Synthesis of Propylene-co-Styrenic Monomer Copolymers via Arylation of Chlorinated PP and Their Compatibilization for PP/PS Blend
Abstract
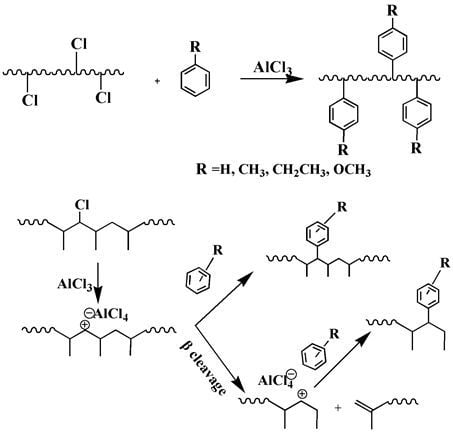
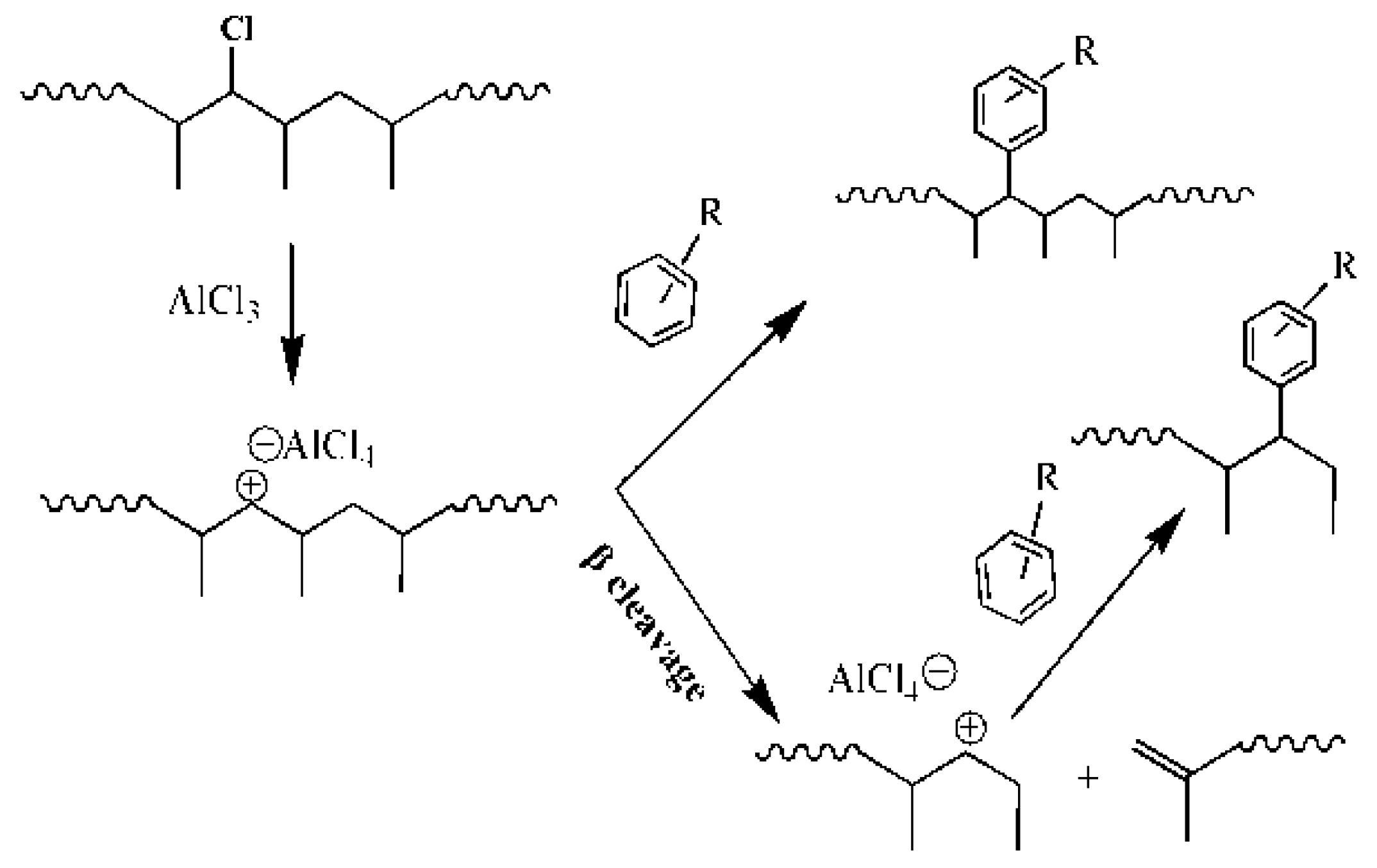
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Propylene-co-Styrenic Monomer Copolymers
2.3. Preparation of Blends
2.4. Instrumentation
