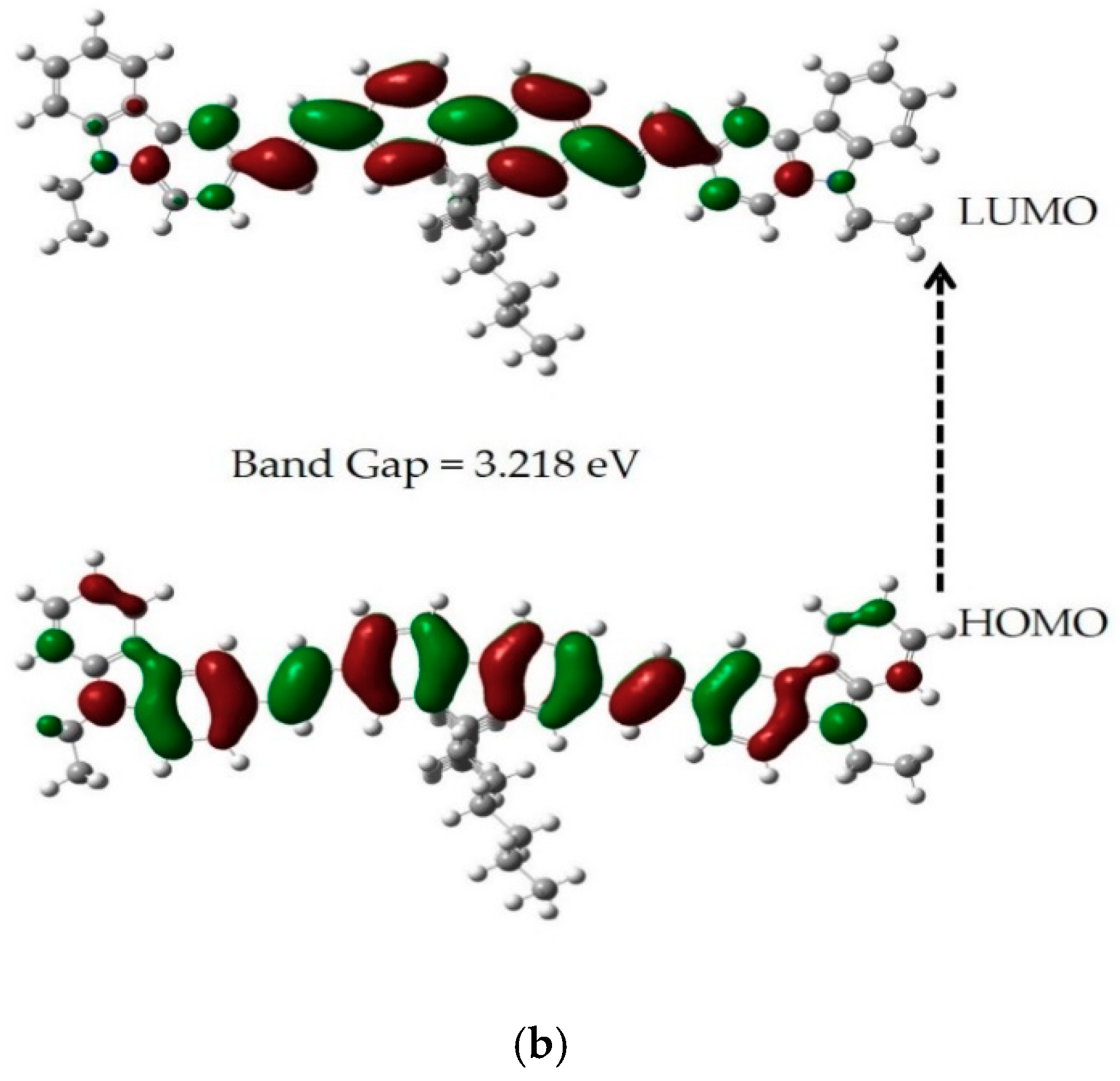
3.2. Time-Dependent DFT Calculation of HOMO-LUMO
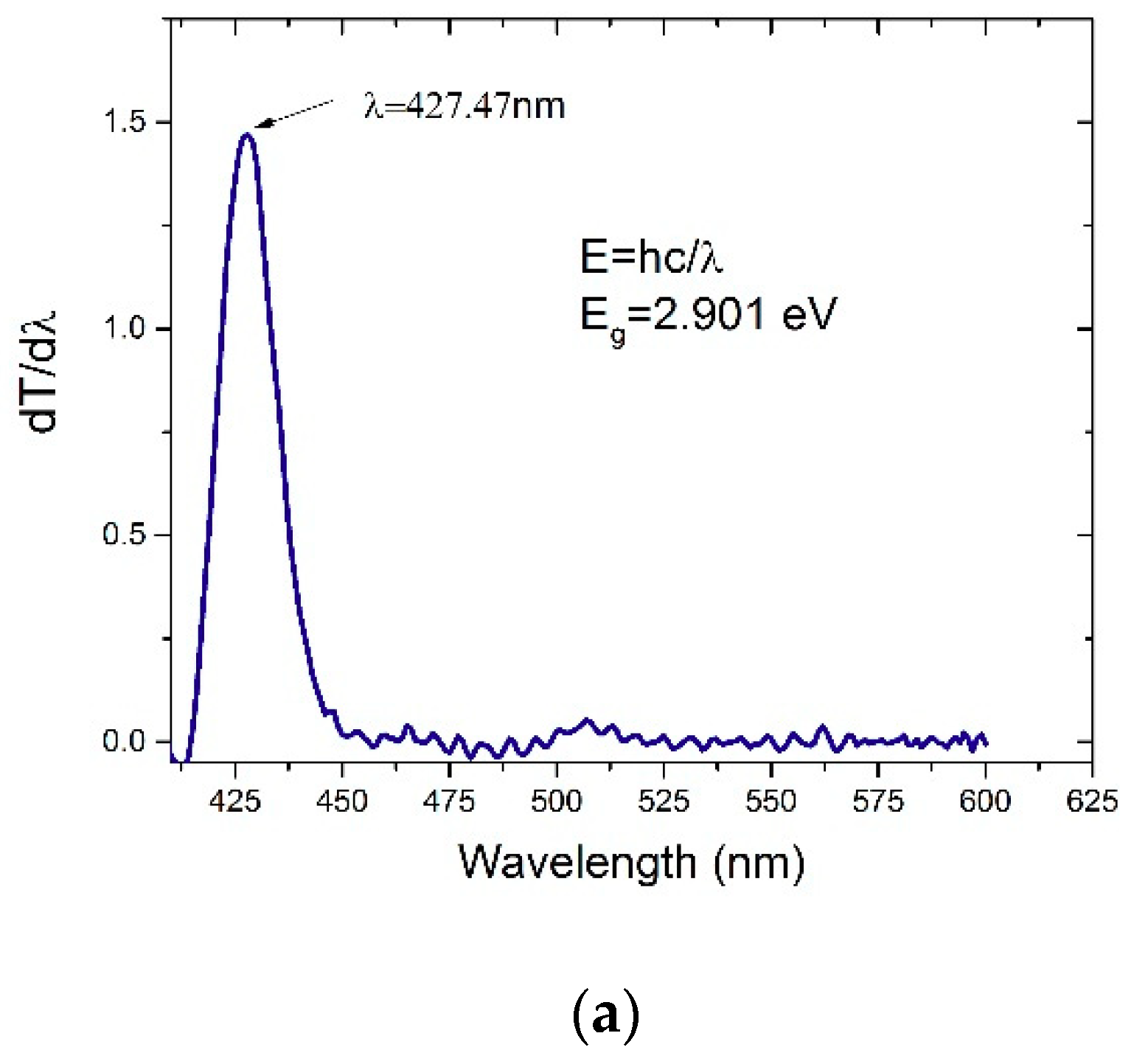
3.3. Time-Dependent DFT of CO’s Energies
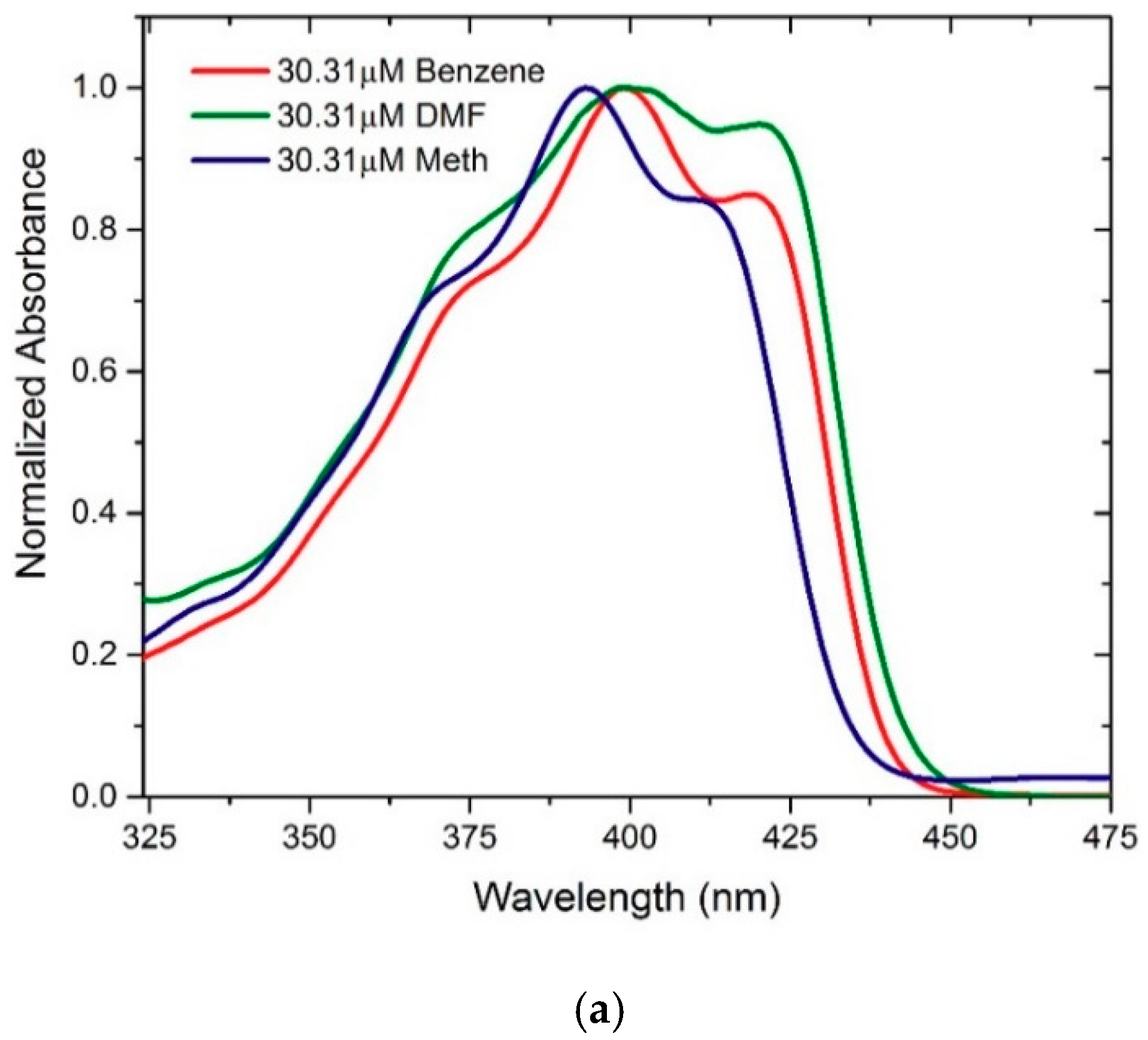
3.4. Experimental Absorption Spectra in Solution
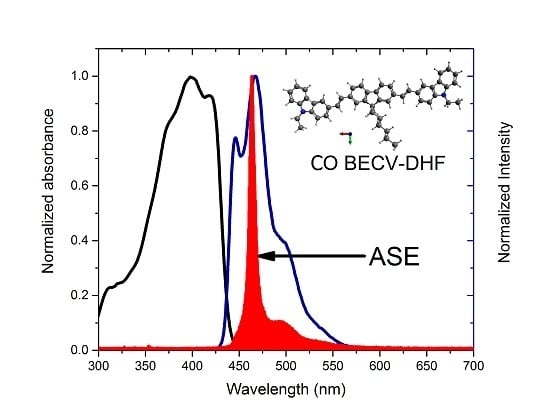
3.5. Absorption and Fluorescence Spectra of CO in Toluene
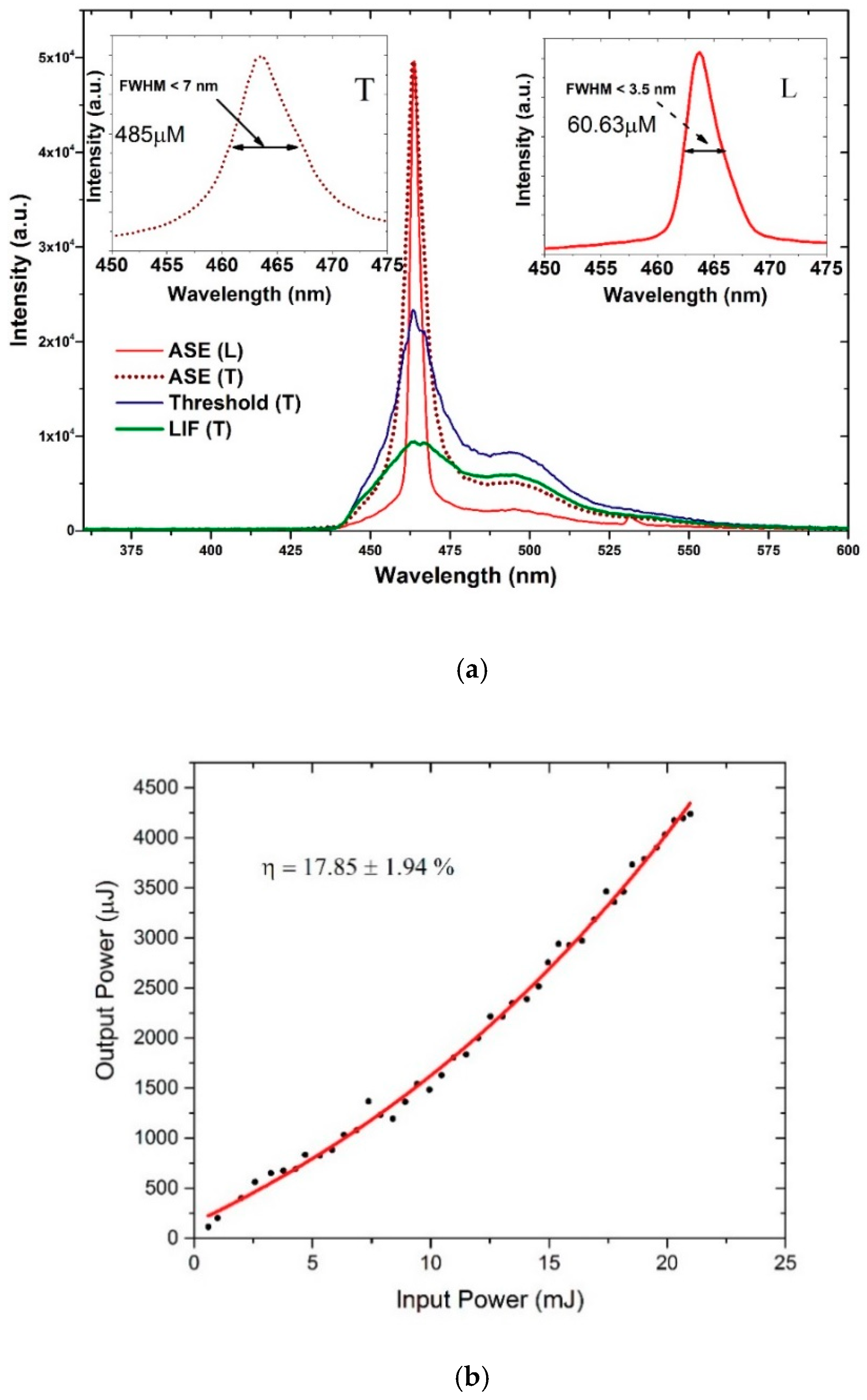
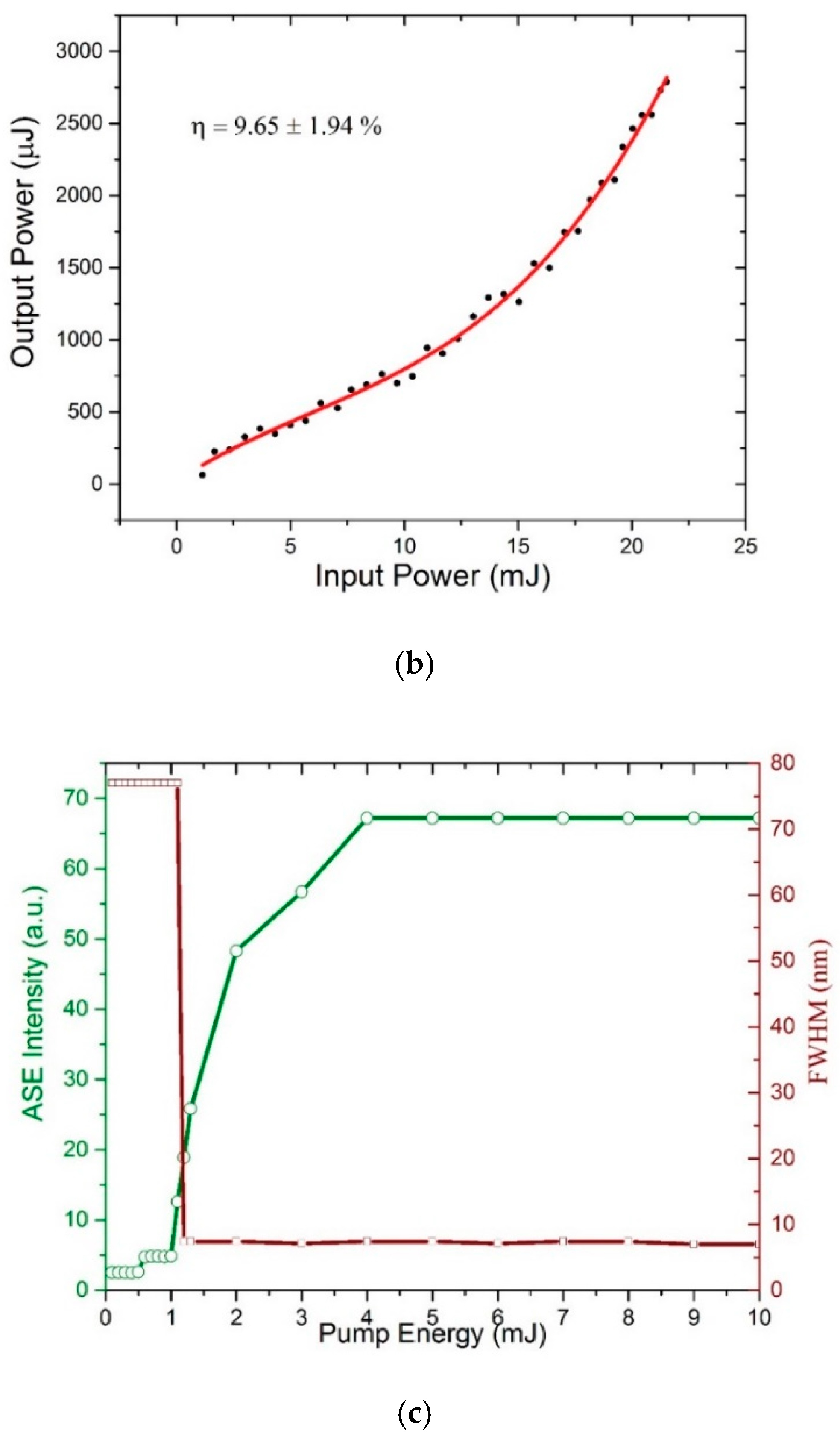
3.6. ASE (Mirrorless Laser) from CO BECV-DHF in Toluene and Acetone
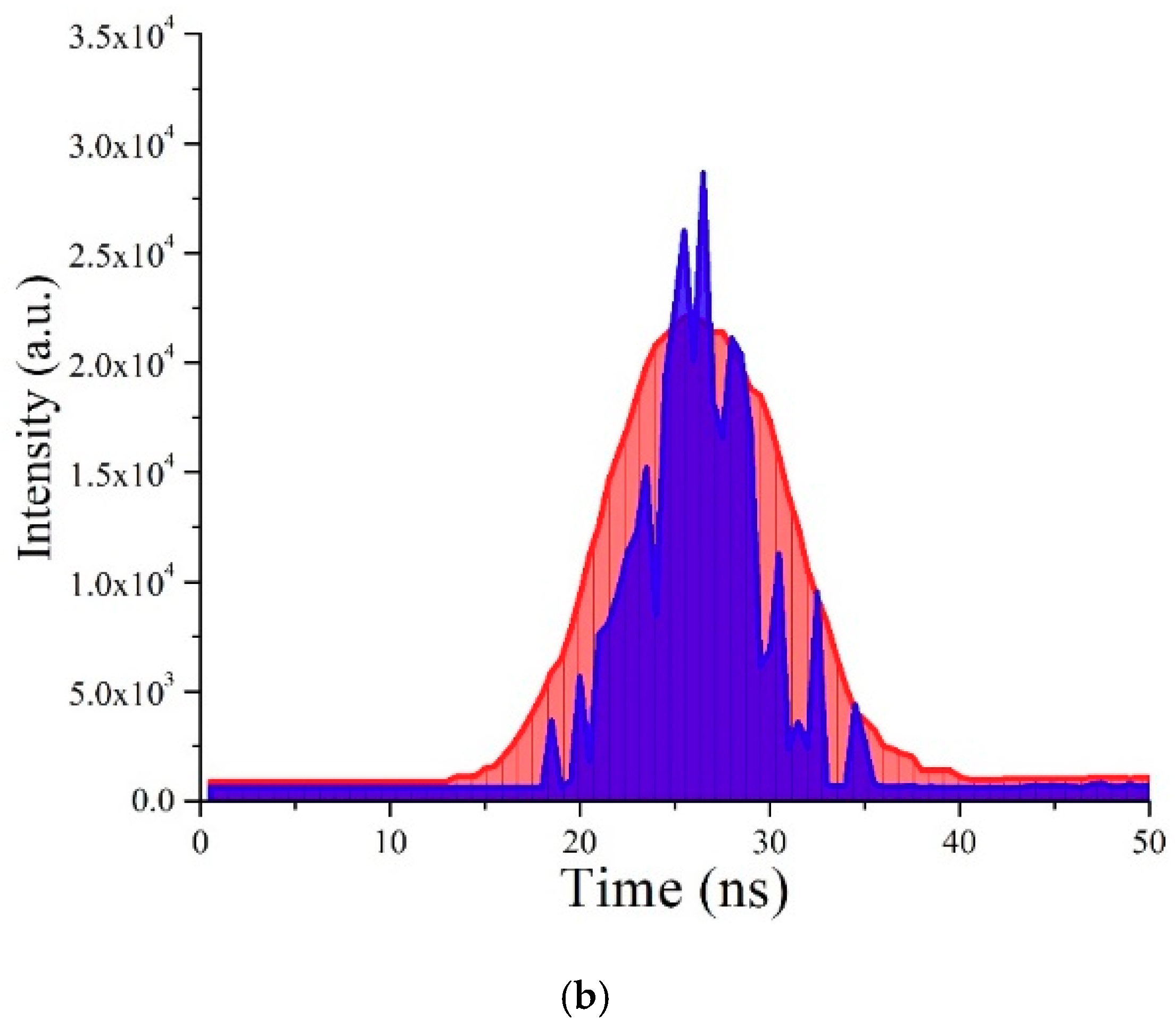
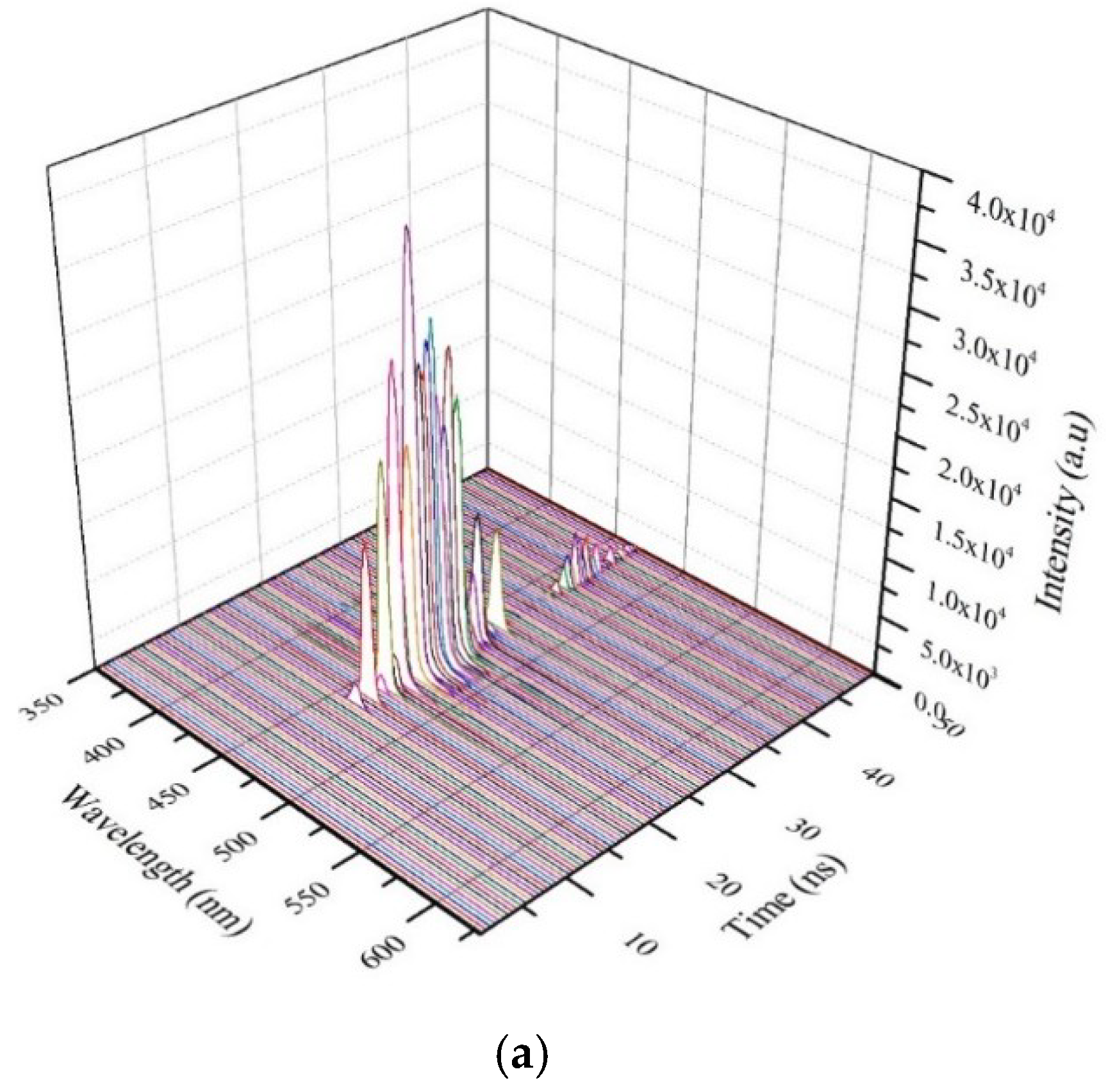
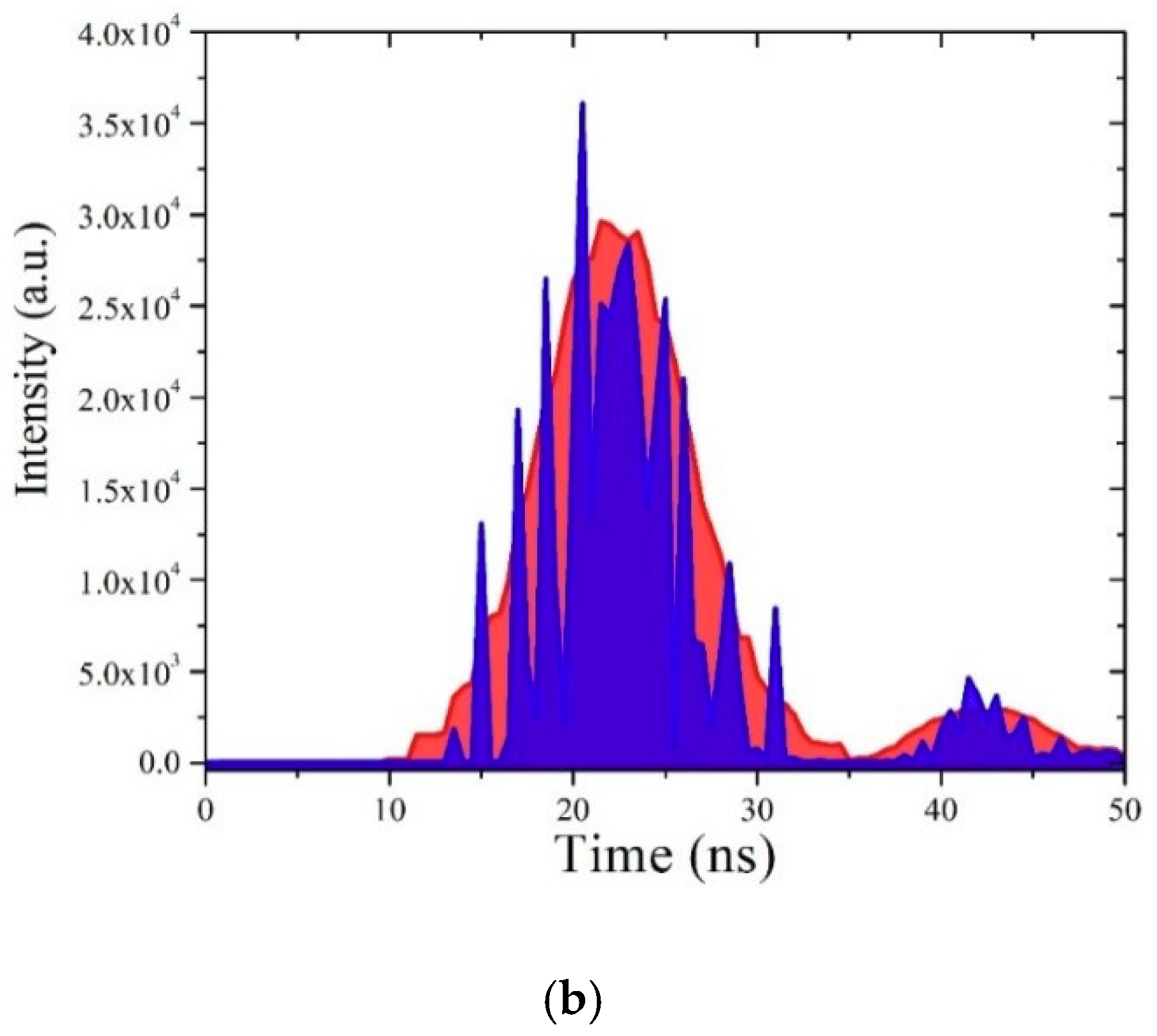
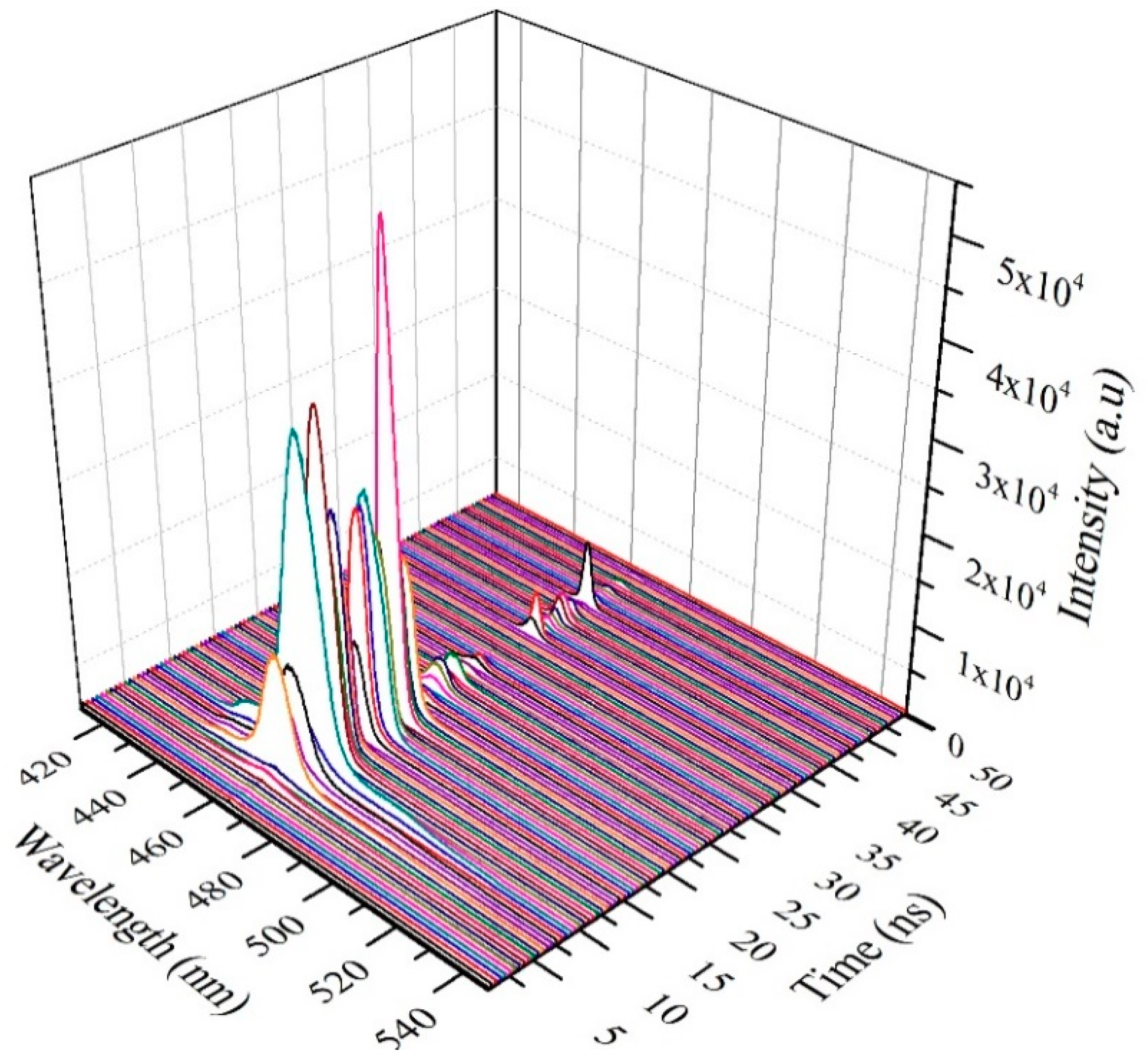
3.7. TRS of the CO BECV-DHF in Toluene
4. ASE Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kranzelbinder, G.; Leising, G. Organic solid-state lasers. Rep. Prog. Phys. 2000, 63, 729. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef] [PubMed]
- Hide, F.; DÍaz-GarcÍa, M.A.; Schwartz, B.J.; Heeger, A.J. New developments in the photonic applications of conjugated polymers. Acc. Chem. Res. 1997, 30, 430–436. [Google Scholar] [CrossRef]
