3.2. Column Studies—Laboratory Scale
The fine grain pectin-based biosorbent, used previously in batch studies [
24], was tested in column experiments for zinc ions removal. The column processes in comparison to batch ones are less space-consuming and easy to automate, thus usually more desirable in the industry. The shape of our hybrid beads enabled their usage in such dynamic systems without misgiving of column clogging or high pressure drop. Presented in
Figure 1, the breakthrough curve of zinc(II) ions sorption on studied biosorbent has no specific, sharp breakpoint and the Zn(II) concentration in effluent increases slowly to achieve its value in inlet (c/c
0 = 1). The limits for Zn(II) in wastewater, which may be introduced into environment, usually vary between 1.5 and 2.61 mg/L [
8,
9,
10]. The breakthrough point in this work was then assumed as volume of the effluent, when the Zn concentration in effluent reaches 2 mg/L (limit given by legal regulations in Poland [
9]). This was marked in the figure as a horizontal line at c/c
0 = 0.067 (since initial (c
0) and limit (c
B) concentration equaled 30 mg/L and 2 mg/L, respectively). This breakthrough point was used in all further experiments as end point of sorption experiment.
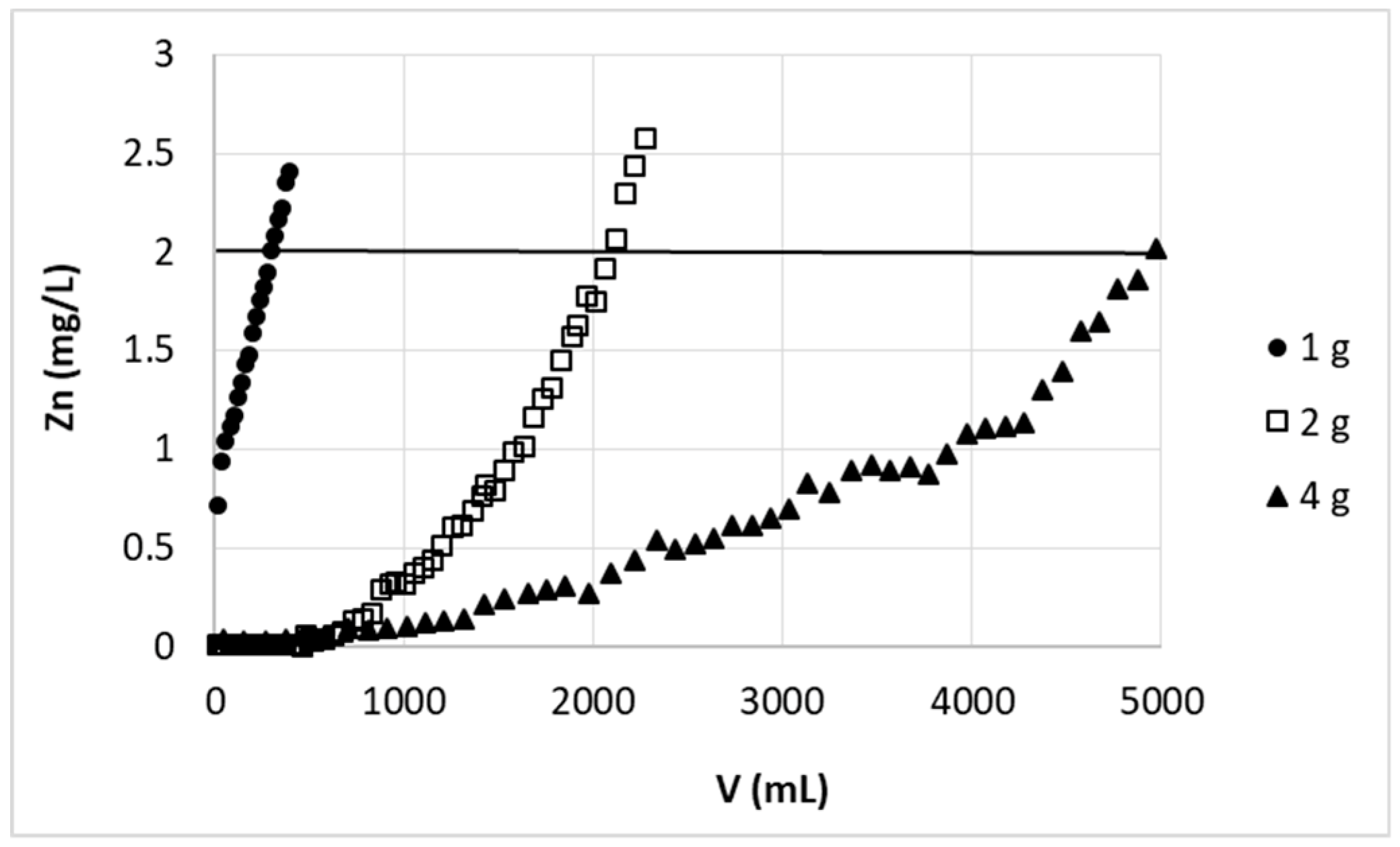
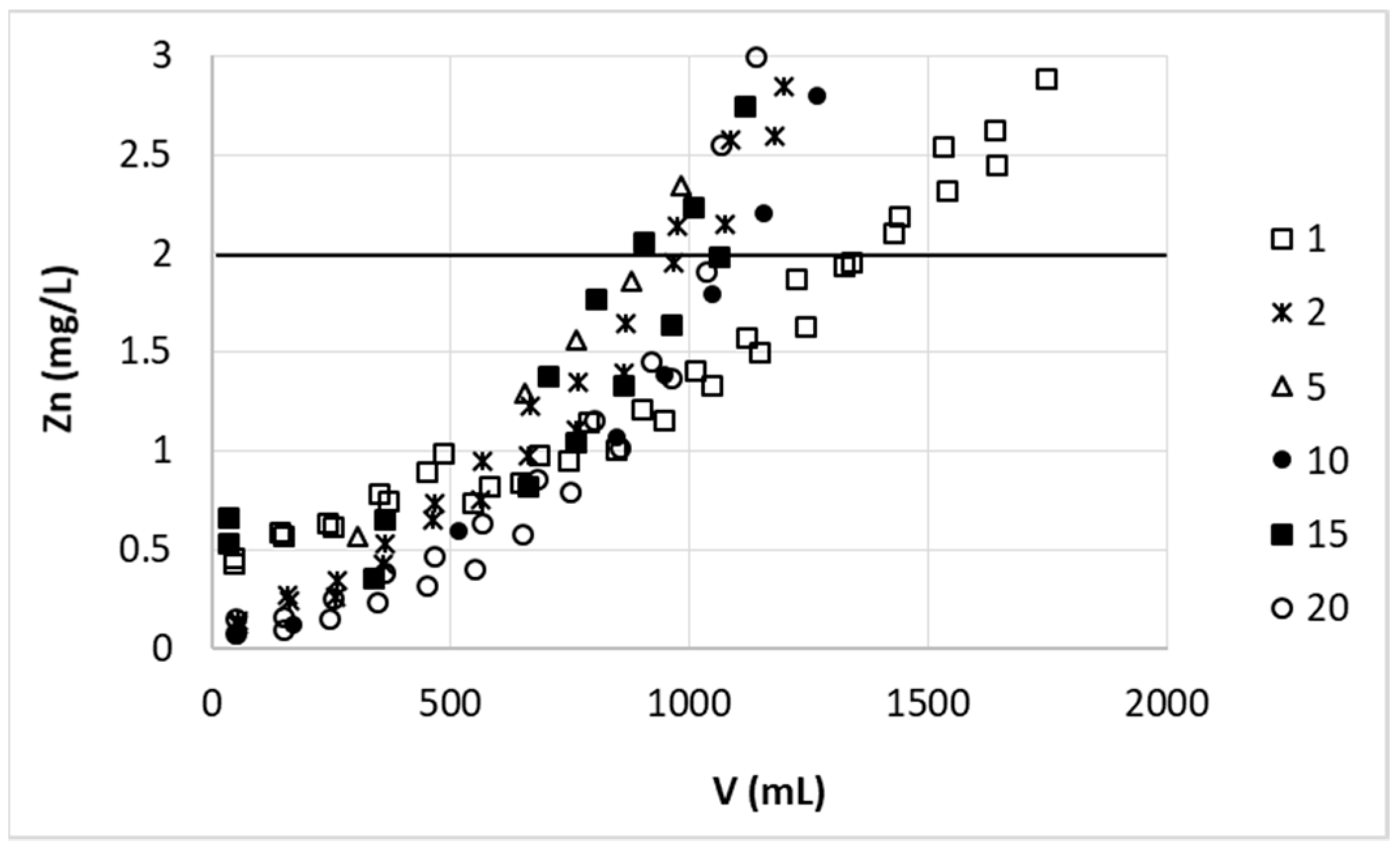
It is clear that the increase in the bed height increased the number of active sites for the binding of metal ions; the breakthrough point thus is achieved after a longer time or greater volume of effluent. This dependency is however not always proportional and the relationship between bed height change and received ratio of its breakthrough volume or breakthrough time (i.e., (h
2/h
1):(V
2/V
1) or (h
2/h
1):( t
2/t
1 ) may be as well < 1 [
26,
27]; > 1 [
28] or equal 1 [
29]). In our case the amount of purified solution increased significantly (~6.5-fold) with increasing bed height from 2.9 cm to 5.7 cm (V: 320 mL and 2069 mL, respectively) (
Figure 2), giving the ratio of (h
1/h
2):(V
1/V
2) definitely higher than 1. Increasing in bed height (from 5.7 cm to 11.2 cm) resulted in further increasing of breakthrough point volume, but this growth is not as spectacular as previously—an almost double increase of sorbent in the column resulted in app 2.5 times more purified solution (the ratio (h
