Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors
Abstract
1. Introduction
2. Experimental
2.1. Materials
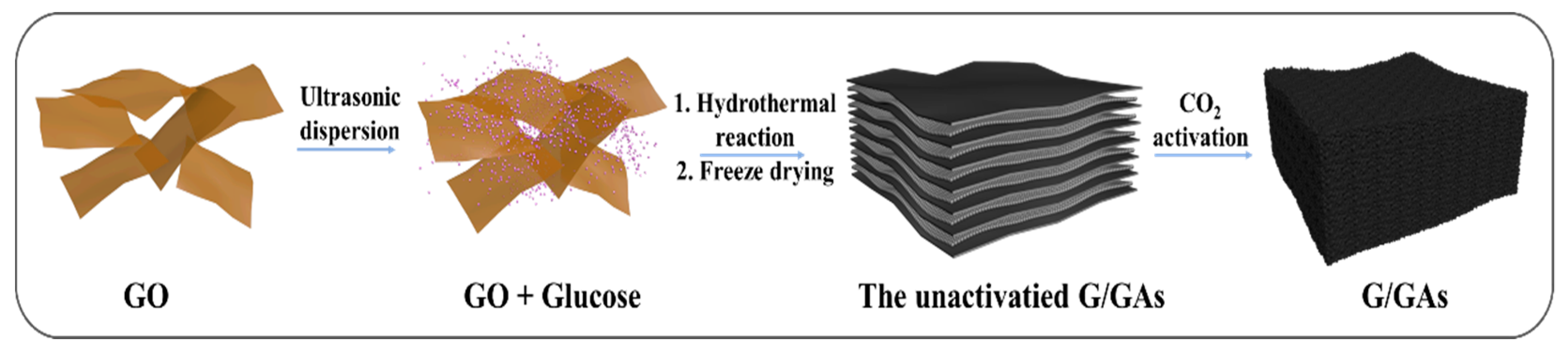
2.2. Preparation of G/GAs
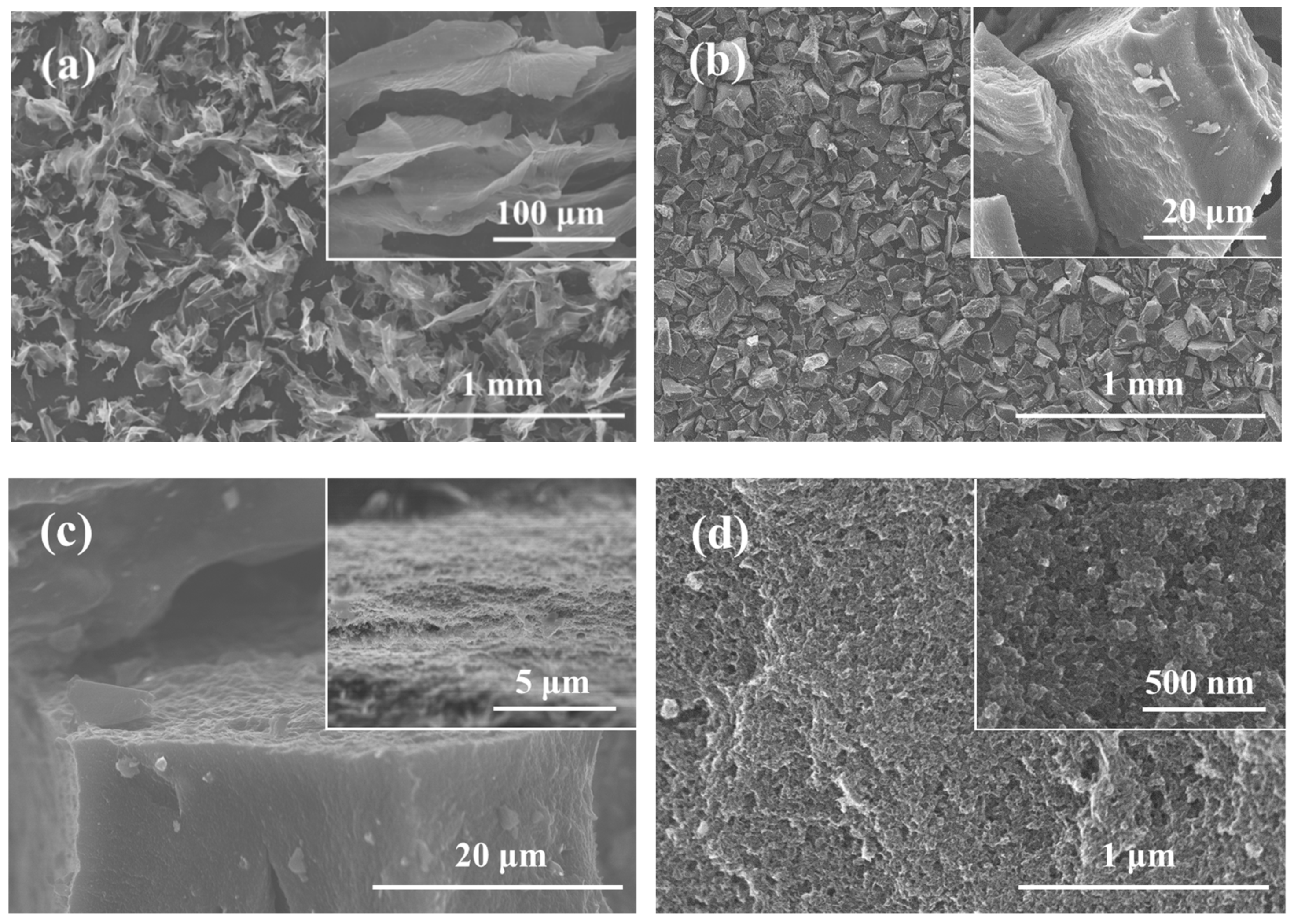
2.3. Characterization
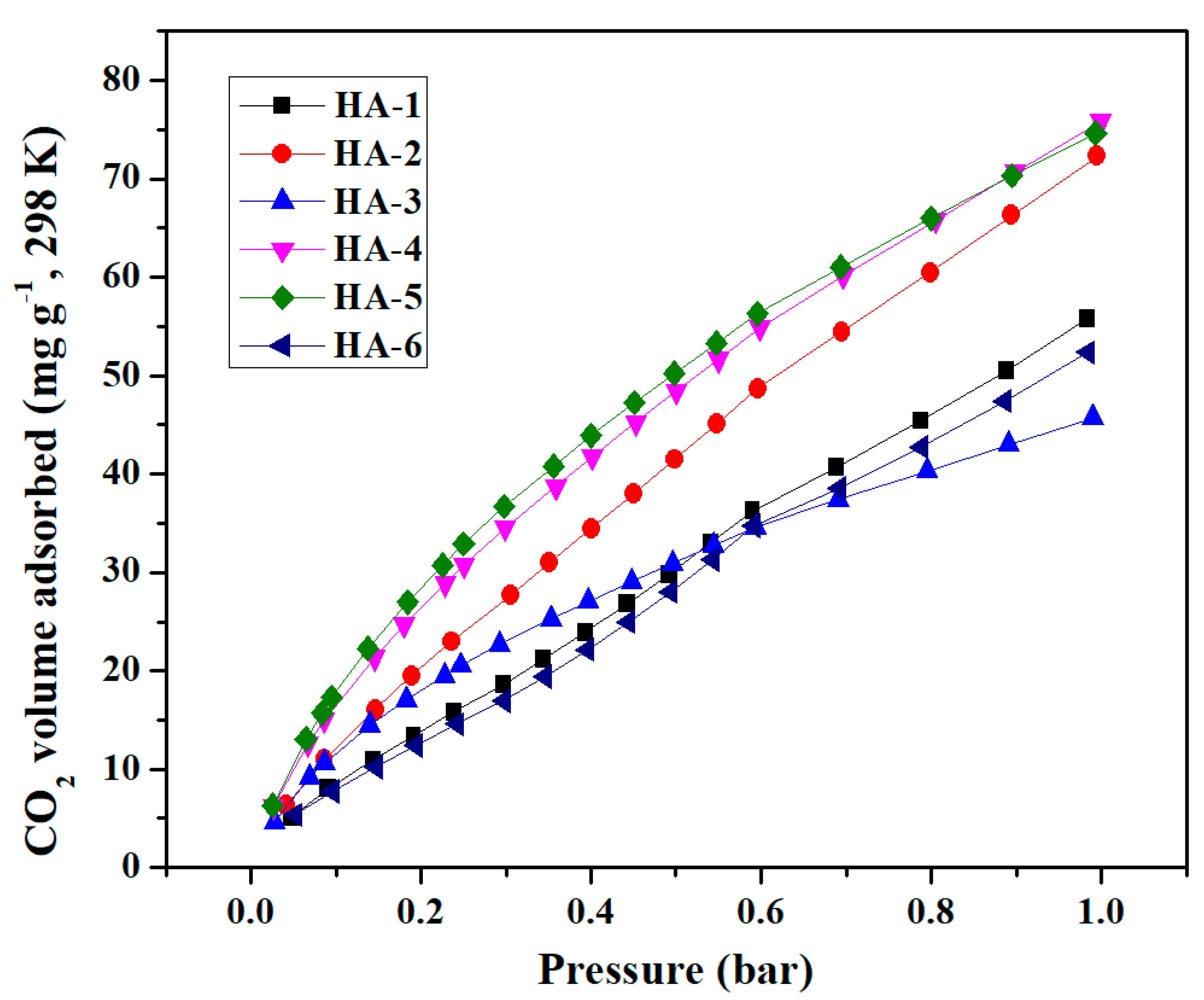
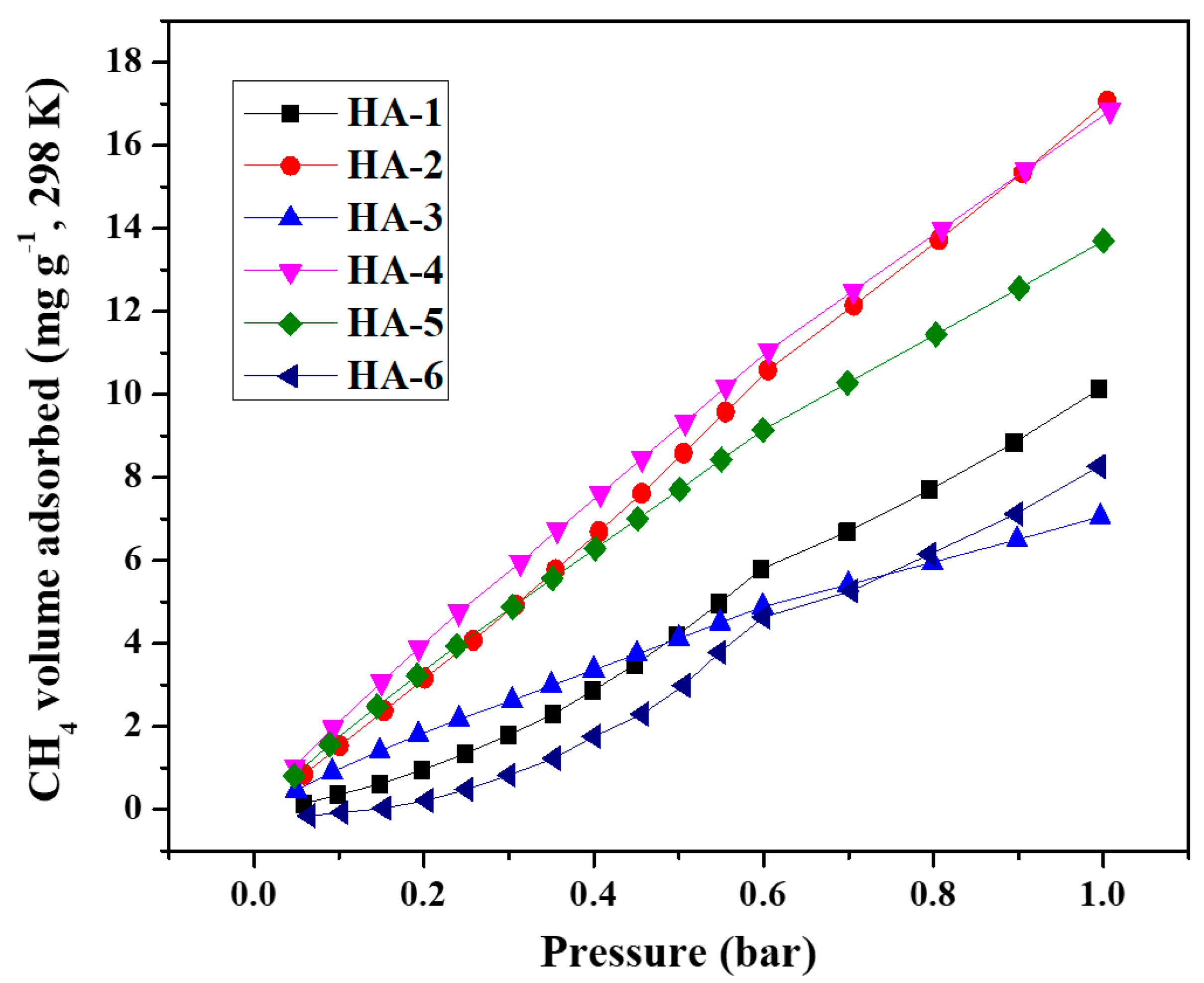
2.4. CO2, CH4 and H2 adsorption measurements
2.5. Electrochemical measurements
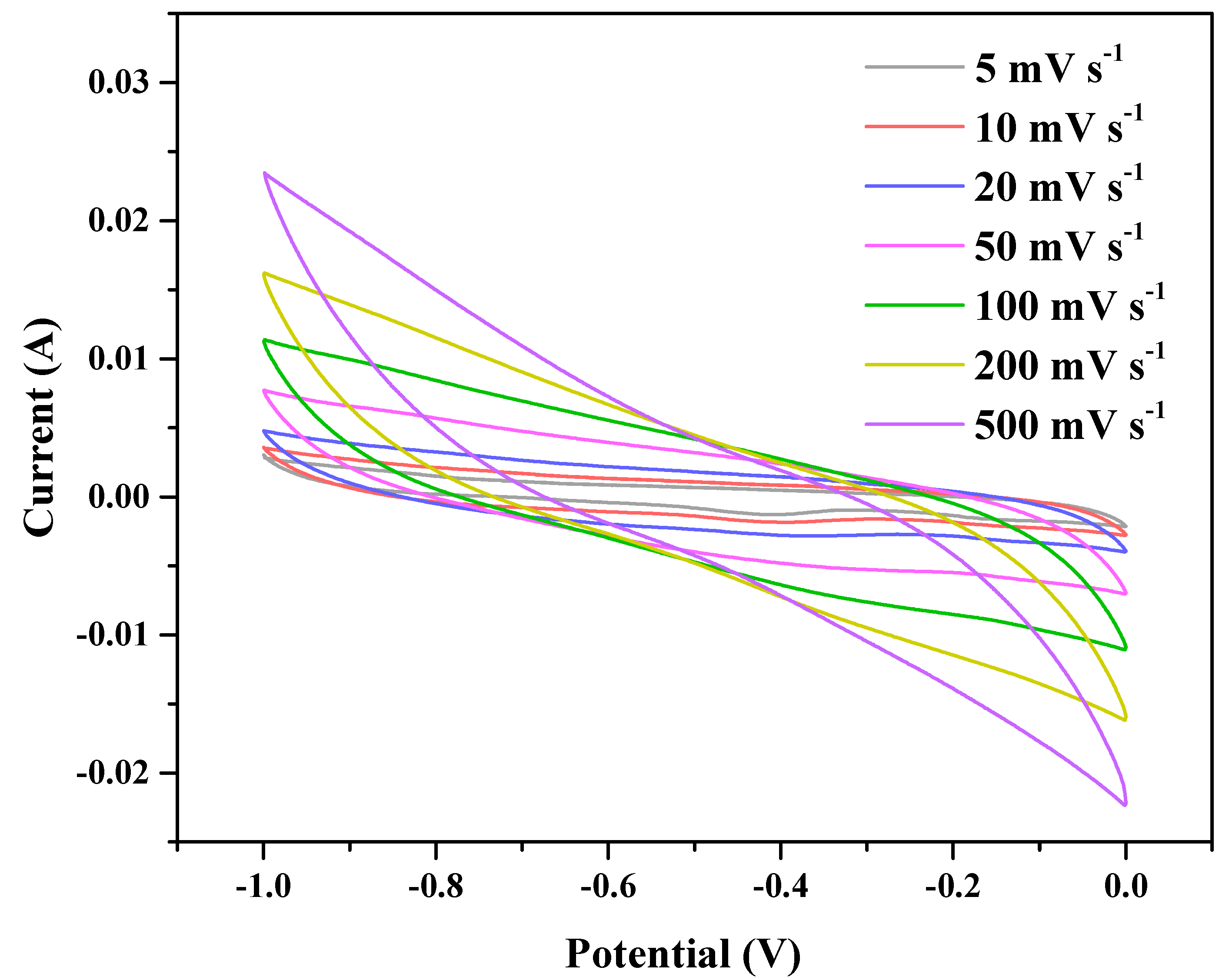
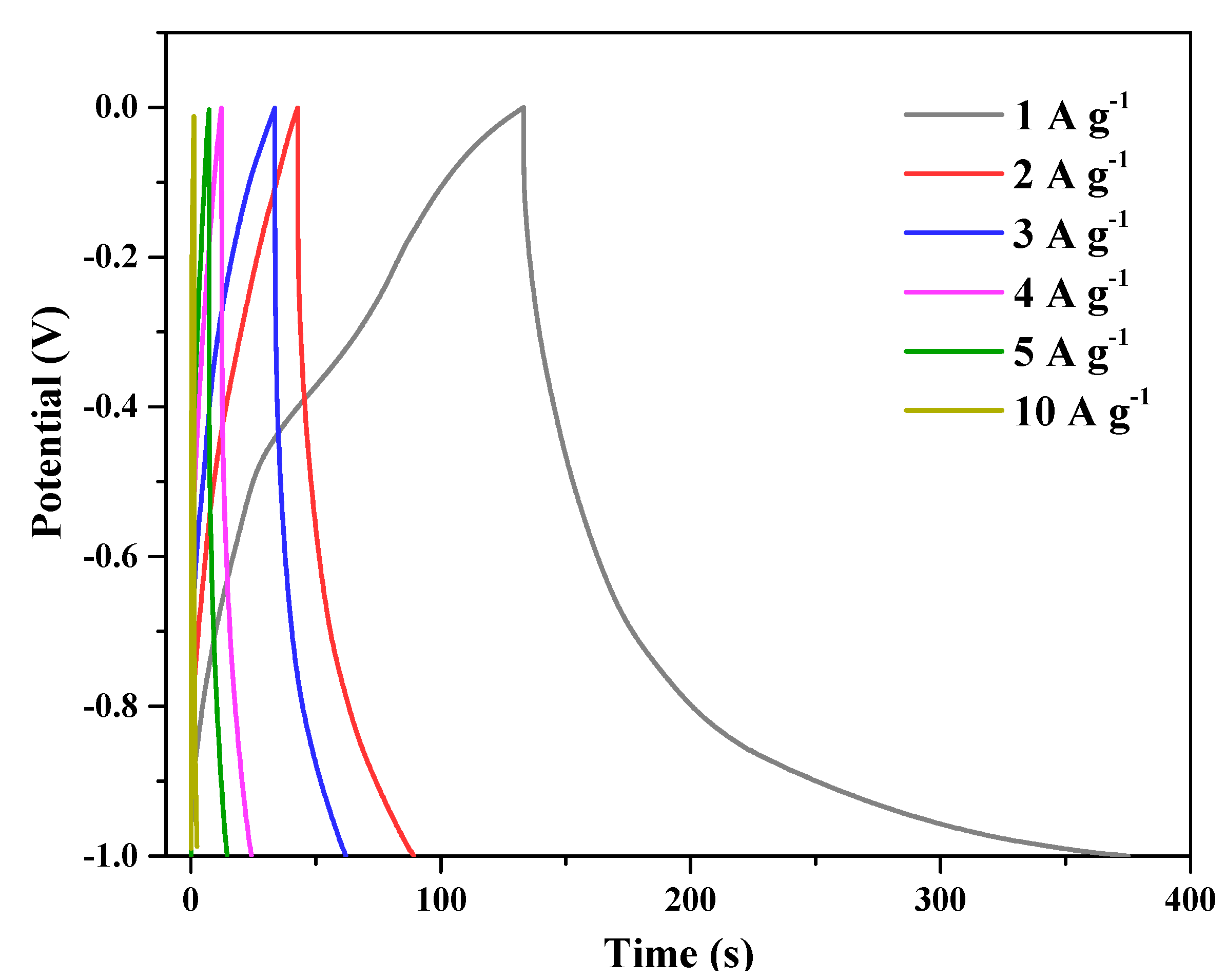
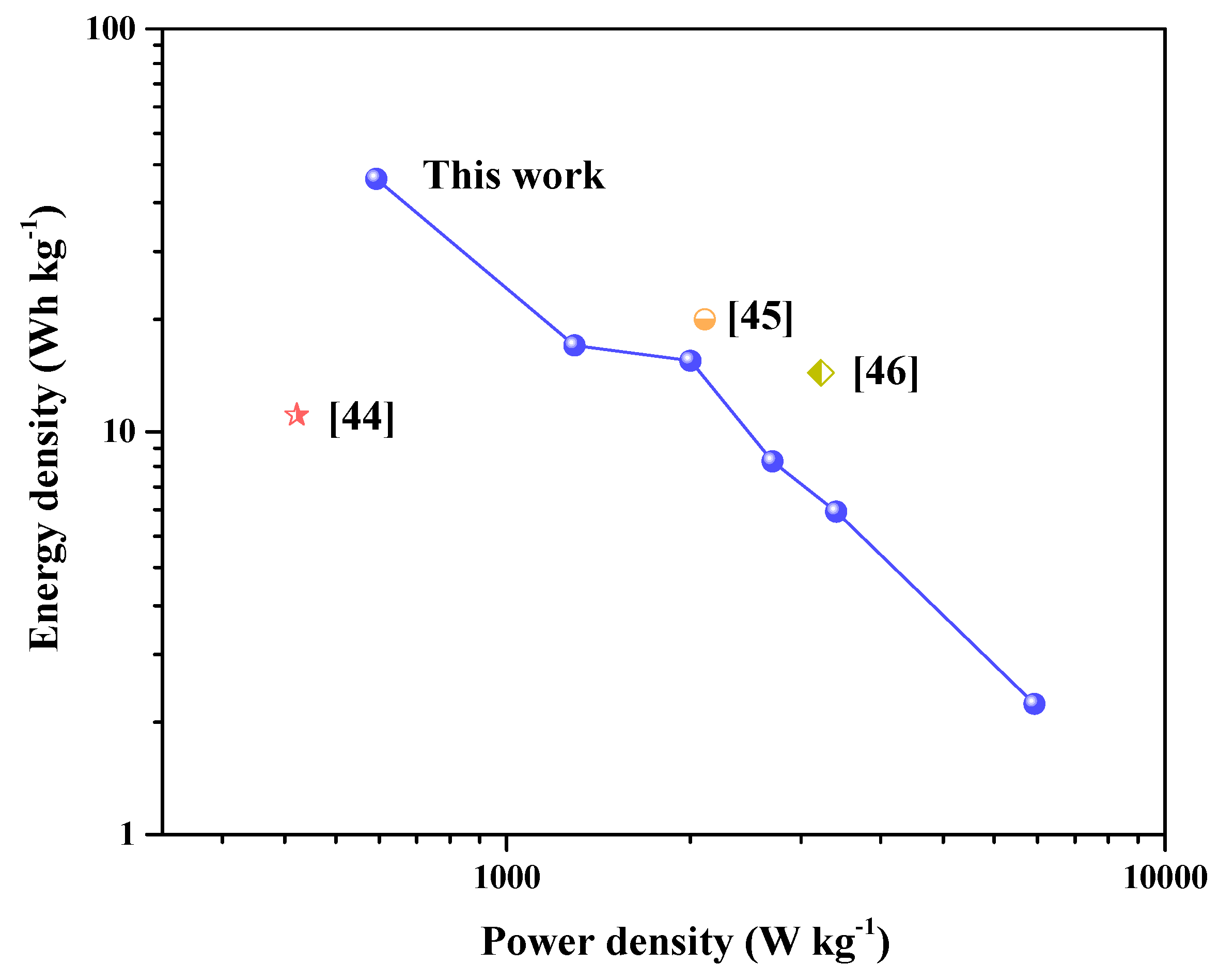
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, X.X.; Cheng, P.; Wang, H.J.; Xu, H.; Dang, L.Q.; Liu, Z.H.; Lei, Z.B. Activation of graphene aerogel with phosphoric acid for enhanced electrocapacitive performance. Carbon 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Liu, M.R.; Peng, C.; Yang, W.K.; Guo, J.J.; Zheng, Y.X.; Chen, P.Q.; Huang, T.; Xu, J. Pd nanoparticles supported on three-dimensional graphene aerogels as highly efficient catalysts for methanol electrooxidation. Electrochim. Acta 2015, 178, 838–846. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast sol-gel synthesis of graphene aerogel materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Luo, Q.Z.; Huang, Q.; Chen, Z.; Yao, L.; Fu, Q.M.; Fu, P.; Lin, Z.D. Temperature dependence of the pore structure in polyvinylidene fluoride (PVDF)/graphene composite membrane probed by electrochemical impedance spectroscopy. Polymers 2018, 10, 1123. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Gadipelli, S.; Li, Z.N.; Zhao, T.T.; Yang, Y.C.; Yildirim, T.; Guo, Z.X. Graphitic nanostructures in a porous carbon framework significantly enhance electrocatalytic oxygen evolution. J. Mater. Chem. A 2017, 5, 24686–24694. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.X.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.L.; Deng, S.G. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2018, 355, 309–319. [Google Scholar] [CrossRef]
- Xing, L.B.; Hou, S.F.; Zhou, J.; Zhang, J.L.; Si, W.J.; Dong, Y.H.; Zhuo, S.P. Three dimensional nitrogen-doped graphene aerogels functionalized with melamine for multifunctional applications in super capacitors and adsorption. J. Solid State Chem. 2015, 230, 224–232. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, W.S. CO2 adsorption on LTA zeolites: Effect of mesoporosity. Appl. Surf. Sci. 2014, 311, 107–109. [Google Scholar] [CrossRef]
- Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous hyper-crosslinked polystyrenes and nanocomposites with high adsorption properties: A review. Polymers 2017, 9, 651. [Google Scholar] [CrossRef]
- Mohammed, M.M.M.; Chun, D.M. Electrochemical performance of few-layer graphene nano-flake supercapacitors prepared by the vacuum kinetic spray method. Coatings 2018, 8, 302. [Google Scholar] [CrossRef]
- Lee, H.I.; Park, S.J. Facile fabrication of electrospun polyacrylonitrile/poly(vinylidene fluoride)-based carbon nanofibers for supercapacitor electrodes. Carbon Lett. 2017, 23, 79–83. [Google Scholar]