- Hide, F.; Diaz-Garcia, M.A.; Schwartz, B.J.; Andersson, M.R.; Pei, Q.; Heeger, A.J. Semiconducting polymers: A new class of solid-state laser materials. Science 1996, 273, 1833–1836. [Google Scholar] [CrossRef]
- Friend, R.; Gymer, R.; Holmes, A.; Burroughes, J.; Marks, R.; Taliani, C.; Bradley, D.; Dos Santos, D.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121. [Google Scholar] [CrossRef]
- Xia, R.; Heliotis, G.; Hou, Y.; Bradley, D.D. Fluorene-based conjugated polymer optical gain media. Org. Electron. 2003, 4, 165–177. [Google Scholar] [CrossRef]
- Tessler, N.; Denton, G.; Friend, R. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Kozlov, V.; Forrest, S. Lasing action in organic semiconductor thin films. Curr. Opin. Solid State Mater. Sci. 1999, 4, 203–208. [Google Scholar] [CrossRef]
- Pei, Q.; Yu, G.; Zhang, C.; Yang, Y.; Heeger, A.J. Polymer light-emitting electrochemical cells. Science 1995, 269, 1086–1088. [Google Scholar] [CrossRef]
- Fish, D.; Young, N.; Childs, M.; Steer, W.; George, D.; McCulloch, D.; Godfrey, S.; Trainer, M.; Johnson, M.; Giraldo, A. 32.1: Invited paper: A comparison of pixel circuits for active matrix polymer/organic LED displays. SID Symp. Digest Tech. Pap. 2002, 33, 968–971. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Polymer solar cells. In Photoresponsive Polymers II; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–86. [Google Scholar]
- Kawase, T.; Sirringhaus, H.; Friend, R.H.; Shimoda, T. Inkjet Printed Via-Hole Interconnections and Resistors for All-Polymer Transistor Circuits. Adv. Mater. 2001, 13, 1601–1605. [Google Scholar] [CrossRef]
- Ahles, M.; Hepp, A.; Schmechel, R.; von Seggern, H. Light emission from a polymer transistor. Appl. Phys. Lett. 2004, 84, 428–430. [Google Scholar] [CrossRef]
- Lai, W.Y.; Xia, R.; He, Q.Y.; Levermore, P.A.; Huang, W.; Bradley, D.D. Enhanced Solid-State Luminescence and Low-Threshold Lasing from Starburst Macromolecular Materials. Adv. Mater. 2009, 21, 355–360. [Google Scholar] [CrossRef]