2/h
3):(V
2/V
3) is still higher, but close to 1). This specific behavior may be due to fact that the lowest applied bed height was very close to minimum bed depth (estimated from BDST model to be 2.19 cm)—minimum bed height, which in studied conditions (c
0 = 30 mg/L, F = 33.97 cm/L), is able to purify solution to established breakthrough concentration (c
B = 2 mg/L). Experiments with lower bed height gave unsatisfactory results, e.g., sorbent bed of 2.9 cm height was not sufficient to complete zinc ions removal (see
Figure 2)—the most purified fraction still contained 0.72 mg/L of zinc. Increasing the bed height resulted in deeper purification of initial portions of effluent (Zn concentration below 0.01 mg/L). As mentioned previously, as the bed depth increases, the amount of biosorbent binding sites and simultaneously contact time of sorbent with Zn(II) ions increases, which resulted in higher removal efficiency.
Received results were used for determination of BDST (Bed Depth Service Time) model parameters which were further used for some results prediction.
Applying the equation 1 and using the data from
Figure 2 (t—calculated as breakthrough point volume V (mL) divided by flow rate (60 mL/h), Z) the dependence t = a × Z + b was presented as linear curve of equation: t = 9.2636 Z −20.251 (r
2 = 0.9981). Slope (a) and intercept (b) of the curve with studied conditions (c
0, F, c
t = c
B) enabled calculation of N
0 and K
a parameters to be 9442 mg/L and 0.0043 L/(mg × h), respectively (
Table 1).
The minimum bed depth necessary for purifying the solution to zinc concentration 2 mg/L in the effluent in studied conditions i.e., Z
0 calculated for t = 0, was obtained as 2.19 cm (
Table 1).
Following the study of Han et al. [
26], changing the flow rate, but keeping all other conditions constant, the slope constant (a) for a different linear flow rate (F) can be directly calculated from the relation:
where a and F are the old slope and influent linear flow rate, respectively, and a’ and F’ are the new slope and influent linear flow rate, respectively.