- Lee, H.; Lee, W.J.; Park, Y.K.; Ki, S.J.; Kim, B.J.; Jung, S.C. Liquid phase plasma synthesis of iron oxide nanoparticles on nitrogen-doped activated carbon resulting in nanocomposite for supercapacitor applications. Nanomaterials 2018, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Bao, W.T.; Jiang, Y.; Wang, L.; Zhang, L.; Sha, O.; Wu, C.Q.; Gao, F.M. Fabrication of Ni-Al LDH/nitramine-N-doped graphene hybrid composites via a novel self-assembly process for hybrid supercapacitors. Chem. Eng. J. 2018, 354, 1132–1140. [Google Scholar] [CrossRef]
- Choi, M.S.; Park, S.; Lee, H.; Park, H.S. Hierarchically nanoporous carbons derived from empty fruit bunches for high performance supercapacitors. Carbon Lett. 2018, 25, 103–112. [Google Scholar]
- Gao, C.Y.; Meng, L.Y.; Piao, S.H.; Choi, H.J. Hollow submicron-sized spherical conducting polyaniline particles and their suspension rheology under applied electric fields. Polymer 2018, 140, 80–88. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, J.; Huang, W.X. Porous graphene oxide prepared on nickel foam by electrophoretic deposition and thermal reduction as high-performance supercapacitor electrodes. Materials 2017, 10, 936–953. [Google Scholar]
- Zhang, Y.M.; Chen, Z.; Zhang, D.D.; Zhao, Y.L.; Wu, P.P.; Wang, F. Diversified applications of polypyrrole/graphene aerogel in supercapacitors and three-dimensional electrode system. Mater. Lett. 2018, 227, 158–160. [Google Scholar] [CrossRef]
- Kshetri, T.; Thanh, T.D.; Singh, S.B.; Kim, N.H.; Lee, J.H. Hierarchical material of carbon nanotubes grown on carbon nanofibers for high performance electrochemical capacitor. Chem. Eng. J. 2018, 345, 39–47. [Google Scholar] [CrossRef]
