Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
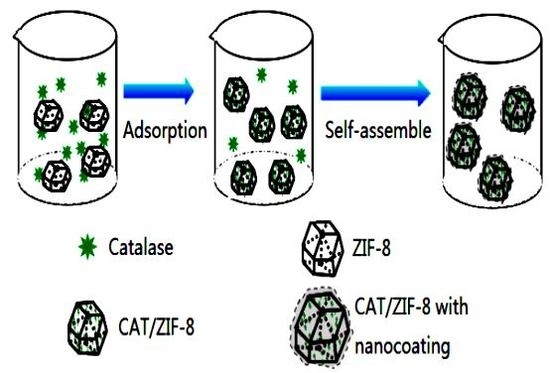
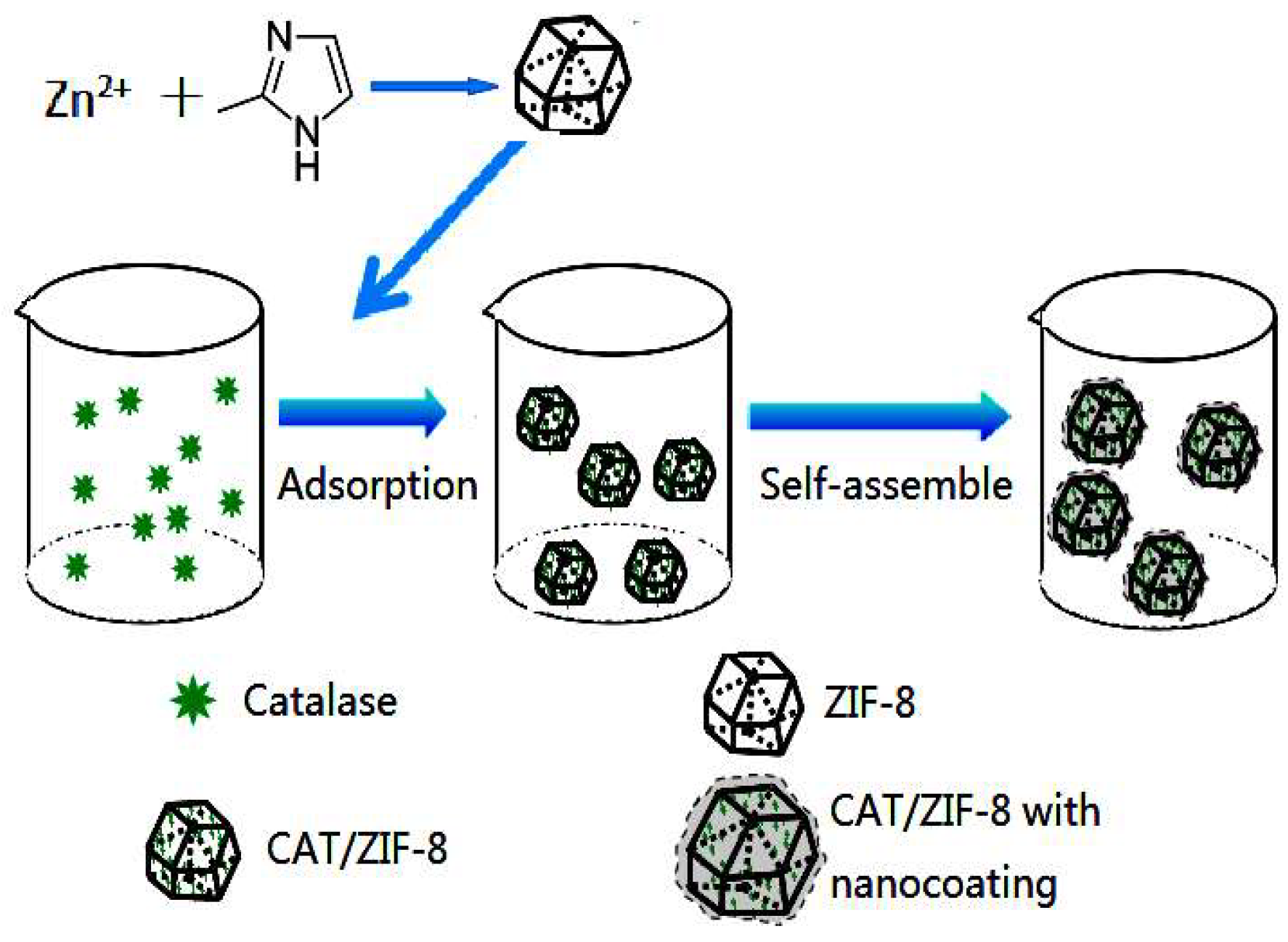
2.2. Synthesis of the ZIF-8 and Catalase/ZIF-8 Composites (CAT/ZIF-8)
2.3. Synthesis of Catalase/ZIF-8 Composites with a Protective Nanocoating
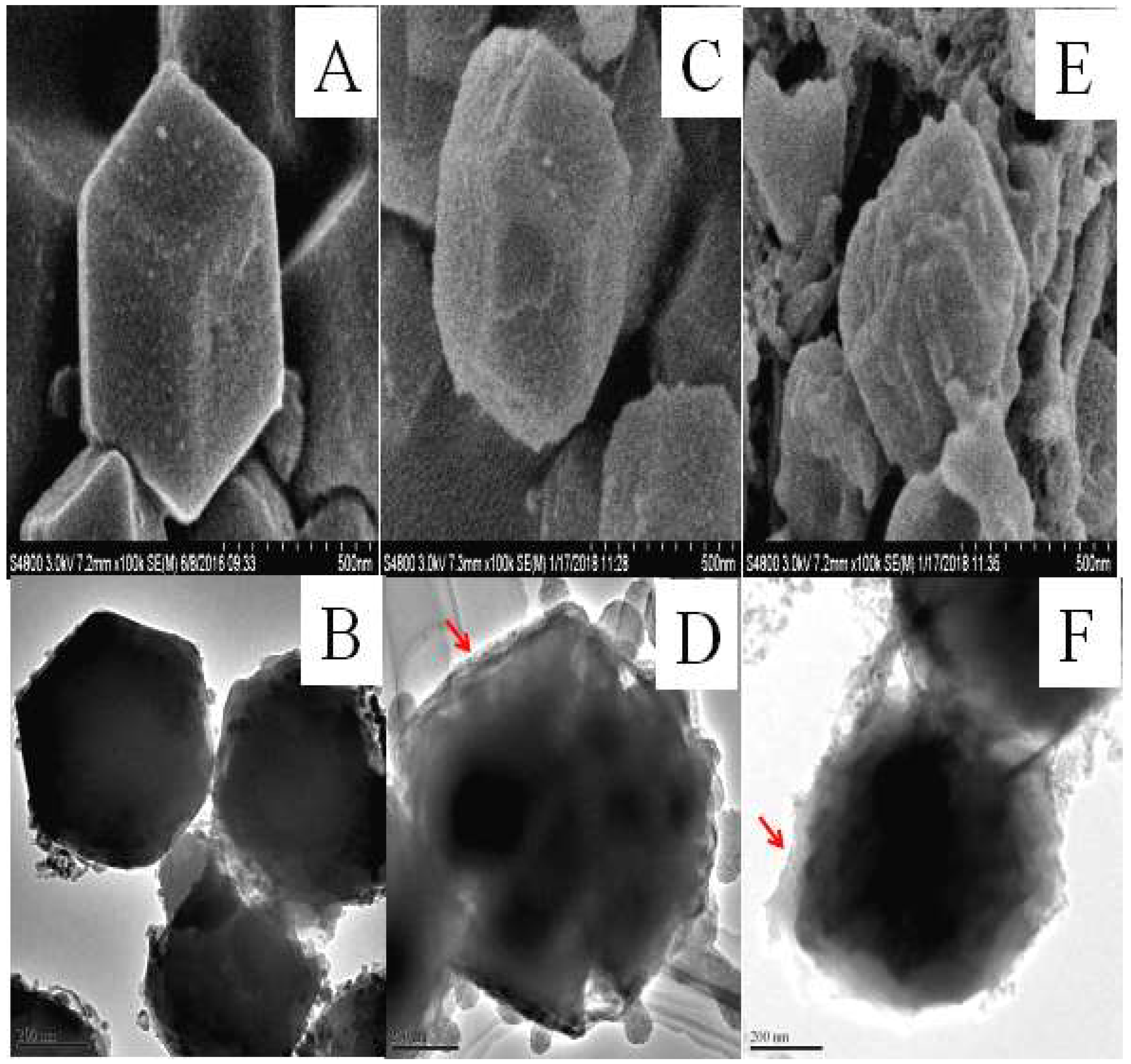
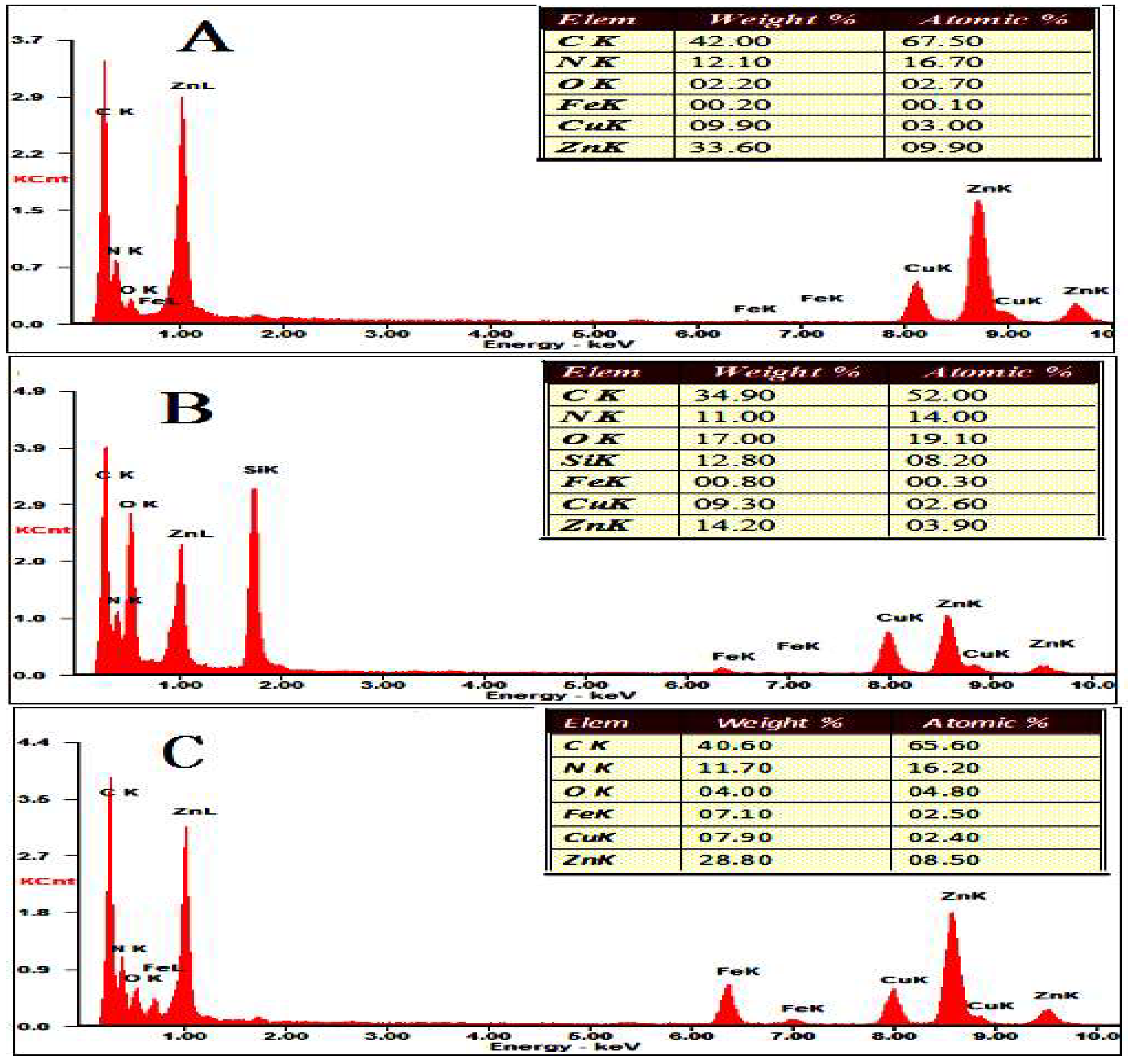
2.4. Characterization Methods
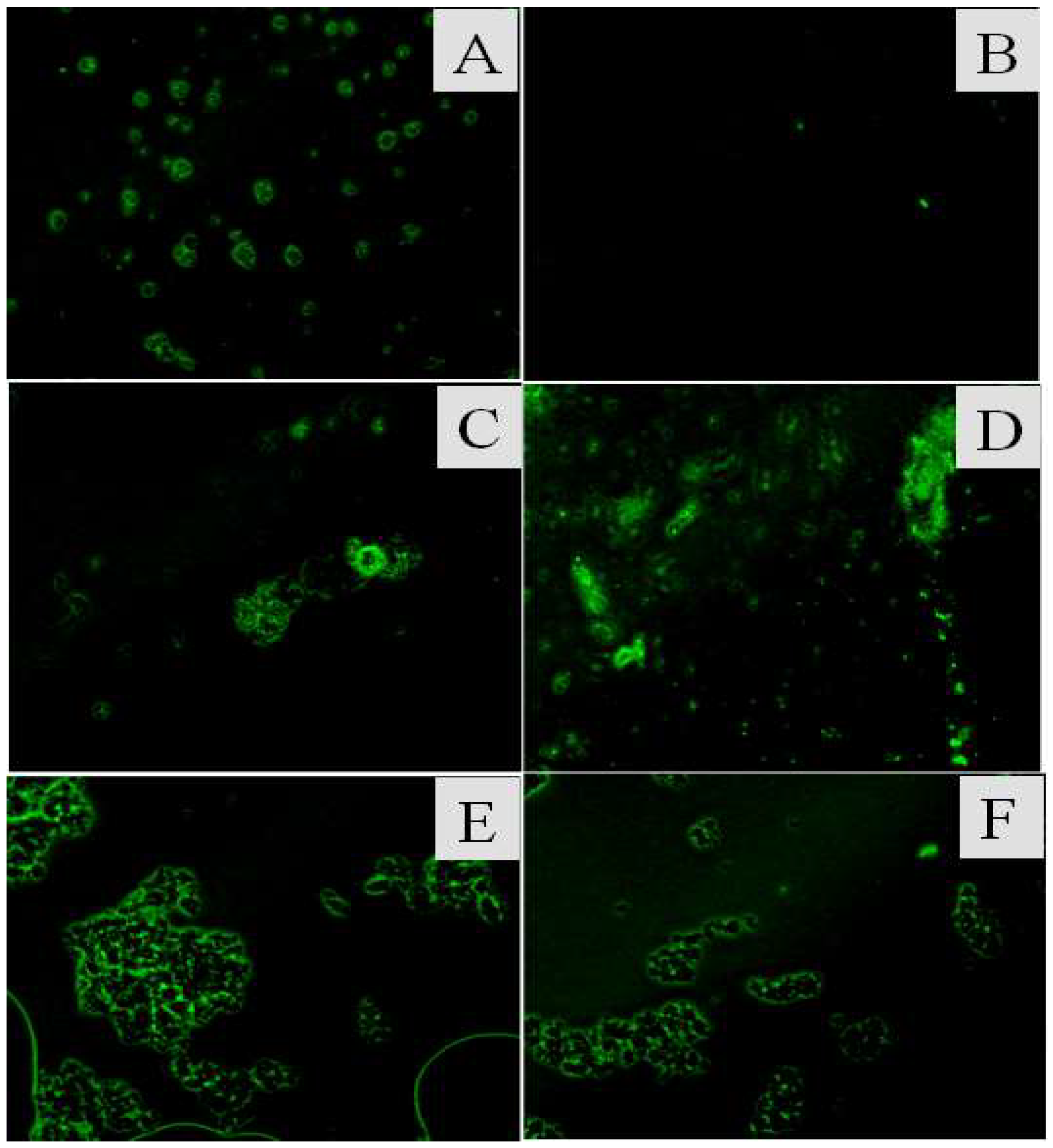
2.5. Labeled Catalase with FITC
2.6. Activity Assay
2.7. Acid Tolerance Measurement (pH 3.0) of SiO2@CAT/ZIF-8 and Fe3+-CA@CAT/ZIF-8
2.8. Stability of Free CAT, CAT/ZIF-8, and CAT/ZIF-8 with Nanocoating
3. Results and Discussion
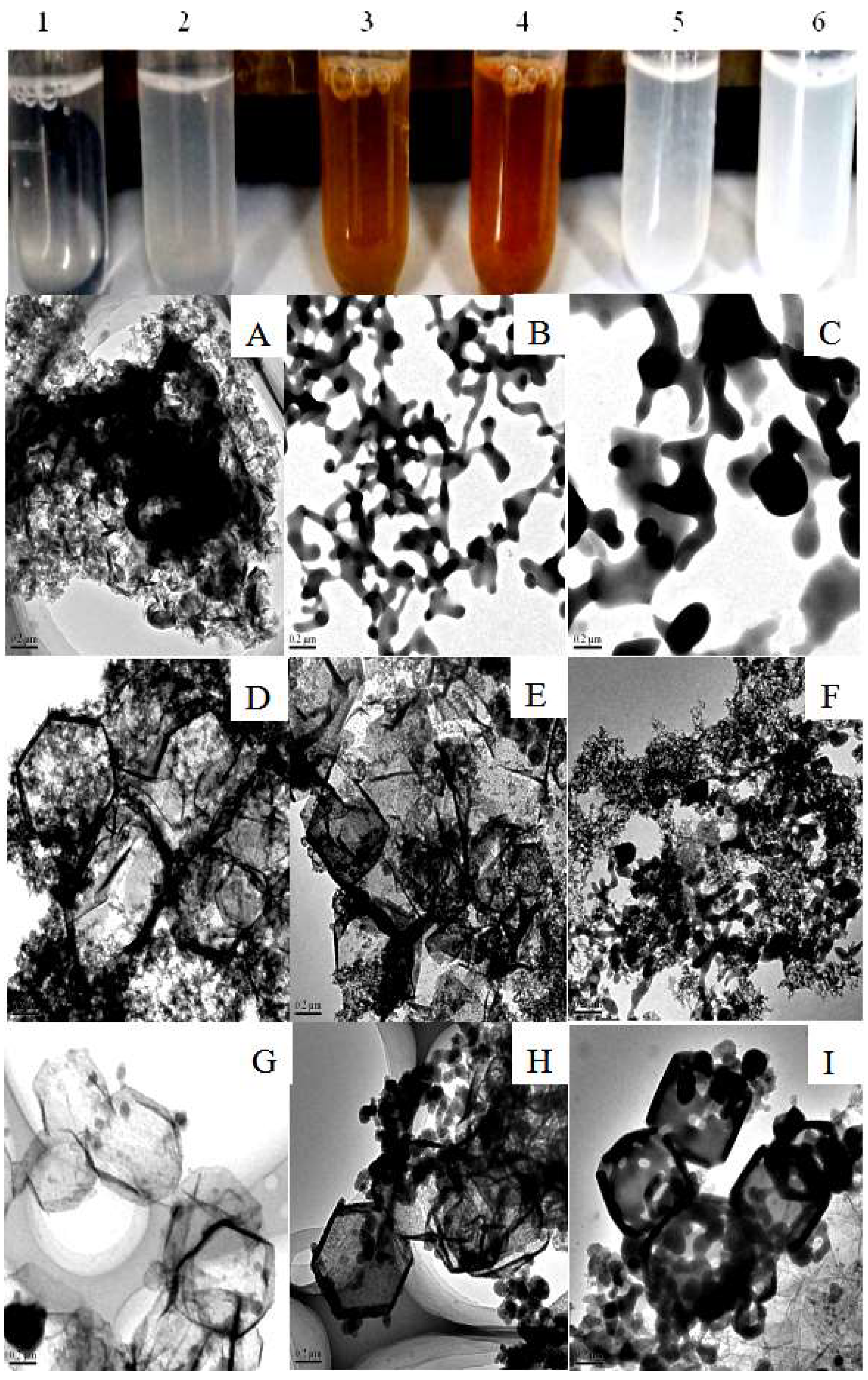
3.1. Synthesis and Characterization of the CAT@ZIF-8 Composites with a Protective Nanocoating
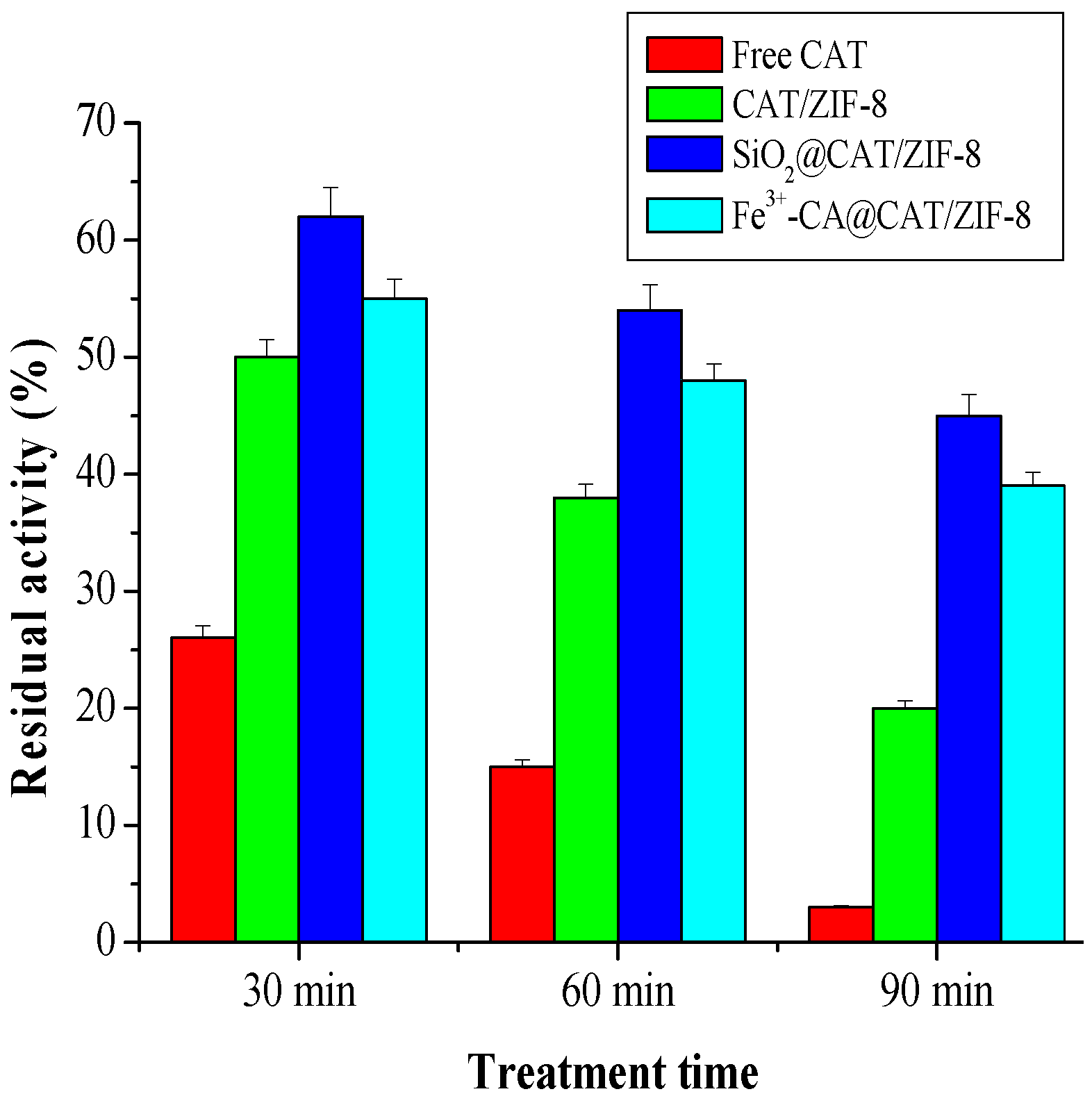
3.2. Acid Resistance Measurement of CAT/ZIF-8, SiO2@CAT/ZIF-8 and Fe3+-TA@CAT/ZIF-8
3.3. Stability of CAT/ZIF-8, Fe3+-TA@CAT/ZIF-8 and SiO2@CAT/ZIF-8
