Sol–Gel-Processed Organic–Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene
Abstract
1. Introduction
2. Experimental Methods
2.1. Synthesis of the O–I Nanohybrid
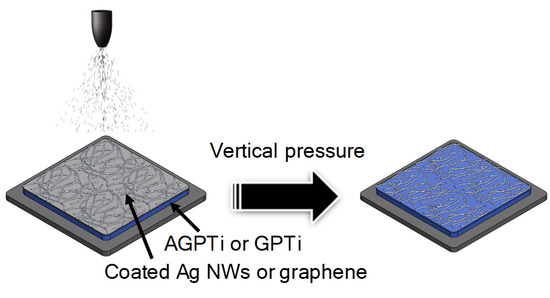
2.2. Fabrication of Conductive Films
2.3. Characterization of the Conductive Films
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gelinck, G.H.; Huitema, H.E.A.; Van Veenendaal, E.; Cantatore, E.; Schrijnemakers, L.; Van der Putten, J.B.P.H.; Geuns, T.C.T.; Beenhakkers, M.; Giesbers, J.B.; Huisman, B.H.; et al. Flexible Active-Matrix Displays and Shift Registers Based on Solution-Processed Organic Transistors. Nat. Mater. 2004, 3, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Lee, C.; Suh, K.Y. Recent Advances in Flexible Sensors for Wearable and Implantable Devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwodiauer, R. 25th Anniversary Article: A Soft Future: From Robots and Sensor Skin to Energy Harvesters. Adv. Mater. 2014, 26, 149–162. [Google Scholar] [CrossRef]
- Beeby, S.P.; Torah, R.N.; Tudor, M.J.; Glynne-Jones, P.; O’Donnell, T.; Saha, C.R.; Roy, S. A Micro Electromagnetic Generator for Vibration Energy Harvesting. J. Micromech. Microeng. 2007, 17, 1257–1265. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, C.K.; Hwang, G.T.; Lee, K.J. Self-Powered Flexible Inorganic Electronic System. Nano Energy 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, W.Z. Nanotechnology-Enabled Energy Harvesting for Self-Powered Micro-/Nanosystems. Angew. Chem. Int. Edit. 2012, 51, 11700–11721. [Google Scholar] [CrossRef]
- Qi, Y.; Kim, J.; Nguyen, T.D.; Lisko, B.; Purohit, P.K.; McAlpine, M.C. Enhanced Piezoelectricity and Stretchability in Energy Harvesting Devices Fabricated from Buckled PZT Ribbons. Nano Lett. 2011, 11, 1331–1336. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lorincz, K.; Malan, D.J.; Fulford-Jones, T.R.F.; Nawoj, A.; Clavel, A.; Shnayder, V.; Mainland, G.; Welsh, M.; Moulton, S. Sensor Networks for Emergency Response: Challenges and Opportunities. IEEE Pervas. Comput. 2004, 3, 16–23. [Google Scholar] [CrossRef]
- Tate, J.; Rogers, J.A.; Jones, C.D.W.; Vyas, B.; Murphy, D.W.; Li, W.J.; Bao, Z.A.; Slusher, R.E.; Dodabalapur, A.; Katz, H.E. Anodization and Microcontact Printing on Electroless Silver: Solution-Based Fabrication Procedures for Low-Voltage Electronic Systems with Organic Active Components. Langmuir 2000, 16, 6054–6060. [Google Scholar] [CrossRef]
- Li, Y.N.; Wu, Y.L.; Ong, B.S. Facile Synthesis of Silver Nanoparticles Useful for Fabrication of High-Conductivity Elements for Printed Electronics. J. Am. Chem. Soc. 2005, 127, 3266–3267. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B.; Prabhumirashi, P.L.; Puntambekar, K.; Geier, M.L.; Hersam, M.C. Inkjet Printing of High Conductivity, Flexible Graphene Patterns. J. Phys. Chem. Lett. 2013, 4, 1347–1351. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet Printing as a Deposition and Patterning Tool for Polymers and Inorganic Particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Tan, H.S.; Guo, Y.L.; Di, C.A.; Yu, G.; Liu, Y.Q.; Lin, M.; Lim, S.H.; Zhou, Y.H.; et al. A Stable Solution-Processed Polymer Semiconductor with Record High-Mobility for Printed Transistors. Sci. Rep. 2012, 2, 754. [Google Scholar] [CrossRef]