- Guo, J.; Park, S.J.; Meng, L.Y.; Jin, X.H. Applications of carbon-based materials in solid phase micro-extraction: A review. Carbon Lett. 2017, 24, 10–17. [Google Scholar]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Masika, E.; Mokaya, R. High surface area metal salt templated carbon aerogels via a simple subcritical drying route: Preparation and CO2 uptake properties. RSC Adv. 2013, 3, 17677–17681. [Google Scholar] [CrossRef]
- Lin, R.J.; Li, Z.N.; Abou El Amaiem, D.I.; Zhang, B.J.; Brett, D.J.L.; He, G.J.; Parkin, I.P. A general method for boosting the supercapacitor performance of graphitic carbon nitride/graphene hybrids. J. Mater. Chem. A 2017, 5, 25545–25554. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Isothermal exfoliation of graphene oxide by a new carbon dioxide pressure swing method. Carbon 2014, 68, 112–117. [Google Scholar] [CrossRef]
- Meng, L.Y.; Jiang, W.Q.; Piao, W.X.; Meng, W. Effect of bio-template on the properties of SiO2/Al2O3 composites for drug delivery. J. Ind. Eng. Chem. 2016, 37, 14–17. [Google Scholar] [CrossRef]
- Liu, J.; Choi, H.J.; Meng, L.Y. A review of approaches for the design of high-performance metal/graphene electrocatalysts for fuel cell applications. J. Ind. Eng. Chem. 2018, 64, 1–15. [Google Scholar] [CrossRef]