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dicosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; MSanders, J.P. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ji, P.J. Enzymes Immobilized on carbon nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torrest, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to ppportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar]
- Cao, L.Q. Immobilised enzymes: Science or art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Carcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Quilles, J.C.J.; Brito, R.R.; Borges, J.P.C.; Aragon, C.; Fernandez-Lorente, G.; Bocchini-Martins, D.A.; Gomes, E.; Da Silva, R.; Boscolo, M.; Guisan, J.M. Modulation of the activity and selectivity of the immobilized lipases by surfactants and solvents. Biochem. Eng. J. 2015, 93, 274–280. [Google Scholar] [CrossRef]
- Chen, Q.; Kenausis, G.L.; Heller, A. stability of oxidases immobilized in silica gels. J. Am. Chem. Soc. 1998, 120, 4582–4585. [Google Scholar] [CrossRef]
- Wang, Q.G.; Yang, Z.M.; Zhang, X.Q.; Xiao, X.D.; Chang, C.K.; Xu, B.A. Supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase. Angew. Chem. Int. Ed. 2007, 46, 4285–4289. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Magner, E. Proteins in mesoporous silicates. Angew. Chem. Int. Ed. Engl. 2008, 47, 8582–8594. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Encapsulation of lipase within metal-organic framework (MOF) with enhanced activity intensified under ultrasound. Enzymes Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kempahanumakkagari, S.; Kumar, V.; Samaddar, P.; Kumar, P.; Ramakrishnappa, T.; Kim, K.H. Biomolecule-embedded metal-organic frameworks as an innovative sensing platform. Biotechnol. Adv. 2018, 36, 467–481. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; OKeeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 239, 424–428. [Google Scholar] [CrossRef]
- Li, P.; Moon, S.Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal–organic framework engenders thermal and long-term stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef]
- Feng, D.W.; Liu, T.F.; Su, J.; Bosch, M.; Wei, W.; Yuan, D.Q.; Chen, Y.P.; Wang, X.K.; Wang, C.; Lian, X.Z.; et al. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef]