- Alsalhi, M.; Ibnaouf, K.; Masilamani, V.; Yassin, O. Excimer state of a conjugate polymer (MEH-PPV) in liquid solutions. Laser Phys. 2007, 17, 1361–1366. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Almotiri, A.R.; Prasad, S.; Aljaafreh, M.J.; Othman, A.H.; Masilamai, V. A Temperature-Tunable Thiophene Polymer Laser. Polymers 2018, 10, 470. [Google Scholar] [CrossRef]
- Abdulaziz Alfahd, S.; Prasad Rajendra, S.; Al-Mujammi, W.; Devaraj, D.; Masilamani, V.; AlSalhi, M. An Efficient Violet Amplified Spontaneous Emission (ASE) from a Conjugated Polymer (PFO-co-pX) in Solution. Materials 2017, 10, 265. [Google Scholar] [CrossRef]
- Müllen, K.; Wegner, G. Electronic Materials: The Oligomer Approach; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sperling, L.H. Introduction to Physical Polymer Science; Wiley Online Library: Hoboken, NJ, USA, 2006; Volume 78. [Google Scholar]
- Brazel, C.S.; Rosen, S.L. Fundamental Principles of Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Devaraj, D.; Masilamani, V. High power amplified spontaneous emission from an oligomer in solution. J. Lumin. 2015, 168, 109–113. [Google Scholar] [CrossRef]