- Lau, P.H.; Takei, K.; Wang, C.; Ju, Y.; Kim, J.; Yu, Z.B.; Takahashi, T.; Cho, G.; Javey, A. Fully Printed, High Performance Carbon Nanotube Thin-Film Transistors on Flexible Substrates. Nano Lett. 2013, 13, 3864–3869. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.S.; Bae, S.H.; Chen, H.J.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices Toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Huang, D.; Liao, F.; Molesa, S.; Redinger, D.; Subramanian, V. Plastic-Compatible Low Resistance Printable Gold Nanoparticle Conductors for Flexible Electronics. J. Electrochem. Soc. 2003, 150, G412–G417. [Google Scholar] [CrossRef]
- Logothetidis, S. Flexible Organic Electronic Devices: Materials, Process and Applications. Mater. Sci. Eng. B-Adv. 2008, 152, 96–104. [Google Scholar] [CrossRef]
- Zarek, M.; Layani, M.; Cooperstein, I.; Sachyani, E.; Cohn, D.; Magdassi, S. 3D Printing of Shape Memory Polymers for Flexible Electronic Devices. Adv. Mater. 2016, 28, 4449–4454. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, G.B.; Loo, Y.L.; Rogers, J.A.; Gao, F.; Fincher, C.R. Large Area, High Resolution, Dry Printing of Conducting Polymers for Organic Electronics. Appl. Phys. Lett. 2003, 82, 463–465. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, S.S.; Jo, J.; Kim, D.Y. Efficient and Flexible ITO-Free Organic Solar Cells Using Highly Conductive Polymer Anodes. Adv. Mater. 2008, 20, 4061–4067. [Google Scholar] [CrossRef]
- Ito, S.; Ha, N.L.C.; Rothenberger, G.; Liska, P.; Comte, P.; Zakeeruddin, S.M.; Pechy, P.; Nazeeruddin, M.K.; Gratzel, M. High-Efficiency (7.2%) Flexible Dye-Sensitized Solar Cells with Ti-Metal Substrate for Nanocrystalline-TiO2 Photoanode. Chem. Commun. 2006, 38, 4004–4006. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Choi, K.H.; Kim, H.K. Room Temperature Flexible and Transparent ITO/Ag/ITO Electrode Grown on Flexile PES Substrate by Continuous Roll-To-Roll Sputtering for Flexible Organic Photovoltaics. J. Phys. D. Appl. Phys. 2009, 42, 235109. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, J.Y.; Yen, W.T. Low Temperature ITO Thin Film Deposition on PES Substrate Using Pulse Magnetron Sputtering. Appl. Surf. Sci. 2008, 254, 3262–3268. [Google Scholar] [CrossRef]
- Huang, L.M.; Chen, C.H.; Wen, T.C. Development and Characterization of Flexible Electrochromic Devices Based on Polyaniline and Poly(3,4-Ethylenedioxythiophene)-Poly(Styrene Sulfonic Acid). Electrochim. Acta 2006, 51, 5858–5863. [Google Scholar] [CrossRef]
- Hwang, B.; Li, X.; Kim, S.H.; Lim, S. Effect of Carbon Nanotube Addition on Mechanical Reliability of Ag Nanowire Network. Mater. Lett. 2017, 198, 202–205. [Google Scholar] [CrossRef]
- Li, X.; Lee, G.S.; Park, S.H.; Kong, H.; An, T.K.; Kim, S.H. Direct Writing of Silver Nanowire Electrodes Via Dragging Mode Electrohydrodynamic Jet Printing for Organic Thin Film Transistors. Org. Electron. 2018, 62, 357–365. [Google Scholar] [CrossRef]
