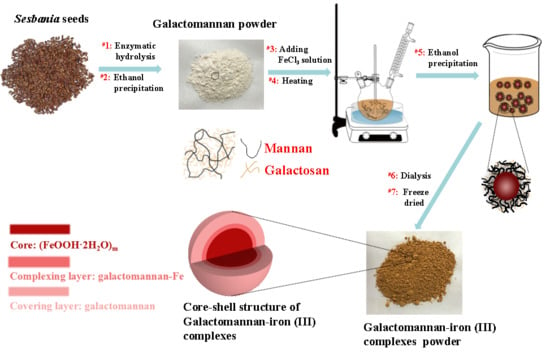
Synthesis and Characterization of an Antioxidative Galactomannan–Iron(III) Complex from Sesbania Seed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Galactomannan–Iron(III) Complexes
2.3. Quantification of the Iron and Polysaccharides Content of the Galactomannan–Iron(III) Complexes
2.4. Molecular Weight Analysis of the Galactomannan–Iron(III) Complexes
2.5. Characterization of the Galactomannan–Iron(III) Complexes
2.6. In Vitro Anti-Radiation Activity of the Galactomannan–Iron(III) Complexes
2.7. Measuring the Iron Release Capability of the Galactomannan–Iron(III) Complexes
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparing of Different Molecule Weight Galactomannan from Sesbania Seeds
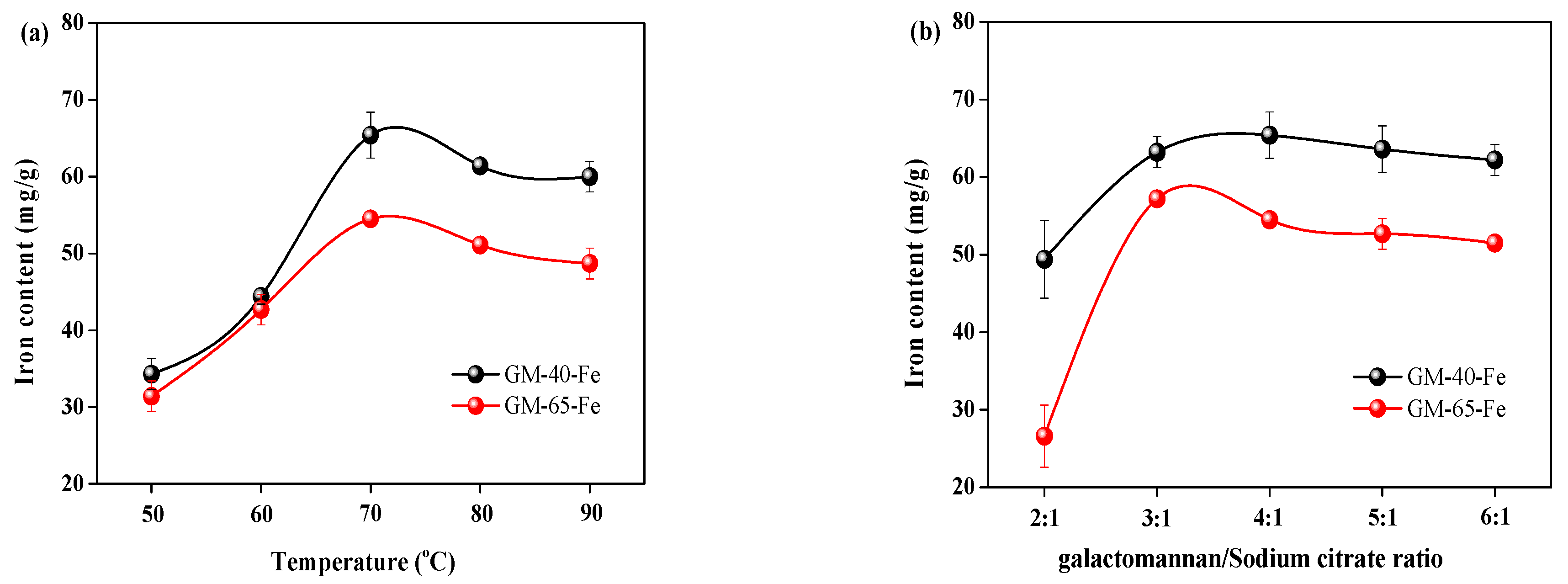
3.2. Determination of Key Factors Contributing to the Iron Content of Synthesized Galactomannan–Iron(III) Complexes
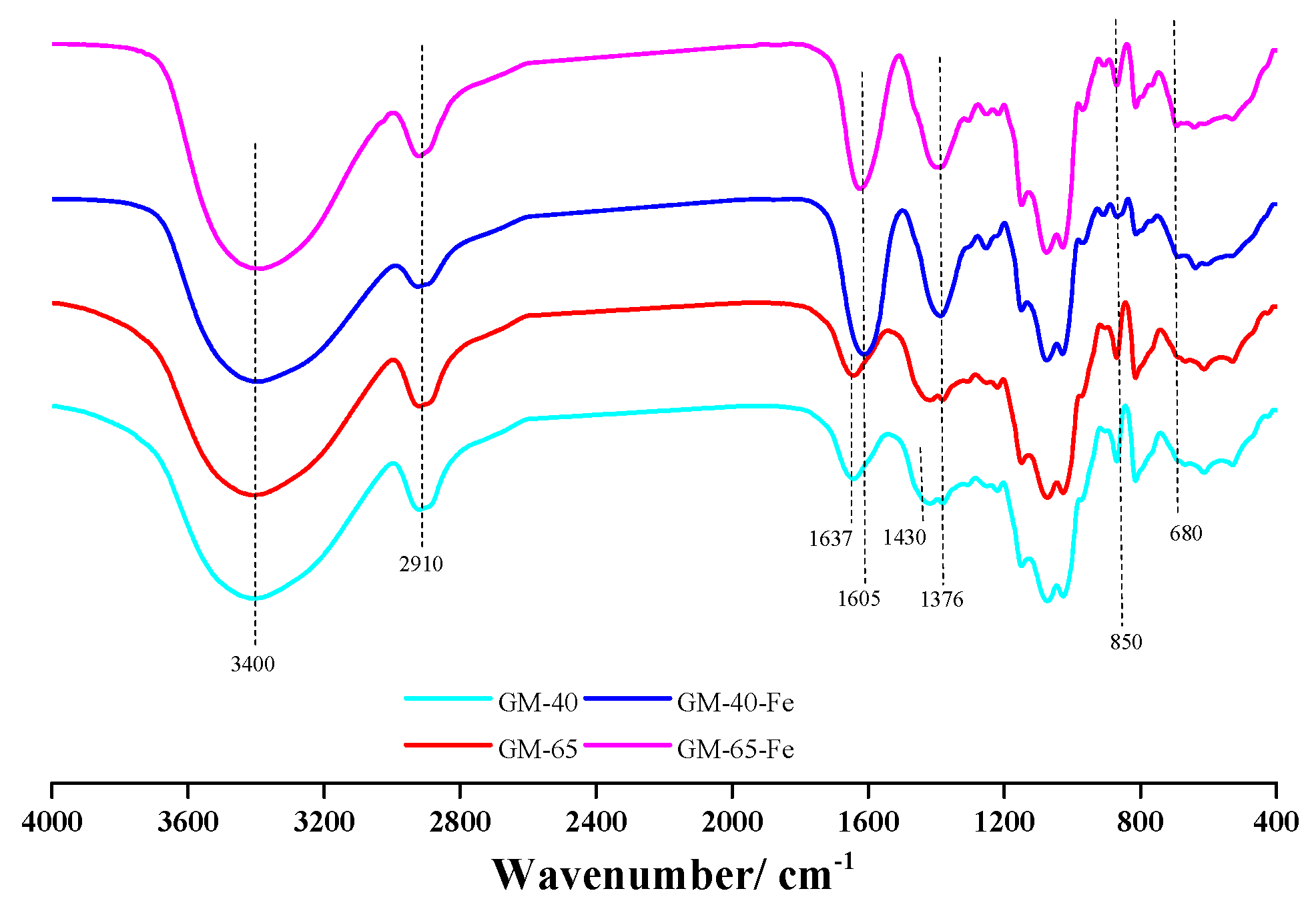
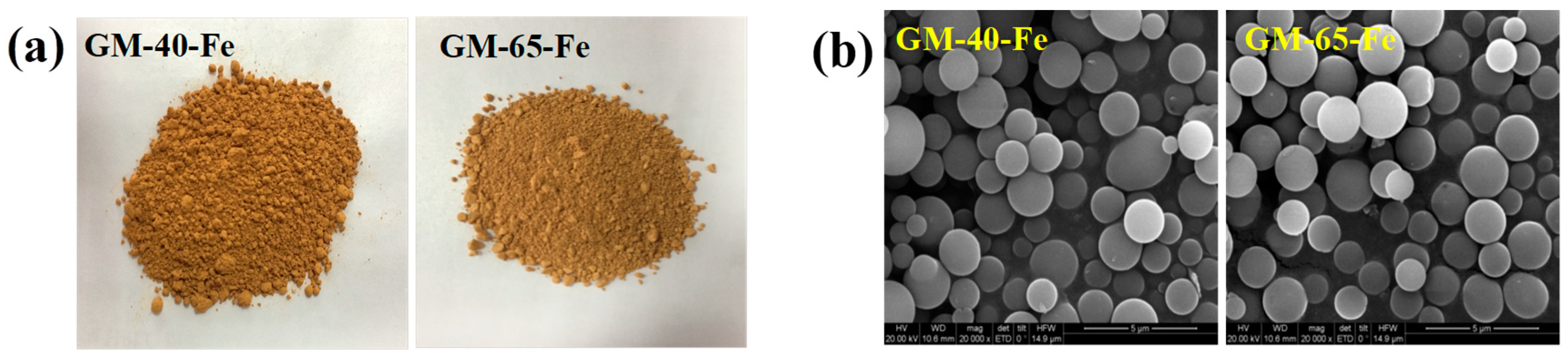
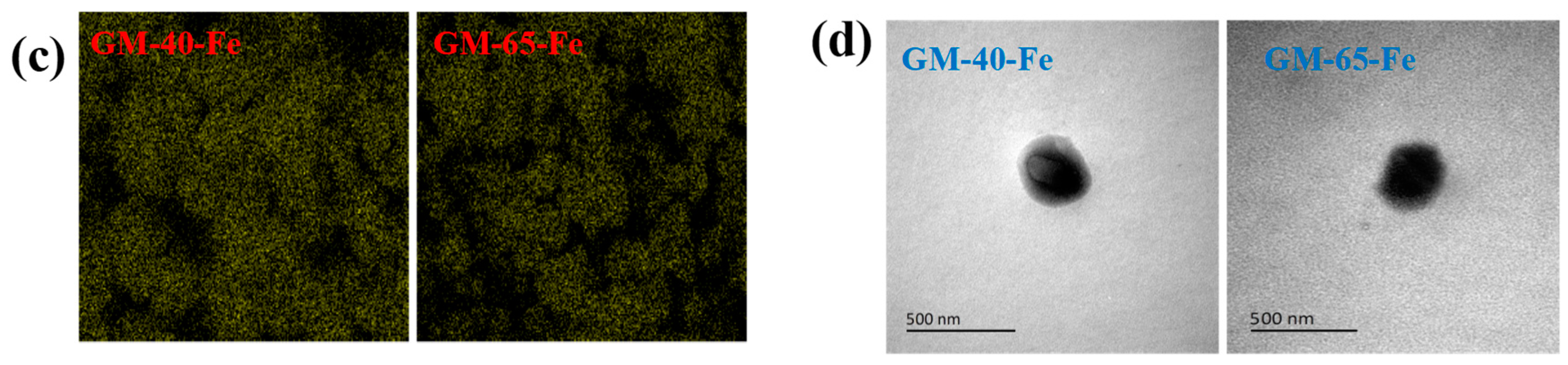
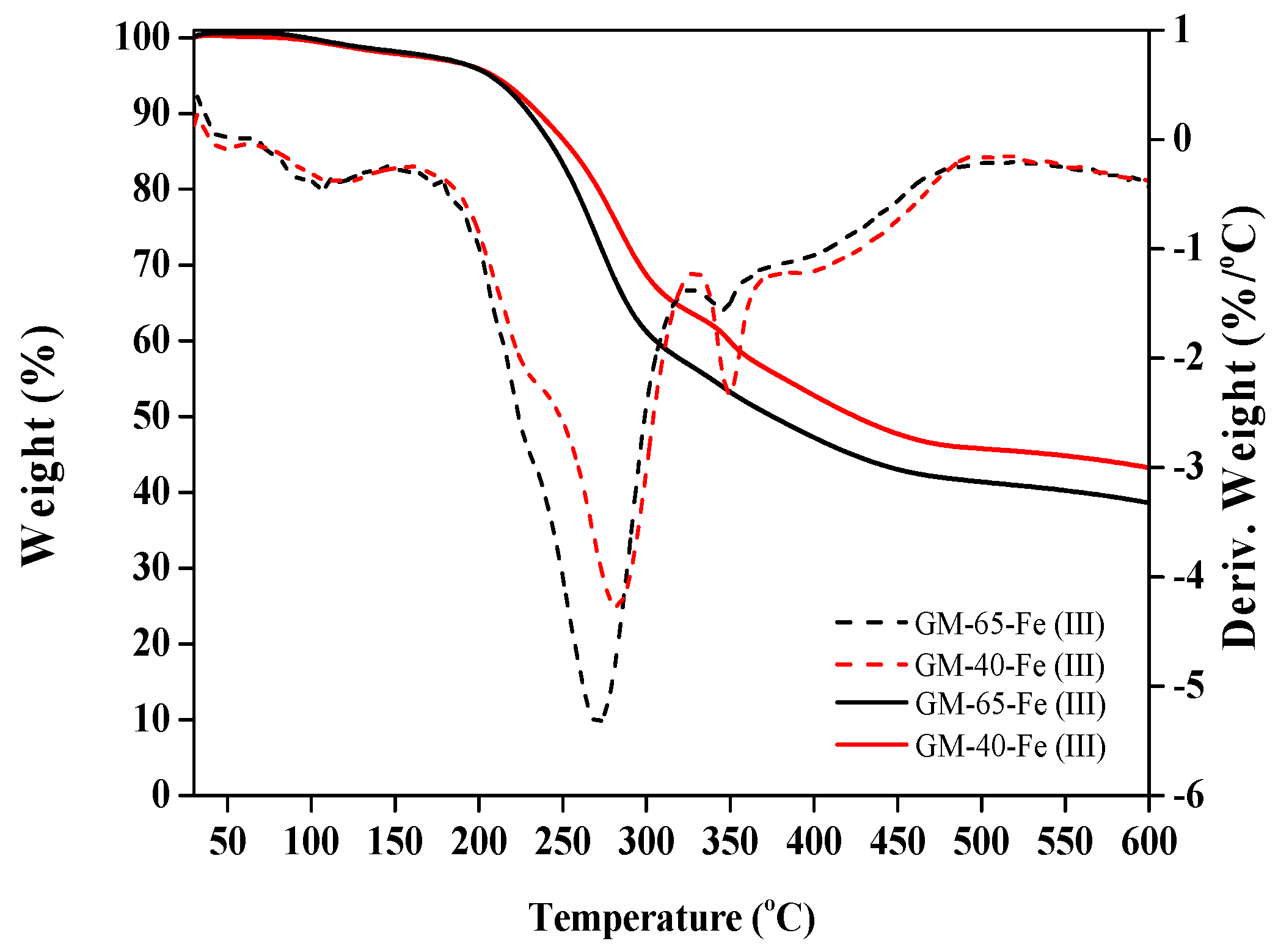
3.3. Characterization of the Galactomannan–Iron(III) Complexes