- Pisignano, D.; Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission and efficient tunable laser emission from a substituted thiophene-based oligomer. Appl. Phys. Lett. 2002, 81, 3534–3536. [Google Scholar] [CrossRef]
- Guilhabert, B.; Laurand, N.; Herrnsdorf, J.; Chen, Y.; Mackintosh, A.R.; Kanibolotsky, A.L.; Gu, E.; Skabara, P.J.; Pethrick, R.; Dawson, M.D. Amplified spontaneous emission in free-standing membranes incorporating star-shaped monodisperse π-conjugated truxene oligomers. J. Opt. 2010, 12, 035503. [Google Scholar] [CrossRef]
- Nagawa, M.; Hibino, R.; Hotta, S.; Yanagi, H.; Ichikawa, M.; Koyama, T.; Taniguchi, Y. Emission gain narrowing from single crystals of a thiophene/phenylene co-oligomer. Appl. Phys. Lett. 2002, 80, 544–546. [Google Scholar] [CrossRef]
- McGehee, M.D.; Heeger, A.J. Semiconducting (conjugated) polymers as materials for solid-state lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A. Ultrafast dynamics of laser from green conjugated-oligomer in solution. Polymer 2019, 169, 106–114. [Google Scholar] [CrossRef]
- Shimizu, K.; Mori, Y.; Hotta, S. Laser oscillation from hexagonal crystals of a thiophene/phenylene co-oligomer. J. Appl. Phys. 2006, 99, 063505. [Google Scholar] [CrossRef]
- Mizuno, H.; Maeda, T.; Yanagi, H.; Katsuki, H.; Aresti, M.; Quochi, F.; Saba, M.; Mura, A.; Bongiovanni, G.; Sasaki, F. Optically Pumped Lasing from Single Crystals of a Cyano-Substituted Thiophene/Phenylene Co-Oligomer. Adv. Opt. Mater. 2014, 2, 529–534. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian09, Revision A; Gaussian. Inc.: Wallingford, CT, USA, 2009; Volume 121, pp. 150–166. [Google Scholar]
- Ullah, H.; Shah, A.-U.-H.A.; Ayub, K.; Bilal, S. Density functional theory study of poly (o-phenylenediamine) oligomers. J. Phys. Chem. C 2013, 117, 4069–4078. [Google Scholar] [CrossRef]
- Renzi, W.; Cordeiro, N.J.; de Santana, H.; Costa, M.F.; da Silva, M.A.; Laureto, E.; Duarte, J.L. Exploring the experimental photoluminescence, Raman and infrared responses and density functional theory results for TFB polymer. Synth. Met. 2018, 236, 24–30. [Google Scholar] [CrossRef]