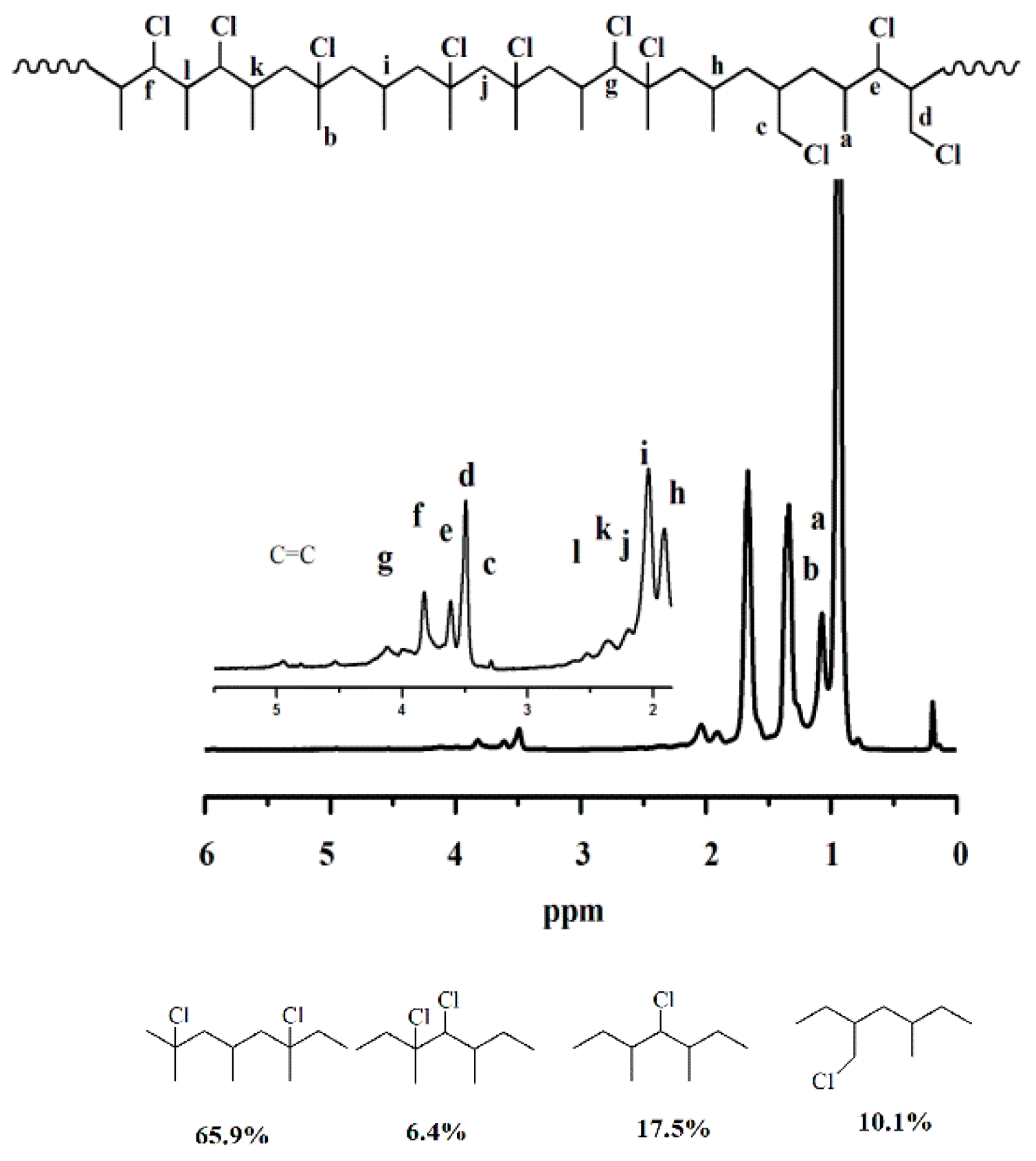
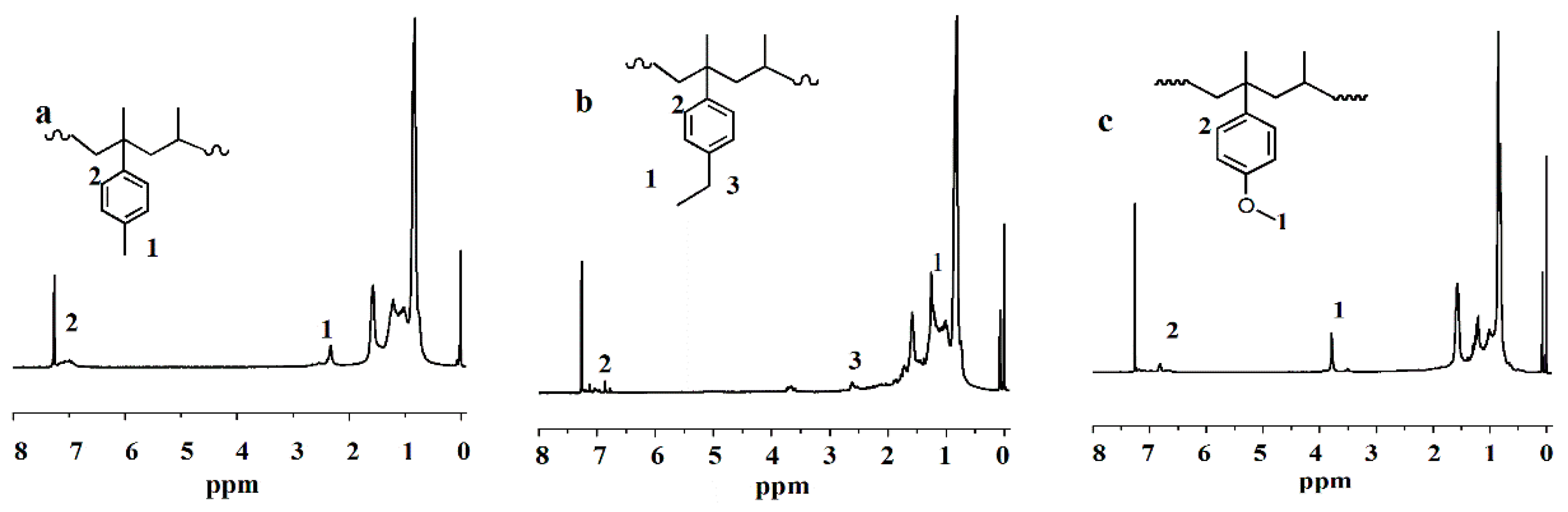
2.4.1. Nuclear Magnetic Resonance
2.4.2. Impact Test
2.4.3. Morphology Observation