- Shaikh, J.S.; Shaikh, N.S.; Kharade, R.; Beknalkar, S.A.; Patil, J.V.; Suryawanshi, M.P.; Kanjanaboos, P.; Hong, C.K.; Kim, J.H.; Patil, P.S. Symmetric supercapacitor: Sulphurized graphene and ionic liquid. J. Colloid Interface Sci. 2018, 527, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.D.; Lu, Y.G.; Li, S.L.; Wang, R.; Liu, Y.L. Extremely low fractions of graphene oxide in carbon foam prepared by a spin-coating method as freestanding supercapacitor electrodes. J. Mater. Sci. 2018, 53, 16476–16483. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, Y.; Li, C.M. Hierarchical graphene-based material for over 4.0 wt% physisorption hydrogen storage capacity. ACS Sustain. Chem. Eng. 2013, 1, 14–18. [Google Scholar] [CrossRef]
- Qin, G.T.; Zhang, Y.P.; Wei, W. Facile synthesis of a nitrogen-doped carbon membrane for CO2 capture. Mater. Lett. 2017, 209, 75–77. [Google Scholar] [CrossRef]
- Doman, A.; Nagy, B.; Nichele, L.P.; Sranko, D.; Madarasz, J.; Laszlo, K. Pressure resistance of copper benzene-1,3,5-tricarboxylate–carbon aerogel composites. Appl. Surf. Sci. 2018, 434, 1300–1310. [Google Scholar] [CrossRef]
- Zhao, X.; Hayner, C.M.; Kung, M.C.; Kung, H.H. Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 2011, 5, 8739–8749. [Google Scholar] [CrossRef]
- Liao, Y.Z.; Cheng, Z.H.; Trunk, M.; Thomas, A. Targeted control over the porosities and functionalities of conjugated microporous polycarbazole networks for CO2-selective capture and H2 storage. Polym. Chem. 2017, 8, 7240–7247. [Google Scholar] [CrossRef]
- Rao, D.W.; Wang, Y.H.; Meng, Z.S.; Yao, S.S.; Chen, X.; Shen, X.Q.; Lu, R.F. Theoretical study of H2 adsorption on metal-doped graphene sheets with nitrogen-substituted defects. Int. J. Hydrogen Energy 2015, 40, 14154–14162. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.R.; Cui, B.; Yin, P.F.; Zhang, C. Design and preparation of biomass-derived carbon materials for supercapacitors: A review. C 2018, 4, 53. [Google Scholar] [CrossRef]