- Bencheikh, F.; Duché, D.; Ruiz, C.M.; Simon, J.-J.; Escoubas, L. Study of optical properties and molecular aggregation of conjugated low band gap copolymers: PTB7 and PTB7-Th. J. Phys. Chem. C 2015, 119, 24643–24648. [Google Scholar] [CrossRef]
- Justino, L.L.; Luísa Ramos, M.; Abreu, P.; Charas, A.; Morgado, J.; Scherf, U.; Minaev, B.F.; Ågren, H.; Burrows, H.D. Structural and electronic properties of poly (9, 9-dialkylfluorene)-based alternating copolymers in solution: An NMR spectroscopy and density functional theory study. J. Phys. Chem. C 2013, 117, 17969–17982. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Expanded theory of H-and J-molecular aggregates: The effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.; Teng, F.; Xu, Z.; Qian, L.; Hou, Y.; Wang, Y.; Xu, X. Solvent and concentration effects on fluorescence emission in MEH-PPV solution. Eur. Polym. J. 2006, 42, 228–233. [Google Scholar] [CrossRef]
- Dias, F.B.; Morgado, J.; Macanita, A.L.; da Costa, F.P.; Burrows, H.D.; Monkman, A.P. Kinetics and thermodynamics of poly (9, 9-dioctylfluorene) β-phase formation in dilute solution. Macromolecules 2006, 39, 5854–5864. [Google Scholar] [CrossRef][Green Version]