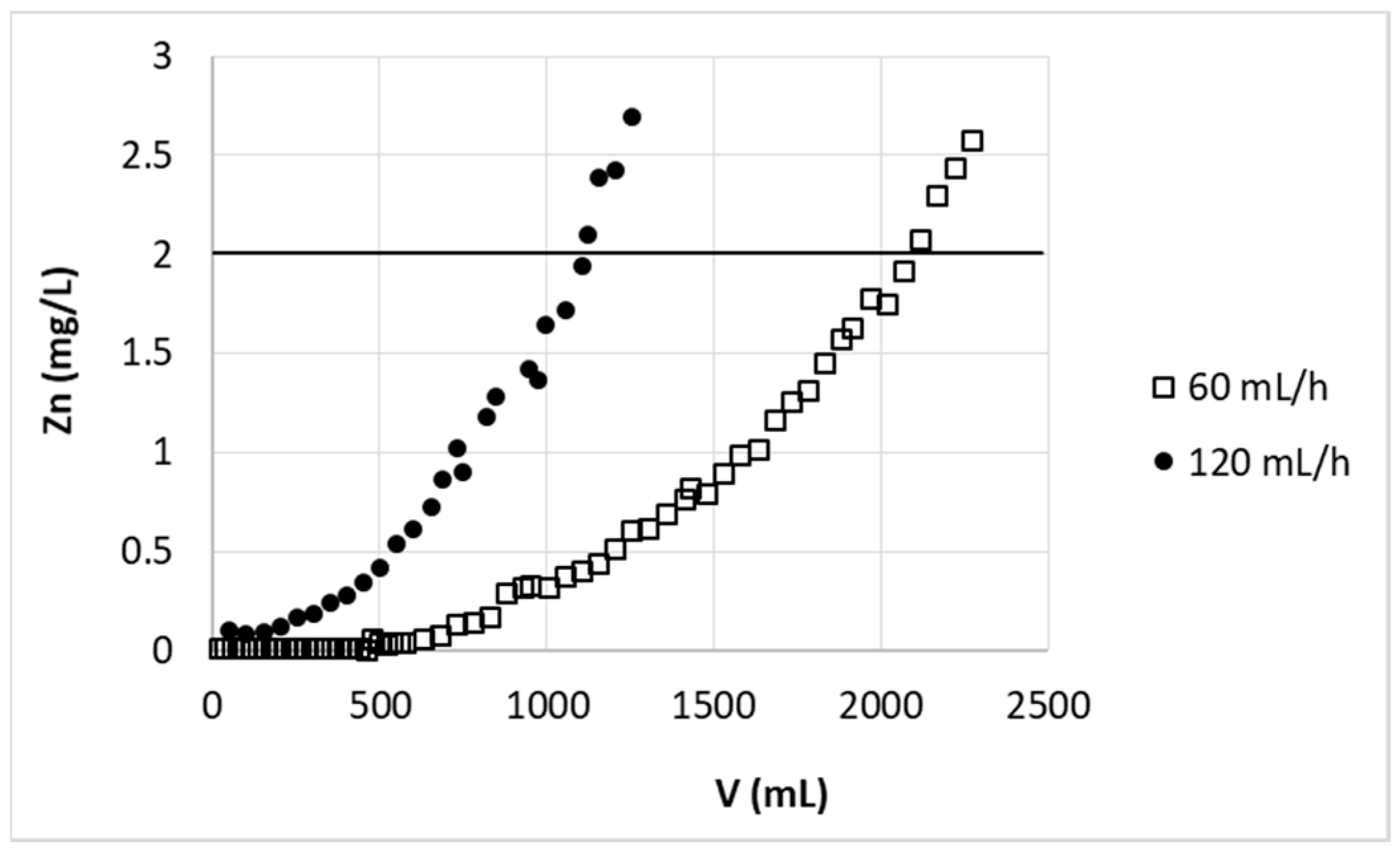
The breakthrough point expected for bed height 5.7 cm and flow rate of F’ = 67.95 cm/h (v = 120 mL/h) equals to t = 6.15 h (or V = 738 mL). Experimental data for that flow rate and bed height 5.7 cm (2g of sorbent) are presented in
Figure 3. The real breakthrough point calculated from these data for 120 mL/h equals to t = 9.20 h (calculated as volume 1104 mL divided by flow rate 120 mL/h) (
Table 1). From the experimental results shown in
Figure 3, it is possible to notice that the two-fold increase of the flow rate (from 60 to 120 mL/h) resulted in double drop of purified solution volume. It is known that at higher flow rate the external film mass transfer diffusion resistance and residence time of adsorbate in column decrease. At higher flow rate the adsorbate leaves the column much before the adsorption equilibrium [
26] and whole adsorption ability of the biosorbent cannot be utilized. Additionally, at higher flow rate, thus at lower residence time of adsorbate in column, the intraparticle diffusion becomes less effective [
28], which could be the reason of error of above breakthrough point estimation at higher flow rate based on BDST model. The BDST model ignores the intraparticle mass transfer resistance and neglects the external film resistance. It is based on the assumption that the rate of surface reaction between metal ions and the adsorbent is limiting step [
22]. The obtained results proved that in this case the intraparticle diffusion influences the zinc(II) ions sorption on pectin-based biosorbent.
The striping of metal ions from sorbent is one of the most important issues, which prejudges the possibility of sorbent reuse. The main aim of this process is desorption of previously adsorbed elements to possibly the highest concentrate without sorbent degradation. Desorption should be complete and as little as possible of gentle eluent should be used. Our previous batch studies shown similar efficiency of various 0.1 M (in conversion to H
+ concentration) acid solutions (0.1 M HCl, 0.1 M HNO
3, 0.05 M H
2SO
4) in removal of zinc(II) ions from studied pectin-based biosorbent [
24]. Application of these solutions allowed fast and almost complete (above 95%) removal of zinc ions sorbed on studied material in batch studies. For column desorption studies 0.1M solution of nitric acid was selected.