- Tabrizi, A.G.; Arsalani, N.; Mohammadi, A.; Ghadimi, L.S.; Ahadzadeh, I. High-performance asymmetric supercapacitor based on hierarchical nanocomposites of polyaniline nanoarrays on graphene oxide and its derived N-doped carbon nanoarrays grown on graphene sheets. J. Colloid Interface Sci. 2018, 531, 369–381. [Google Scholar] [CrossRef]
- Yan, Y.Z.; Tang, H.L.; Wu, F.; Wang, R.; Pan, M. One-step self-assembly synthesis α-Fe2O3 with carbon-coated nanoparticles for stabilized and enhanced supercapacitors electrode. Energies 2017, 10, 1296–1308. [Google Scholar]
- Chang, P.; Qin, Z.H. Hierarchical porous carbon materials with ultrahigh specific surface area prepared from coal for supercapacitors. Carbon Lett. 2018, 25, 117–121. [Google Scholar]
- Hu, X.J.; Bai, D.C.; Wu, Y.Q.; Chen, S.B.; Ma, Y.; Lu, Y.; Chao, Y.Z.; Bai, Y.X. A facile synthesis of reduced holey graphene oxide for supercapacitors. Chem. Commun. 2017, 53, 13225–13228. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Park, S.K.; Nakhanivej, P.; Kim, Y.; Hwang, S.M.; Park, H.S. Hierarchically structured graphene-carbon nanotube-cobalt hybrid electrocatalyst for seawater battery. J. Power Sources 2017, 372, 31–37. [Google Scholar] [CrossRef]