- Li, P.; Kelt, R.C.; Moon, S.Y.; Wang, T.C.; Deria, P.; Peters, A.W.; Kalhr, B.M.; Park, H.J.; Al-Juaid, S.S.; Hupp, J.T.; et al. Synthesis of nanocrystals of Zr-based metal–organic frameworks with Csq-Net: Significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 2015, 51, 10925–10928. [Google Scholar] [CrossRef] [PubMed]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal–organic frameworks: A novel host platform for enzymatic catalysis and detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Lian, X.Z.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.L.; Banerjee, S.; Lallar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [PubMed]
- Wu, Y.; Zhou, M.; Zhang, B.; Wu, B.; Li, J.; Qiao, J.; Guan, X.; Li, F. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112. [Google Scholar] [PubMed]
- Wu, X.L.; Yang, C.; Ge, J.; Liu, Z. Polydopamine tethered enzyme/metal–organic framework composites with high stability and reusability. Nanoscale 2015, 7, 18883–18886. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Côté, Z.N.; Choi, A.P.; Huang, J.Y.; Uribe-Romo, R.; Chae, F.J.; ÓKeeffe, H.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc. 2009, 13, 3875–3877. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, S.; He, D.; He, X.; Wang, K.; Li, L.; Yang, X.; Li, H. A versatile stimulus-responsive metal–organic framework for size/morphology tunable hollow mesoporous silica and pH-triggered drug delivery. J. Mater. Chem. B 2017, 5, 2126–2132. [Google Scholar] [CrossRef]
- Hidalgo, A.; Betancor, L.; Lopez-Gallego, F.; Moreno, R.; Berenguer, J.; Fernández-Lafuente, R.; Guisan, R.J.M. Design of an immobilized preparation of catalase from Thermus thermophilus to be used in a wide range of conditions.: Structural stabilization of a multimeric enzyme. Enzyme Microb. Technol. 2003, 33, 278–285. [Google Scholar]
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Hydrogen peroxide in biocatalysis. A dangerous liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Betancor, L.; Hidalgo, A.; Fernandez-Lorente, G.