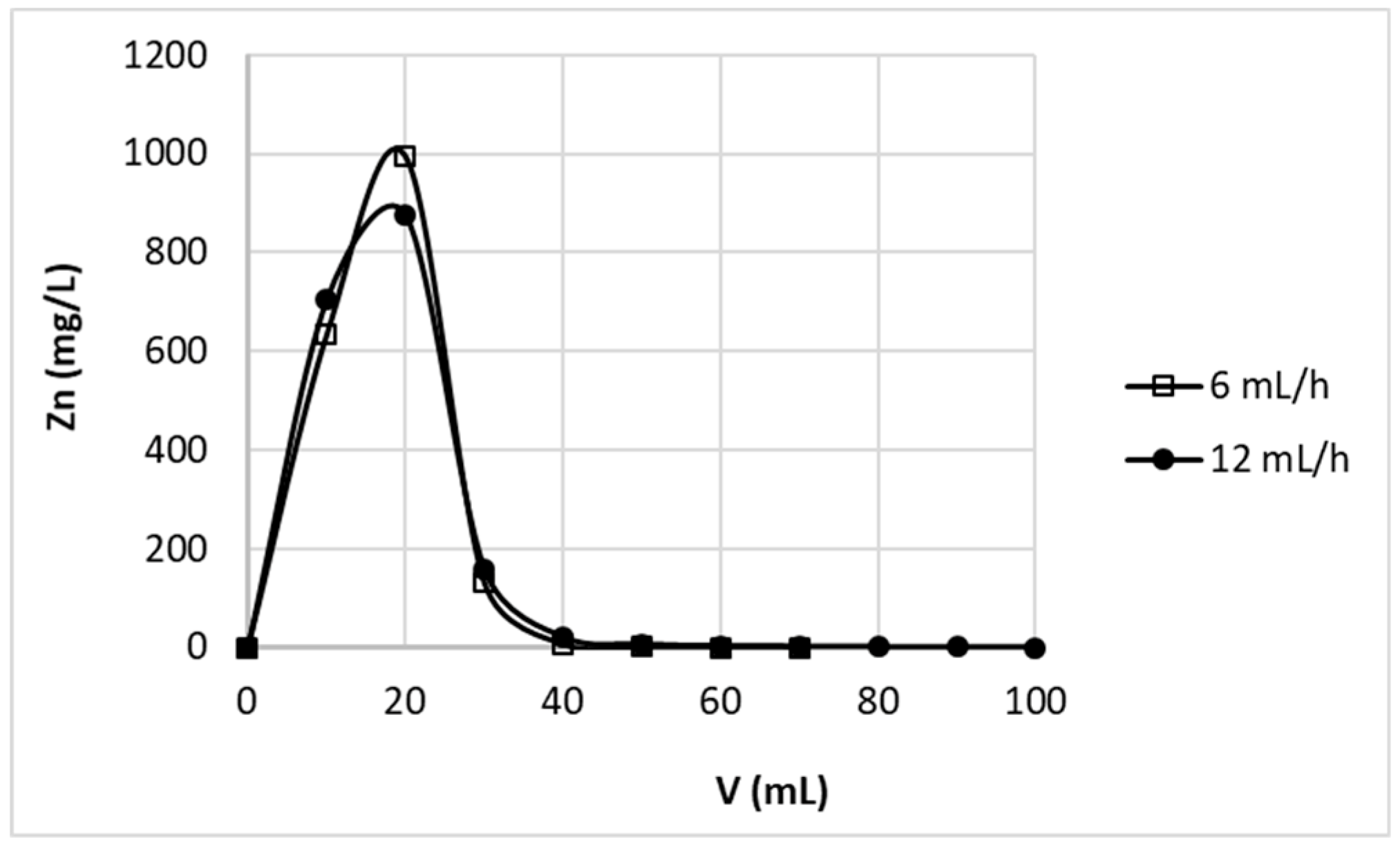
Figure 4 shows the elution curves obtained for bed height 2.9 cm and two various flow rates 6 mL/h and 12 mL/h. The results did not differ with flow rate change, therefore it may be stated that in studied conditions the residence time of protons in the column was sufficient to achieve equilibrium of Zn
2+–H
+ exchange. Complete elution of zinc ions was achieved with first 40 mL of the effluent, wherein most of the zinc is removed with first 30 mL of the effluent.
In the case of higher bed height (5.7 cm) a little more eluent (50 mL) was necessary for complete zinc removal (
Figure 5). Taking into account that in studied conditions (c
0 = 30 mg/L, flow rate 60 mL/h) 2069 mL of solution may be purified to desired level, it may be calculated that zinc ions were concentrated above 40 times in one sorption–desorption step. The time required for performing mentioned concentration of zinc(II) ions mainly consists of sorption step (~34.5 h); desorption step takes ~4 h. Therefore it may be stated that in this case sorption is the step, which prejudges the time needed for concentration of zinc(II) ions. Different dependence may be observed, when the faster flow rate (120 L/h) in sorption step was applied. In this case 1104 mL of purified solution was obtained in 9.2 h. That means 22 fold concentration of zinc(II) ions in about 13.5 h was achieved. In this case duration of both sorption (9.2 h) and desorption (4 h) step was significant.
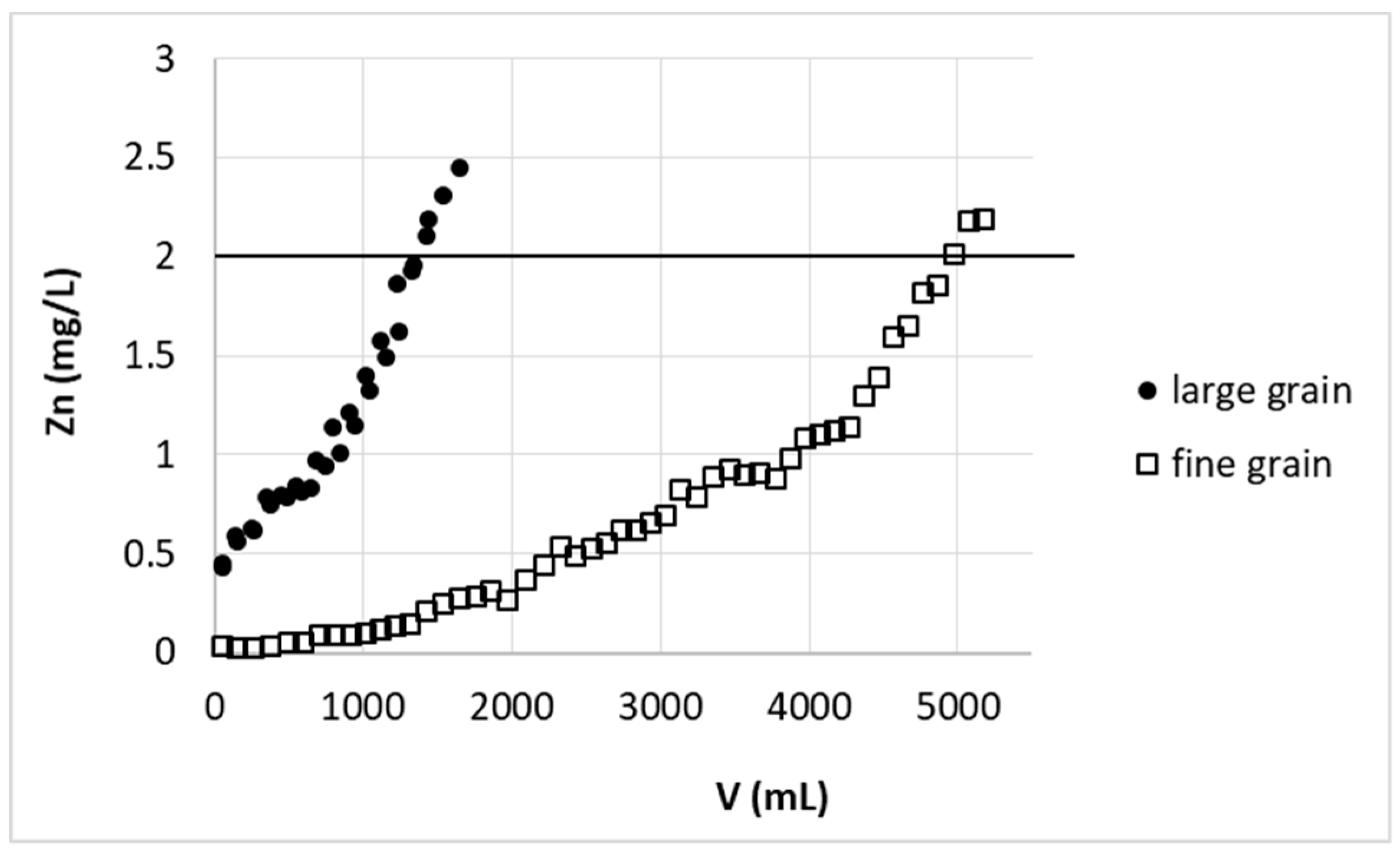
As mentioned above for the large-scale study, due to some technical problems, sorbent in the form of greater beads was prepared. The removal of zinc(II) ions on this biosorbent was compared with data received for biosorbent of fine grain (
Figure 6).