- Lee, H.-J.; Song, Y.-S.; An, T.K.; Choi, W.-K.; Kim, S.-R. Ultrasmooth transparent conductive hybrid films of reduced graphene oxide and single-walled carbon nanotube by ultrasonic spraying. Synth. Met. 2016, 221, 340–344. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.Q.; Woodard, T.L.; Zhang, Y.B.; Sun, D.Z.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing Anaerobic Digestion of Complex Organic Waste with Carbon-Based Conductive Materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar]
- Deng, B.; Hsu, P.C.; Chen, G.C.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.X.; Guo, Y.F.; Lin, L.; Zhou, Y.; et al. Roll-to-Roll Encapsulation of Metal Nanowires Between Graphene and Plastic Substrate for High-Performance Flexible Transparent Electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Kim, K.W.; Joo, S.W.; Seo, J.; Lee, J.; An, T.K.; Kim, S.H. Facile Method for Enhancing Conductivity of Printed Carbon Nanotubes Electrode via Simple Rinsing Process. Org. Electron. 2017, 47, 174–180. [Google Scholar] [CrossRef]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the Long-Term Stability of Perovskite Solar Cells with a Porous Al2O3 Buffer Layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.W.; Wang, J.; Pan, Z.X.; Zhao, K.; Zhang, H.; Li, Y.; Zhao, Y.X.; Mora-Sero, I.; Bisquert, J.; Zhong, X.H. Amorphous TiO2 Buffer Layer Boosts Efficiency of Quantum Dot Sensitized Solar Cells to over 9%. Chem. Mater. 2015, 27, 8398–8405. [Google Scholar] [CrossRef]
- Kavan, L.; Steier, L.; Gratzel, M. Ultrathin Buffer Layers of SnO2 by Atomic Layer Deposition: Perfect Blocking Function and Thermal Stability. J. Phys. Chem. C 2017, 121, 342–350. [Google Scholar] [CrossRef]
- Zhan, C.X.; Yu, G.Q.; Lu, Y.; Wang, L.Y.; Wujcik, E.; Wei, S.Y. Conductive Polymer Nanocomposites: A Critical Review of Modern Advanced Devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Manthiram, A.; Li, L.J. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy. Mater. 2015, 5, 1401302. [Google Scholar] [CrossRef]
- Ababtain, K.; Babu, G.; Susarla, S.; Gullapalli, H.; Masurkar, N.; Ajayan, P.M.; Arava, L.M.R. Porous Graphene Current Collectors Filled with Silicon as High-Performance Lithium Battery Anode. Mater. Res. Express 2018, 5, 014004. [Google Scholar] [CrossRef]
- Kim, N.; Kim, S.H.; Kim, J. Colloidally Stable Organic- Inorganic Hybrid Nanoparticles Prepared Using Alkoxysilane-Functionalized Amphiphilic Polymer Precursors and Mechanical Properties of Their Cured Coating Film. J. Ind. Eng. Chem. 2018, 68, 209–219. [Google Scholar] [CrossRef]
- Kim, J.; Wainaina, J.; Na, J.S. Synthesis of Amphiphilic Silica/Polymer Composite Nanoparticles as Water-Dispersible Nano-Absorbent for Hydrophobic Pollutants. J. Ind. Eng. Chem. 2011, 17, 681–690. [Google Scholar] [CrossRef]
- Sun, Y.G.; Yin, Y.D.; Mayers, B.T.; Herricks, T.; Xia, Y.N. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, Z.G.; Liu, W.M. Biomimetic Transparent and Superhydrophobic Coatings: From Nature and Beyond Nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Ji, Q.M.; Kamachi, Y.; Mori, T.; Shieh, F.K.; Wu, K.C.W.; Ariga, K.; Yamauchi, Y. Templated Synthesis for Nanoarchitectured Porous Materials. B. Chem. Soc. Jpn. 2015, 88, 1171–1200. [Google Scholar] [CrossRef]