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Preparation of a stable biocatalyst of bovine liver catalase using immobilization and postimmobilization techniques. Biotechnol. Prog. 2003, 19, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Kauldhar, B.S.; Dhau, J.S.; Sooch, B.S. Covalent linkage of alkalothermophilic catalase onto functionalized cellulose. RSC Adv. 2016, 6, 39364–39375. [Google Scholar] [CrossRef]
- Li, J.; Li, L.S.; Xu, L. Hierarchically macro/mesoporous silica spheres for catalase immobilization and catalysis. Mater. Lett. 2017, 193, 67–69. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de Novo approach: Size-selective sheltering of catalase in metal-organic framework microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Feng, Y.; Lin, T.; Tan, Z.; Zhong, C.; Jia, S. Mesoporous metal–organic framework with well-defined cruciate flower-like morphology for enzyme immobilization. ACS Appl. Mater. Interfaces 2017, 9, 10587–10594. [Google Scholar] [CrossRef] [PubMed]
- Seyhan Tukel, S.; Hurrem, F.; Yildirim, D.; Alptekin, O. Preparation of crosslinked enzyme aggregates (CLEA) of catalase and its characterization. J. Mol. Catal. B Enzym. 2013, 97, 252–257. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.X.; Zhou, L.; He, Y.Y.; Ma, L.; Wang, Y.; Yin, L.Y.; Jiang, Y.J. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Kato, K.; Kawachi, Y.; Nakamura, H. Silica–enzyme–ionic liquid composites for improved enzymatic activity. J. Asian Ceram. Soc. 2014, 2, 33–40. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, S.H.; Moon, H.C.; Seo, H.L.; Kim, J.Y.; Hong, S.P.; Lee, B.S.; Kang, E.; Lee, J.H.; Ryu, D.H.; et al. Antimicrobial spray nanocoating of supramolecular Fe(III)-tannic acid metal-organic coordination complex: Applications to shoe insoles and fruits. Sci. Rep. 2017, 7, 6980. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Ren, S.Z.; Lin, T.; Feng, Y.X.; Jia, S.R. Shielding effects of Fe3+-tannic acid Films for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chem. Eng. J. 2018, 343, 629–637. [Google Scholar] [CrossRef]