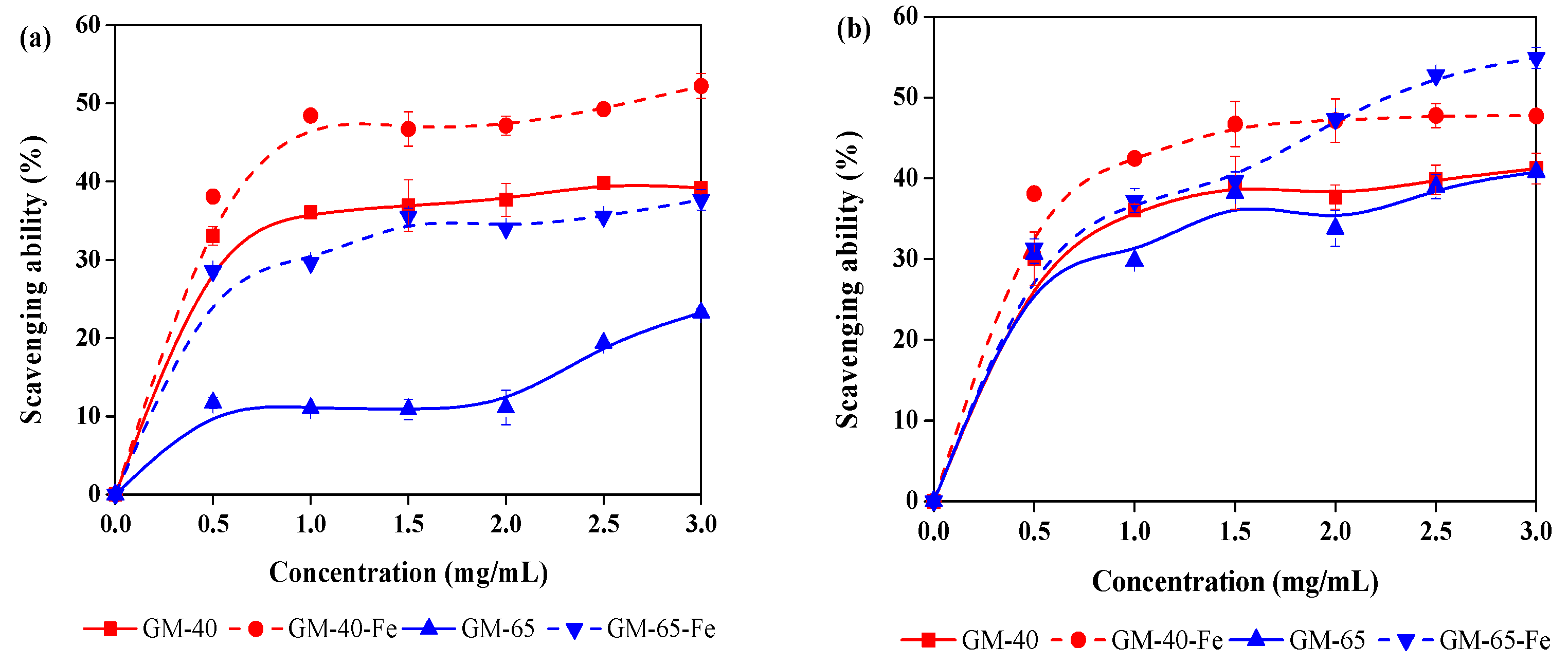
3.4. Antioxidant Activity of Galactomannan–Iron(III) Complexes
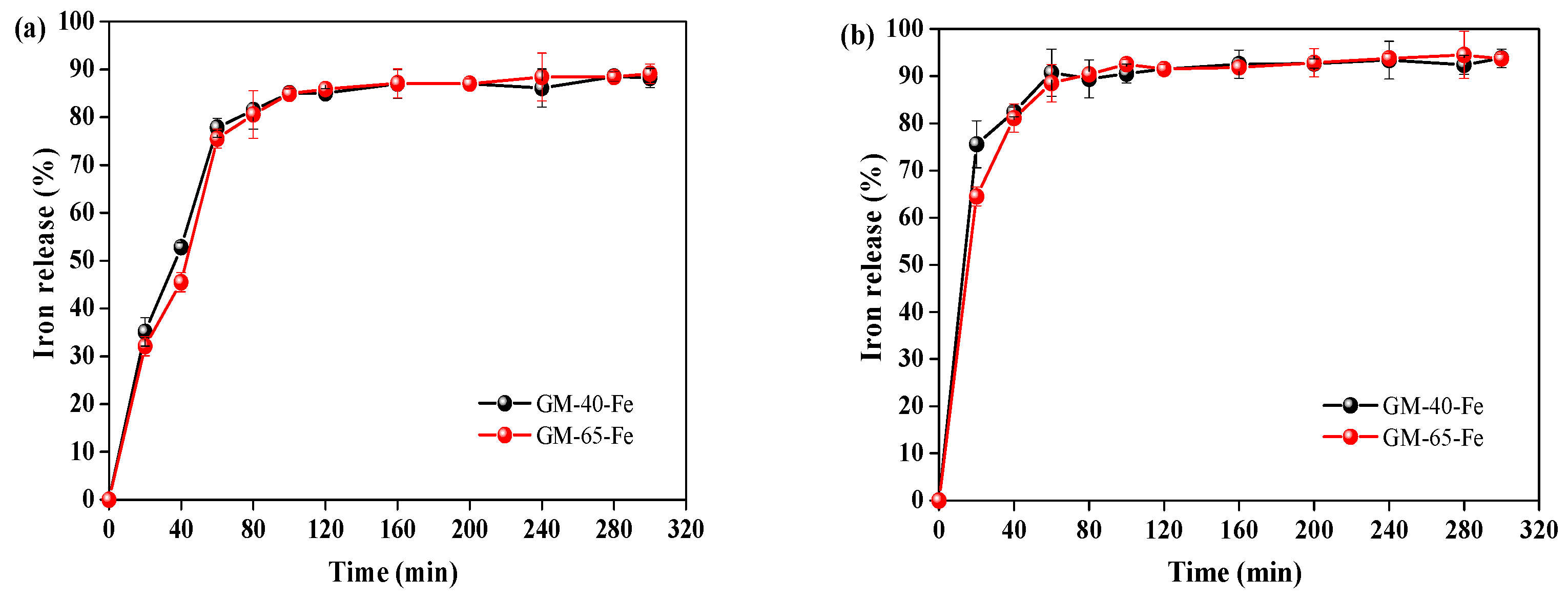
3.5. Bioavailability of Galactomannan–Iron(III) Complexes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tapiero, H.; Gate, L.; Tew, K.D. Iron: Deficiencies and requirements. Biomed. Pharmacother. 2001, 55, 324–332. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Wang, Y.; Xing, L. Synthesis and characterization of a new Inonotus obliquus polysaccharide–iron(III) complex. Int. J. Biol. Macromol. 2015, 75, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Qian, X.H.; Qian, X.L. Astragalus polysaccharide upregulates hepcidin and reduces iron overload in mice via activation of p38 mitogen-activated protein kinase. Biochem. Biophs. Res. Commun. 2016, 472, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xu, L.; Meng, Y.; Liu, Y.; Li, J.; Zu, Y.; Zhu, M. Preparation and characterization of a novel Astragalus membranaceus polysaccharide–iron(III) complex. Int. J. Biol. Macromol. 2016, 93, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Al-Momen, A.K.; Al-Meshari, A.; Al-Nuaim, L.; Saddique, A.; Abotalib, Z.; Khashogji, T.; Abbas, M. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 69, 121–124. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, C.; Zhao, H.; Jing, J.; Gong, N.; Lu, W. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide–iron(III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef]
- Saini, R.K.; Manoj, P.; Shetty, N.P.; Srinivasan, K.; Giridhar, P. Dietary iron supplements and Moringa oleifera leaves influence the liver hepcidin messenger RNA expression and biochemical indices of iron status in rats. Nutr. Res. 2014, 34, 630–638. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Yu, P.; Zhan, Q.; Wang, J.; Chi, Y.; Wang, P. A novel low molecular weight Enteromorpha polysaccharide–iron(III) complex and its effect on rats with iron deficiency anemia (IDA). Int. J. Biol. Macromol. 2018, 108, 412–418. [Google Scholar] [CrossRef]
- Bailie, G.R.; Schuler, C.; Leggett, R.E.; Levin, R. Oxidative effect ferumoxytol and iron dextran on urinary bladder contraction and impact of antioxidants. Free Radic. Antioxid. 2013, 3, 7–10. [Google Scholar] [CrossRef]
- Somsook, E.; Hinsin, D.; Buakhrong, P.; Teanchai, R.; Mophan, N.; Pohmakotr, M.; Shiowatana, J. Interactions between iron(III) and sucrose, dextran, or starch in complexes. Carbohydr. Res. 2005, 61, 281–287. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, L.; Yang, S.; Zhao, F.; Xu, L.; Yong, Q. Isolation, characterization and in vitro anticancer activity of an aqueous galactomannan from the seed of Sesbania cannabina. Int. J. Biol. Macromol. 2018, 113, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, L.; Lai, C.; Zhou, M.; Xi, X.; Yu, S.; Yong, Q. In vitro proliferation of Bifidobacterium adolescentis by galactomanno-oligosaccharides from Sesbania cannabina. J. For. Eng. 2017, 2, 64–69. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory (NREL), U.S. Department of Energy: Golden, CO, USA, 2011.
- Xie, Y.; Huang, C.; Li, X.; Lai, C.; Yu, S.; Yong, Q. Effect of agitation speed and aeration rate on fermentation synthesis of β-mannanase with Trichoderma reesei. J. For. Eng. 2017, 2, 92–96. [Google Scholar]