- Geng, H.Z.; Kim, K.K.; So, K.P.; Lee, Y.S.; Chang, Y.; Lee, Y.H. Effect of Acid Treatment on Carbon Nanotube-Based Flexible Transparent Conducting Films. J. Am. Chem. Soc. 2007, 129, 7758. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Shin, E.W.; Kim, J.S.; Chung, J.S.; Kim, E.J. Fast and Simple Fabrication of a Large Transparent Chemically-Converted Graphene Film by Spray-Coating. Carbon 2010, 48, 1945–1951. [Google Scholar] [CrossRef]
- Madaria, A.R.; Kumar, A.; Ishikawa, F.N.; Zhou, C.W. Uniform, Highly Conductive, and Patterned Transparent Films of a Percolating Silver Nanowire Network on Rigid and Flexible Substrates using a Dry Transfer Technique. Nano Res. 2010, 3, 564–573. [Google Scholar] [CrossRef]
- Gaskell, P.E.; Skulason, H.S.; Rodenchuk, C.; Szkopek, T. Counting Graphene Layers on Glass via Optical Reflection Microscopy. Appl. Phys. Lett. 2009, 94, 143101. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.; Kim, H. Smooth-Surface Silver Nanowire Electrode with High Conductivity and Transparency on Functional Layer Coated Flexible Film. Thin Solid Films 2015, 589, 403–407. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical Conductivity of Compacts of Graphene, Multi-Wall Carbon Nanotubes, Carbon Black, and Graphite Powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Gaynor, W.; Burkhard, G.F.; McGehee, M.D.; Peumans, P. Smooth Nanowire/Polymer Composite Transparent Electrodes. Adv. Mater. 2011, 23, 2905–2910. [Google Scholar] [CrossRef]
- Shin, K.H.; Jang, Y.; Kim, B.S.; Jang, J.; Kim, S.H. Highly Conductive Reduced Graphene Oxide Produced via Pressure-Assisted Reduction at Mild Temperature for Flexible and Transparent Electrodes. Chem. Commun. 2013, 49, 4887–4889. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B.; Lim, S.; Zhang, C.D.; Frisbie, H.; Francis, L.F.; Hersam, M.C. Gravure Printing of Graphene for Large-area Flexible Electronics. Adv. Mater. 2014, 26, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Gunes, F.; Shin, H.J.; Biswas, C.; Han, G.H.; Kim, E.S.; Chae, S.J.; Choi, J.Y.; Lee, Y.H. Layer-by-Layer Doping of Few-Layer Graphene Film. ACS Nano 2010, 4, 4595–4600. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Kim, N.; Youn, S.; An, T.K.; Kim, J.; Lim, S.; Kim, S.H. Sol–Gel-Processed Organic–Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene. Polymers 2019, 11, 158. https://doi.org/10.3390/polym11010158
Li X, Kim N, Youn S, An TK, Kim J, Lim S, Kim SH. Sol–Gel-Processed Organic–Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene. Polymers. 2019; 11(1):158. https://doi.org/10.3390/polym11010158
Chicago/Turabian StyleLi, Xinlin, Nahae Kim, Seongwook Youn, Tae Kyu An, Juyoung Kim, Sooman Lim, and Se Hyun Kim. 2019. "Sol–Gel-Processed Organic–Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene" Polymers 11, no. 1: 158. https://doi.org/10.3390/polym11010158
APA StyleLi, X., Kim, N., Youn, S., An, T. K., Kim, J., Lim, S., & Kim, S. H. (2019). Sol–Gel-Processed Organic–Inorganic Hybrid for Flexible Conductive Substrates Based on Gravure-Printed Silver Nanowires and Graphene. Polymers, 11(1), 158. https://doi.org/10.3390/polym11010158