- Shi, J.F.; Tian, Y.; Liu, H.; Yang, D.; Zhang, S.H.; Wu, Y.H.; Jiang, Z.Y. Shielding of enzyme by stable and protective organosilica layer on monolithic scaffolds for continuous bioconversion. Ind. Eng. Chem. Res. 2017, 56, 10615–10622. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Zhou, Z.; Hartmann, M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhong, L.; Bilal, M.; Tan, Z.; Hou, Y.; Jia, S.; Cui, J. Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation. Polymers 2019, 11, 27. https://doi.org/10.3390/polym11010027
Feng Y, Zhong L, Bilal M, Tan Z, Hou Y, Jia S, Cui J. Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation. Polymers. 2019; 11(1):27. https://doi.org/10.3390/polym11010027
Chicago/Turabian StyleFeng, Yuxiao, Le Zhong, Muhammad Bilal, Zhilei Tan, Ying Hou, Shiru Jia, and Jiandong Cui. 2019. "Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation" Polymers 11, no. 1: 27. https://doi.org/10.3390/polym11010027
APA StyleFeng, Y., Zhong, L., Bilal, M., Tan, Z., Hou, Y., Jia, S., & Cui, J. (2019). Enzymes@ZIF-8 Nanocomposites with Protection Nanocoating: Stability and Acid-Resistant Evaluation. Polymers, 11(1), 27. https://doi.org/10.3390/polym11010027