Unfortunately, because of the more than 2-fold increase in bead dimensions, a 3.7 times decrease in the amount of solution purified was required to reach the desired limit. To explain this behavior in detail further research is needed, which is not the subject of this manuscript, but we roughly expect that it is associated with an increase in the importance of intramolecular diffusion in sorption on large grains. The most accessible external surface of the beads is lower for the same bed volume of larger grain sorbent.
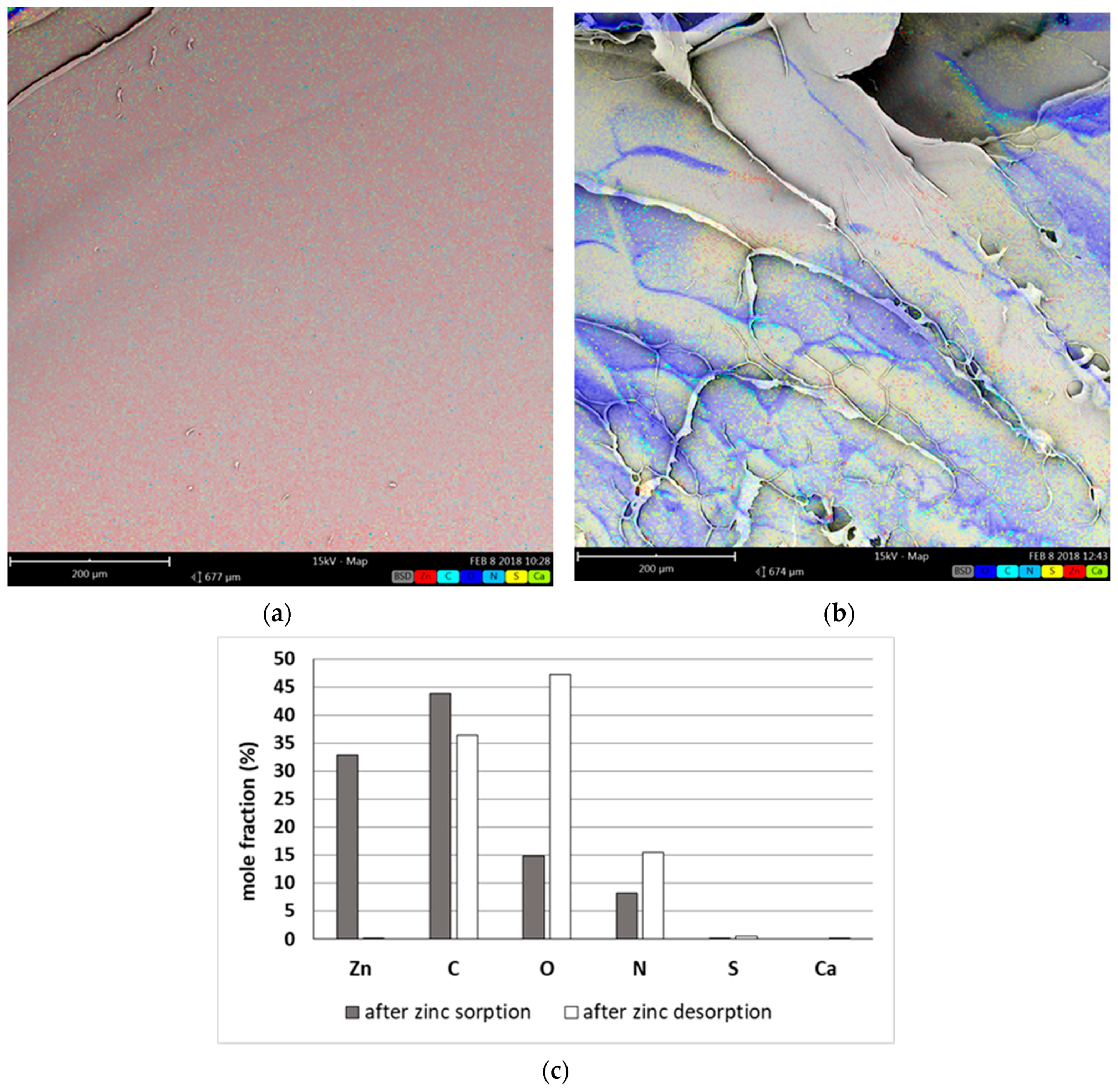
To find out if Zn
2+ are removed due to adsorption on external or internal surface of bead we have applied the EDS analysis (
Figure 7). The swollen beads after sorption and desorption processes were cut in half, lyophilized and next corresponding elements (zinc, calcium, and main sorbent elements—carbon, oxygen, nitrogen, and sulfur) distribution was studied using EDS.
Zinc distribution after sorption was rather uniform throughout the cross-section (
Figure 7a), therefore it may be stated that sorption of zinc occurred in grain whole volume i.e., both external and internal surface of bead took part in zinc(II) ion removal. The analysis of grain after desorption (
Figure 7b,c) proved on the other hand the completeness of zinc(II) ion stripping—the content of zinc in the grain was close to zero. The latter has been further confirmed by sorption–desorption cycle mass balance (see
Table 2).