- Huang, C.; Tang, S.; Zhang, W.; Tao, Y.; Lai, C.; Li, X.; Yong, Q. Unveiling the structural properties of lignin-carbohydrate complexes in bamboo residues and its functionality as antioxidants and immunostimulants. ACS Sustain. Chem. Eng. 2018, 6, 12522–12531. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, J.; Lu, H.; Cai, J.; Zou, J.; Li, X.; Sun, Z.; He, H. Fabrication of magnetic wood and its magnetic and electromagnetic wave absorption properties. J. For. Eng. 2017, 2, 24–29. [Google Scholar]
- Chen, L.; Ma, N.; Park, Y.; Jin, S.; Hwang, H.; Jiang, D.; Jung, Y.M. Highly sensitive determination of iron(III) ion based on phenanthroline probe: Surface-enhanced Raman spectroscopy methods. Spectrochim. Acta A 2018, 197, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Kitagawa, M.; Kumada, Y.; Hashimoto, N.; Shiiba, M.; Katoh, S.; Terashima, M. Production kinetics of angiotensin-I converting enzyme inhibitory peptides from bonito meat in artificial gastric juice. Process Biochem. 2006, 41, 505–511. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, C.F.; Yue, M.B.; Zhang, C.Z.; Huang, W.; Zhu, J.H. New application of hierarchical zeolite in life science: Fast trapping nitrosamines in artificial gastric juice by alkaline-tailored HZSM-5. Mater. Lett. 2007, 61, 3154–3158. [Google Scholar] [CrossRef]