- Tan, S.C.; Li, J.J.; Zhou, L.J.; Chen, P.; Shi, J.T.; Xu, Z.Y. Modified carbon fiber paper-based electrodes wrapped by conducting polymers with enhanced electrochemical performance for supercapacitors. Polymers 2018, 10, 1072. [Google Scholar] [CrossRef]
- Park, M.H.; Yun, Y.S.; Cho, S.Y.; Kim, N.R.; Jin, H.J. Waste coffee grounds-derived nanoporous carbon nanosheets for supercapacitors. Carbon Lett. 2016, 19, 66–71. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.Y.; Li, J.; Wang, L.J. Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor. Int. J. Biol. Macromol. 2018, 115, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, Y.A.; Kim, B.H. Capacitive properties of hierarchically structured carbon nanofiber/graphene/MnO2 hybrid electrode with nitrogen and oxygen heteroatoms. Carbon 2016, 107, 783–791. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, F.; Qian, L.H.; Xiao, J.W.; Wang, S.; Liu, Y.Q. Facile synthesis of 3D MnO2-graphene and carbon nanotube-graphene composite networks for high-performance, flexible, all-solid-state asymmetric supercapacitors. Adv. Energy Mater. 2014, 4, 1400064–1400072. [Google Scholar] [CrossRef]
- Liu, Y.C.; He, D.W.; Wu, H.L.; Duan, J.H.; Zhang, Y.N. Hydrothermal self-assembly of manganese dioxide/manganese carbonate/reduced graphene oxide aerogel for asymmetric supercapacitors. Electrochim. Acta 2015, 164, 154–162. [Google Scholar] [CrossRef]






| Samples | SBET a (m2 g−1) | VTotal b (cm3 g−1) | VMeso c (cm3 g−1) | DBET d (nm) | CO2 Uptake e (mg g−1) | CH4 Uptake f (mg g−1) | H2 Uptake g (mg g−1) |
|---|---|---|---|---|---|---|---|
| H-1 | 519 | 1.14 | 1.29 | 8.76 | 36.34 | 5.17 | 0.59 |