Successful desorption allowed for study of lifetime of the pectin-based biosorbent. Although it is very important issue, which determines the biosorption process economics and ecological profits, such studies are rarely found in literature, and if done, usually performed only for few cycles [
22,
30]. In this study, 20 consecutive sorption–desorption cycles were performed on sorbent of large grains prepared in large-scale and using 0.1 M nitric acid solution as stripping agent. Results coming from a few cycles (including the first and the last one) are presented in
Figure 8.
After the first cycle some drop (of approximately 25%) in zinc removal capabilities of our biosorbent occurred, but in the next 19 cycles no further decrease was observed, just some scatter of results. The initial drop in zinc removal efficiency could be due to the fact that during the first sorption the sorbent was in Ca
2+ doped form (calcium salt was used to prepare the sorbent) and starting from the second cycle it was in H
+ form what was the aftermath of desorption with acid solution. Therefore no significant pH change could be observed in the first case in opposite to the second and next sorption steps where the Zn(II) ions were exchanged on H
+ ions. In these cases the pH of the solution during the sorption step decreased from 6 (inlet) to even 3.3 (outlet). Although our previous studies [
25] showed no significant differences between sorption capacity of pectin-guar gum biosorbent from solutions of pH 6 and 3, a significant drop in sorption efficiency occurred only when pH dropped from 3 to 2. Since 3.3 was the final pH in the effluent, local pH decrease in some part of column could be greater, influencing the zinc sorption on studied material. Additionally, although no damage or shape changes of H
+ form sorbent was observed during these 19 cycles, the sorbent grains seemed to be of lower density so they were less pressed in the column. This made more space between the grains and caused bed height increase of ~20%. From one side the residence time of zinc(II) ions in the sorbent bed increased and could enhance the sorption, but on the other side the diffusion of ions into the surface of the sorbent could play more significant role than in the case of tightly packed biosorbent and efficiency of sorption could decrease. The changes in arrangement of pectin-based beads in the columns could be a reason of scatter of results obtaining for the same cycle number and two parallel columns (
Figure 8). The most important finding from this experiment was that a lot of (at least 20) sorption–desorption cycles could be performed on one portion of pectin-based sorbent without loss of its effectiveness (if counted from the second cycle). This prompts to further studies on lifetime of the biosorbent as an important factor to judge economic and proecological profitability of the process, on the other hand, it allowed for planning the large-scale tests using the same portion of sorbent.
Additionally some interesting observations were made—the sorbent after desorption (in H
+ form) kept the shape and mechanical strength, despite the lack of calcium ions as binding agent, what was confirmed by SEM EDS analysis (
Figure 7b,c). The explanation of this phenomenon will be the topic of separate research.
The mass balance of sorption–desorption cycles presented in
Figure 8 was also prepared (
Table 2). A good recovery (97.8–102.8%) of the element confirmed the stripping method to be applicable for Zn(II) removal from studied biosorbent. In some cases sorption step was performed longer than shown in the
Figure 8, therefore the amount of sorbed zinc may vary from cycle to cycle.
3.3. Column Studies—Large-Scale
The aim of the large-scale studies was to confirm the efficiency of zinc(II) ions sorption on studied biosorbent in semipilot scale and additionally check if phenomena, which could not be observed in laboratory scale and may influence the sorption effectiveness. Including, for instance, the aspect of bead compression that could prevent the desired flow rate achievement, especially during drop-feed column work [
23].
Our biosorbent was in the form of round beads (diameter ~3.5 mm in wet form), so if only mechanical strength was adequate the compression should not happen. Other threat concerning also mechanical strength of our beads was their stability in the conditions of higher flow rates, comparable with those applied in the industry. Generally during all large-scale studies no problems with high flow rate (even 200 L/h (442.5 cm/h)) were observed, independently on drop-fed or reverse-fed column work. Also no significant pressure drop on our bed occurred. The beads did not change their shape or volume during the experiments, therefore it was confirmed that they are suitable for industrial application from the mechanical strength point of view.
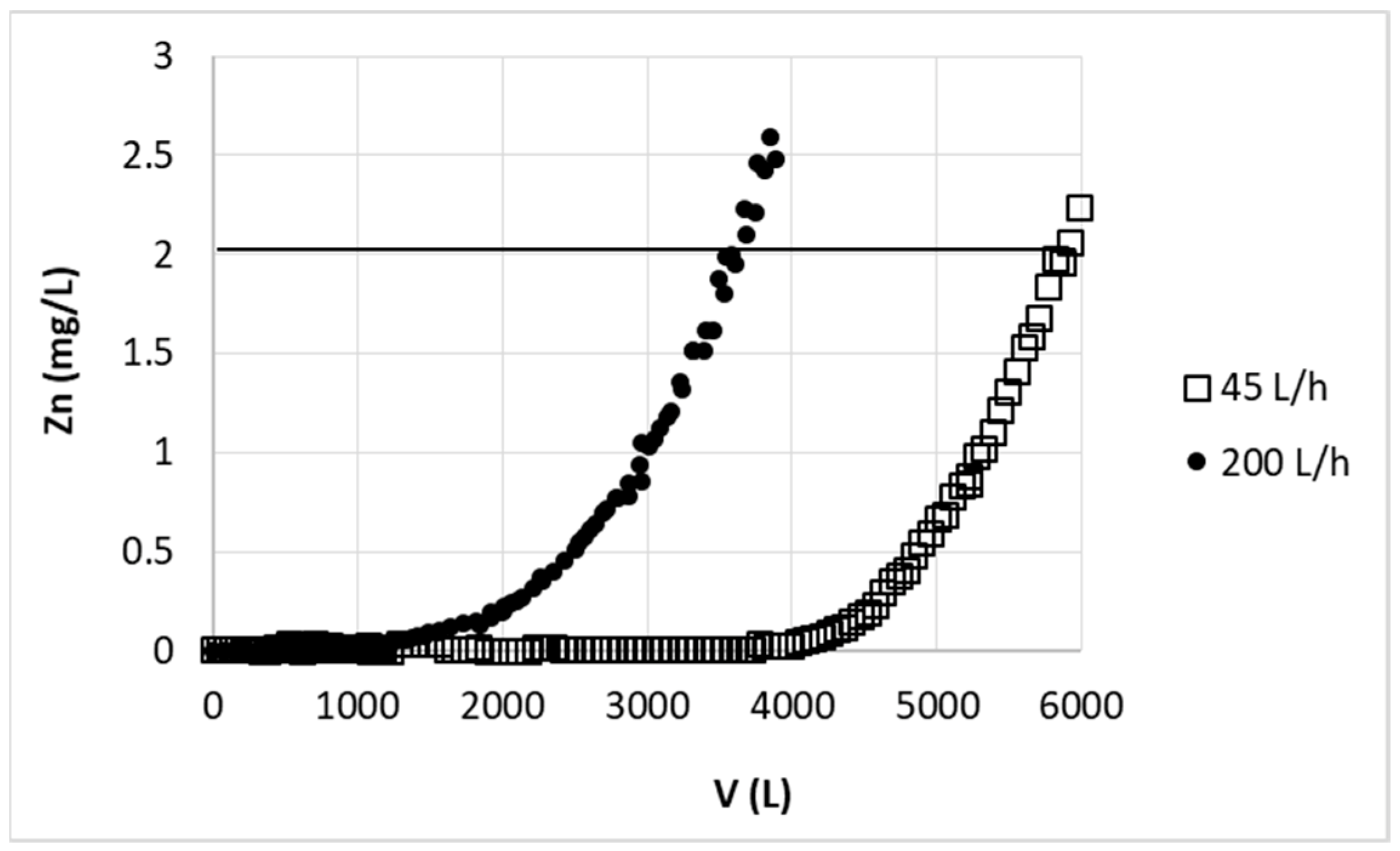
The efficiency of zinc(II) ion sorption in large-scale was first tested using two various flow rates and model zinc solution. The first applied flow rate (200 L/h, 442.5 cm/h) was roughly and simply calculated by multiplying the linear flow rate from small scale (33.97 cm/h) by the same enlargement as bed height increase (11.2 cm to 120 cm). The linear flow rate should equal to 363.5 cm/h and taking into account new dimension of column (240 cm) flow rate should be 164 L/h. However since in wider column beads of sorbent are better packed than in the case of narrow laboratory scale column (16 kg of biosorbent packed into column occupied about 54 L instead of 62 L, as calculated form the proportion of 5 g:19.43 mL to 16 kg:62 L), thus amount of active sites per centimeter of bed height in large column is greater than in the case of laboratory scale column. Therefore the calculated flow rate was increased to 200 L/h. As the result 3600 L of the solution purified to desired breakthrough point (2 mg/L) was obtained (see