- Tang, M.; Wang, D.; Hou, Y.; Buchili, P.; Sun, L. Preparation, characterization, bioavailability in vitro and in vivo of tea polysaccharides–iron complex. Eur. Food Res. Technol. 2013, 236, 341–350. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, H.; Wang, Z.; Liu, S.; Regenstein, J.M. Response surface methodology for the synthesis of an Auricularia auriculajudae polysaccharides-CDDP complex. Int. J. Biol. Macromol. 2016, 93, 333–343. [Google Scholar] [CrossRef]
- Chaidedgumjorn, A.; Toyoda, H.; Woo, E.R.; Lee, K.B.; Kim, Y.S.; Toida, T.; Imanari, T. Effect of (1→3)-and (1→4)-linkages of fully sulfated polysaccharides on their anticoagulant activity. Carbohydr. Res. 2002, 337, 925–933. [Google Scholar] [CrossRef]
- Peng, X.B.; Li, Q.; Ou, L.N.; Jiang, L.F.; Zeng, K. GC-MS, FT-IR analysis of black fungus polysaccharides and its inhibition against skin aging in mice. Int. J. Biol. Macromol. 2010, 47, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Wang, K.P.; Chen, Z.X.; Zhang, Y.; Wang, P.P.; Wang, J.H.; Dai, L.Q. Molecular weight and proposed structure of the Angelica sinensis polysaccharide–iron complex. Chin. J. Chem. 2008, 26, 1068–1074. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, J.; Zhao, B.; Yao, J.; Wang, Y. Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int. J. Biol. Macromol. 2010, 46, 59–66. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Tian, J.; Zeng, T.; Wang, X.; Yao, J.; Lei, Z. Synthesis and characterization of phosphorylated galactomannan: The effect of DS on solution conformation and antioxidant activities. Carbohydr. Polym. 2014, 113, 325–335. [Google Scholar] [CrossRef]
- Chen, S.K.; Tsai, M.L.; Huang, J.R.; Chen, R.H. In vitro antioxidant activities of low-molecular-weight polysaccharides with various functional groups. J. Agric. Food Chem. 2009, 57, 2699–2704. [Google Scholar] [CrossRef]
- Huang, C.; Fang, L.; Lai, C.; Tang, S.; Liang, C.; Li, X.; Yong, Q. Effects of different pretreatments on antioxidant activities of Moso bamboo lignin. J. For. Eng. 2018, 3, 73–80. [Google Scholar]

| Mw (g/mol) | Mn (g/mol) | PDI 1 | Iron Content 2 (mg/g) | Galactomannan Content 2 | |
|---|---|---|---|---|---|
| GM-40 | 14,700 | 12,000 | 1.23 | None | 90.9 ± 0.3 |
| GM-65 | 4890 | 4380 | 1.12 | None | 96.5 ± 0.2 |
| GM-40-Fe(III) | 16,100 | 15,700 | 1.02 | 65.4 ± 0.1 | 74.5 ± 0.1 |
| GM-65-Fe(III) | 5320 | 5270 | 1.01 | 57.2 ± 0.1 | 80.0 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Tao, Y.; Li, M.; Zhang, W.; Fan, Y.; Yong, Q. Synthesis and Characterization of an Antioxidative Galactomannan–Iron(III) Complex from Sesbania Seed. Polymers 2019, 11, 28. https://doi.org/10.3390/polym11010028
Huang C, Tao Y, Li M, Zhang W, Fan Y, Yong Q. Synthesis and Characterization of an Antioxidative Galactomannan–Iron(III) Complex from Sesbania Seed. Polymers. 2019; 11(1):28. https://doi.org/10.3390/polym11010028
Chicago/Turabian StyleHuang, Caoxing, Yuheng Tao, Min Li, Weiyu Zhang, Yimin Fan, and Qiang Yong. 2019. "Synthesis and Characterization of an Antioxidative Galactomannan–Iron(III) Complex from Sesbania Seed" Polymers 11, no. 1: 28. https://doi.org/10.3390/polym11010028
APA StyleHuang, C., Tao, Y., Li, M., Zhang, W., Fan, Y., & Yong, Q. (2019). Synthesis and Characterization of an Antioxidative Galactomannan–Iron(III) Complex from Sesbania Seed. Polymers, 11(1), 28. https://doi.org/10.3390/polym11010028