| H-2 | 522 | 2.37 | 2.53 | 18.18 | 32.12 | 5.07 | 0.53 |
| H-3 | 476 | 1.86 | 2.01 | 15.63 | 29.83 | 3.43 | 0.42 |
| H-4 | 412 | 2.09 | 2.20 | 20.23 | 23.50 | 2.26 | 0.36 |
| H-5 | 292 | 1.74 | 1.82 | 23.75 | 19.02 | 2.10 | 0.30 |
| H-6 | 256 | 1.69 | 1.76 | 26.32 | 17.82 | 2.14 | 0.21 |
| Samples | SBET a (m2 g−1) | VTotal b (cm3 g−1) | VMicro c (cm3 g−1) | VMeso d (cm3 g−1) | DBET e (nm) | CO2 Uptake f (mg g−1) | CH4 Uptake g (mg g−1) | H2 Uptake h (mg g−1) |
|---|---|---|---|---|---|---|---|---|
| HA-1 | 749 | 1.74 | 0 | 1.67 | 9.28 | 56.4 | 10.0 | 10.5 |
| HA-2 | 763 | 3.06 | 0 | 3.22 | 16.04 | 73.0 | 16.8 | 12.1 |
| HA-3 | 632 | 2.23 | 0 | 2.34 | 14.10 | 46.2 | 7.1 | 7.2 |
| HA-4 | 560 | 1.79 | 0.04 | 1.84 | 12.78 | 76.5 | 16.6 | 10.2 |
| HA-5 | 522 | 1.59 | 0.06 | 1.60 | 12.17 | 75.3 | 13.5 | 10.0 |
| HA-6 | 544 | 1.21 | 0.14 | 1.13 | 8.89 | 52.9 | 8.2 | 3.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.-K.; Jin, B.; Meng, L.-Y. Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers 2019, 11, 40. https://doi.org/10.3390/polym11010040
Liu K-K, Jin B, Meng L-Y. Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers. 2019; 11(1):40. https://doi.org/10.3390/polym11010040
Chicago/Turabian StyleLiu, Kang-Kai, Biao Jin, and Long-Yue Meng. 2019. "Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors" Polymers 11, no. 1: 40. https://doi.org/10.3390/polym11010040
APA StyleLiu, K.-K., Jin, B., & Meng, L.-Y. (2019). Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers, 11(1), 40. https://doi.org/10.3390/polym11010040