Figure 9). Taking into account that in this case sorption parameters (bed height and linear flow rate) were proportionally enlarged from small scale, simple proportion was used to compare result from laboratory and large-scale: using 5 g of sorbent average volume of purified solution (cycle 2–20) was ~0.95 L, using 16 kg it should be 16000 × 0.95/5 = 3040 L. The experimental results were then almost 20% better than estimated. Additionally it should be noted that points obtained in two consecutive experiments overlapped with each other, thus the scatter of the results was significantly lower than during the laboratory studies. The enhancement in zinc sorption efficiency and in results repeatability could be due to more stable arrangement of beads in the column as well as smaller space between the grains, so the external diffusion of ions from the solution into the sorbent surface took less time.
Reduction of the flow rate to 45 L/min caused, that the amount of solution purified to the desired concentration of zinc (2 mg/L) increased to 5900 L (
Figure 9). Reduction of the flow rate, and thus extending the process time, increased the problem with bubbles of air in the column. This important observation was enabled thanks to the column transparency.
Generally the bubbles of gas appeared during all experiments (
Figure 10b); the column was then periodically degassed. Gas bubbles that happened to be bubbles of air (confirmed by gas chromatography), were released through vent valves on the top of the column by tapping the column from down to up. Formed air bubbles partially blocked the access to the surface of the biosorbent causing slight decrease of the sorption efficiency, after degassing the efficiency could return to normal level. This may be observed in
Figure 10a, e.g., from app 5000 L of effluent the process was performed in the presence of great amounts of air bubbles till about 5200 L when the concentration of zinc in the effluent equaled 1.4 mg/L. After removal of gas from the column the concentration of zinc in effluent decreased to 0.8 mg/L and the process was continued to regassing of the column. At ~6000 L and zinc concentration 3.5 g/L the column was again degassed and the concentration of zinc decreased to 2.3 mg/L, thus the sorption efficiency increased. Therefore this issue is important to be taken into account when industrial application is considered. The solution may be the periodical vibration of the column with automatic vent valves system. There are also some industrial solutions for removal of microbubbles from treated solution before it reaches the device (e.g., heat exchanger or column). Our sorption station was equipped with microbubbles gas separator, it was the next device after the peristaltic pump, however it did not prevent gas release from solution in the column. This could be due to the fact that air was not in the form of microbubbles, but it was dissolved in the solution. Gas dissolution process could be promoted by pumping the cold water into our IBC containers. Even though during slow warming to room temperature the solution of air in Zn(II) solution could become supersaturated, we had no technical possibilities to remove the air from solution in the 1000 L container. The solubility of air in the aqueous solution, beside the temperature and pressure, also depends on the composition of solution. Additionally the factors such as vibes or stirring (or other kinetic distortion of solution) may also induce the gas release especially from saturated or supersaturated solution. Conditions such as slight temperature increase, solution composition and pH changes, pressure drop, and kinetic distortion of the solution (pumping, flow through the elbows, nozzles, and between the sorbent grains) occurred in the column during the sorption and desorption processes and could induce generation of the air bubbles.
To check the possibility of the large-scale result prediction by the BDST model appropriate calculations were made. Due to the change of sorbent grain size some coefficient had to be introduced, therefore the value of breakthrough volume calculated from BDST model was divided by 3.7 as it was found during the comparison of sorption on fine and large grain. Using mentioned coefficient, bed height Z = 120 cm and keeping the other parameters unchanged (c0 = 30 mg/L, ct = cB = 2 mg/L), calculated breakthrough volumes for new linear flow rate F’’ and F’’’ (99.6 cm/h and 442.5 cm/h, respectively), was found to be 4361 L and 3519 L for flow rate of 45 L/h and 200 L/h, respectively. These results did not take into account that experiments were conducted on sorbent in H+ form instead of Ca2+, the proper results should be then decreased of ~25% to 3276 L and 2639 L, respectively. After such computations the results are significantly lower than experimental ones (5900 L and 3600 L), but as mentioned earlier BDST model neglect some processes responsible for sorption efficiency. Additionally introduced coefficients of grain size and sorbent form could be a source of some errors.