- Mujamammi, W.; Prasad, S.; AlSalhi, M.; Masilamani, V. Relaxation oscillation with picosecond spikes in a conjugated polymer laser. Polymers 2016, 8, 364. [Google Scholar] [CrossRef] [PubMed]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A.; Al-Mogren, M.M. Optically Pumped Intensive Light Amplification from a Blue Oligomer. Polymers 2019, 11, 1534. https://doi.org/10.3390/polym11101534
Aljaafreh MJ, Prasad S, AlSalhi MS, Alahmed ZA, Al-Mogren MM. Optically Pumped Intensive Light Amplification from a Blue Oligomer. Polymers. 2019; 11(10):1534. https://doi.org/10.3390/polym11101534
Chicago/Turabian StyleAljaafreh, Mamduh J., Saradh Prasad, Mohamad S. AlSalhi, Zeyad A. Alahmed, and Muneerah M. Al-Mogren. 2019. "Optically Pumped Intensive Light Amplification from a Blue Oligomer" Polymers 11, no. 10: 1534. https://doi.org/10.3390/polym11101534
APA StyleAljaafreh, M. J., Prasad, S., AlSalhi, M. S., Alahmed, Z. A., & Al-Mogren, M. M. (2019). Optically Pumped Intensive Light Amplification from a Blue Oligomer. Polymers, 11(10), 1534. https://doi.org/10.3390/polym11101534