In desorption studies two acids were used—0.1 M nitric acid, as the most commonly used for stripping of various elements, and sulfuric acid of the same proton concentration (i.e., 0.05 M H
2SO
4). The selection of acid solution should depend on further management of solution from desorption process. The sulfuric acid was selected as alternative to nitric one due to possibility of further recovery of zinc from sulfate solution by electrolysis. The electrolysis of zinc from nitric acid solution is not effective. Additionally the accepted limit of the sulfate ions in wastewater is higher than for nitrate ones [
9], thus sulfuric acid as stripping agent may be desirable.
The results shown in
Figure 11 for these acids do not differ significantly—in both cases fast and complete removal of zinc from biosorbent are illustrated by sharp and symmetric peak of elution curve. Taking into account that 5900 L of the solution was purified to desired level and only 120 L of acid solution was used for zinc(II) removal from biosorbent, the zinc(II) ions were concentrated 49 times in one sorption–desorption step. This should allow for further processing of the solution and metal recovery by e.g., electrolysis process.
Satisfactory results of large-scale studies on sorption from model solution inclined us to test the zinc removal from real wastewater from galvanizing plant. The wastewater from galvanizing plant has various amounts of zinc as well as other components diluted. The composition depends on wastewater origin—it could be used plating bath or rinse water from various stage of rinsing. We prepared simulated rinse water by dilution of original weakly acidic bath for zinc electroplating to final Zn concentration 30 mg/L (as in the model solution). This enabled to study the effect of real wastewater components on zinc(II) ions removal on studied biosorbent. In our case the substances were as follows; potassium chloride, boric acid, and a few additional components e.g., brightener, surfactants, etc., which could influence the sorption efficiency.
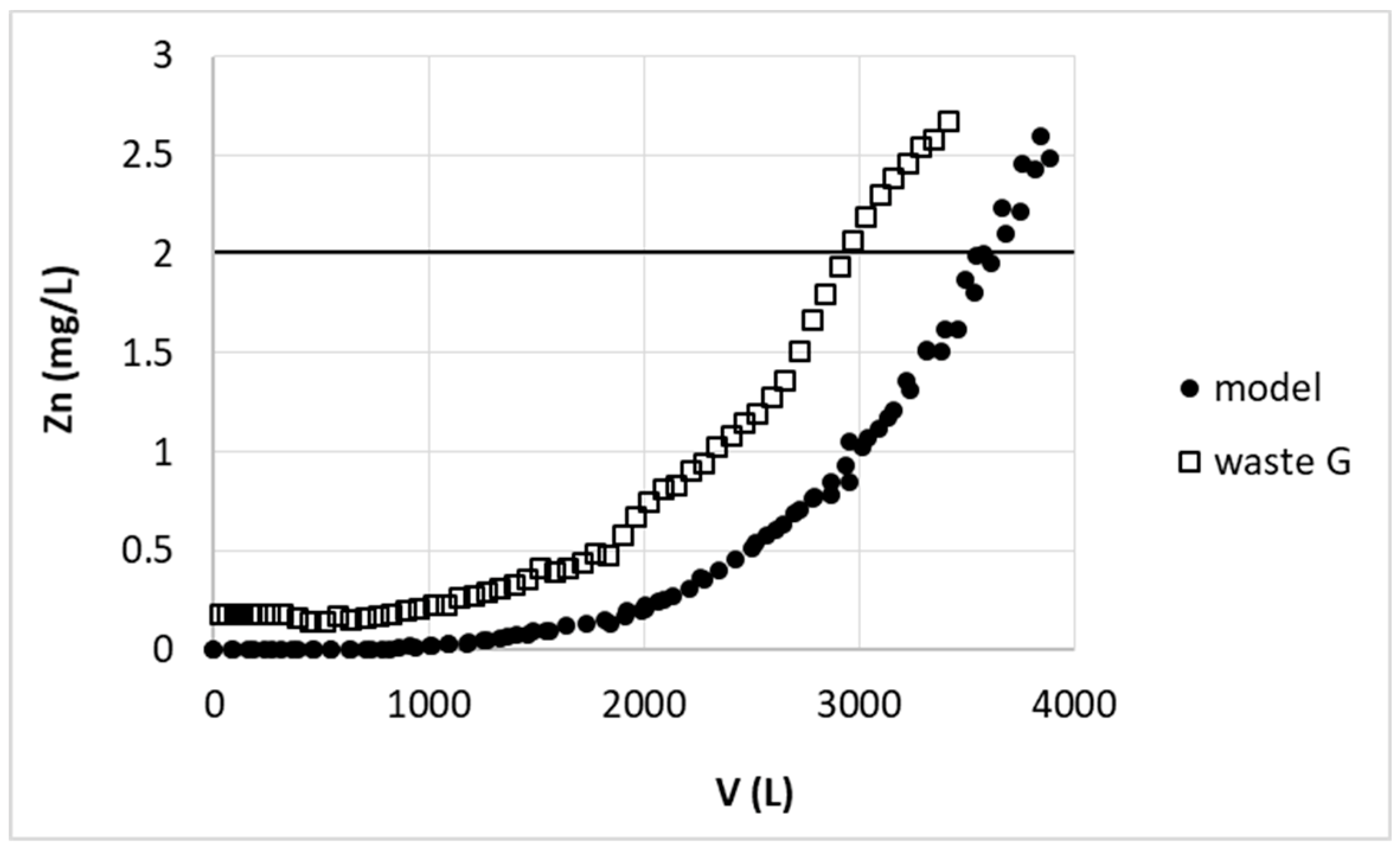
The comparison of the results of zinc ions removal from model (model) and real (waste G) solutions are presented in
Figure 12. Despite of various additional compounds presence, the breakthrough volume for real wastewater was only 17% lower than for model solution. This slight drop in zinc removal efficiency could be due to the fact that substances present in wastewater may act as complexing agent towards zinc(II) ions. These could be among others dextrin, organic acids, aldehydes or polymers with terminal amino groups used as brightener or surfactants like sodium lauryl ether sulfate or dodecanal. Such compounds may partially mask zinc(II) ions and prevent their sorption on pectin biosorbent. Additionally mentioned substances are molecules of rather big size and may clog the access to some active sites of sorbent, thus decrease its sorption capacity.
However the result of rinse water purification seems to be satisfactory and confirmed possibility of our biosorbent usage in large-scale for real wastewater. Additionally our sorption–desorption station was of size that could be already used in small galvanizing plant.
As a next step, very long studies should be performed to estimate real lifetime of the biosorbent. However our results—20 cycles of sorption–desorption process without loss of sorbent efficiency—are, in the case of biosorbent, of great significance.