2.4.4. Dynamic Mechanical Analysis
2.4.5. Thermoanalysis
2.4.6. Rheology Measurement
3. Results
3.1. Nuclear Magnetic Resonance
3.2. Mechanical Properties
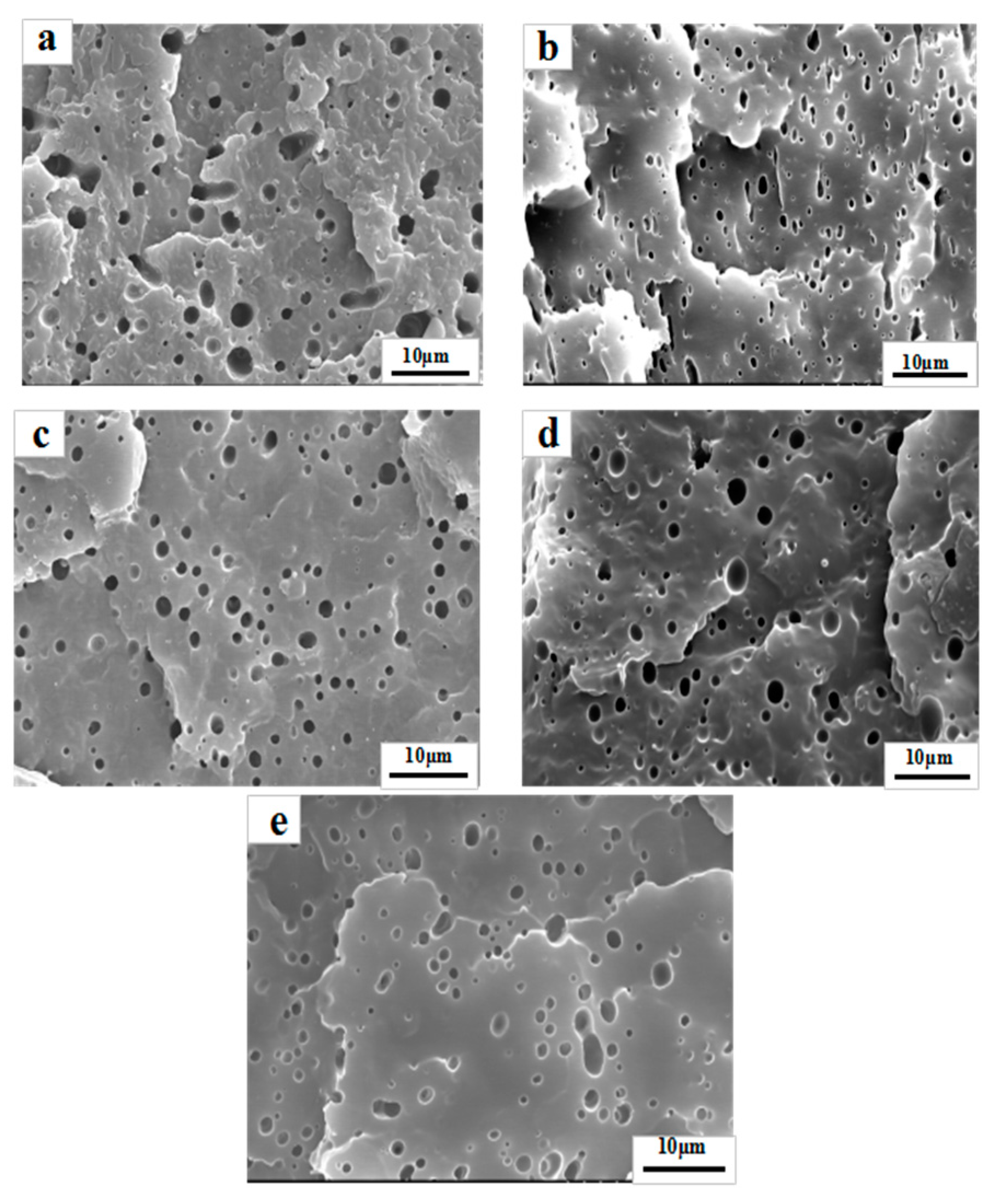
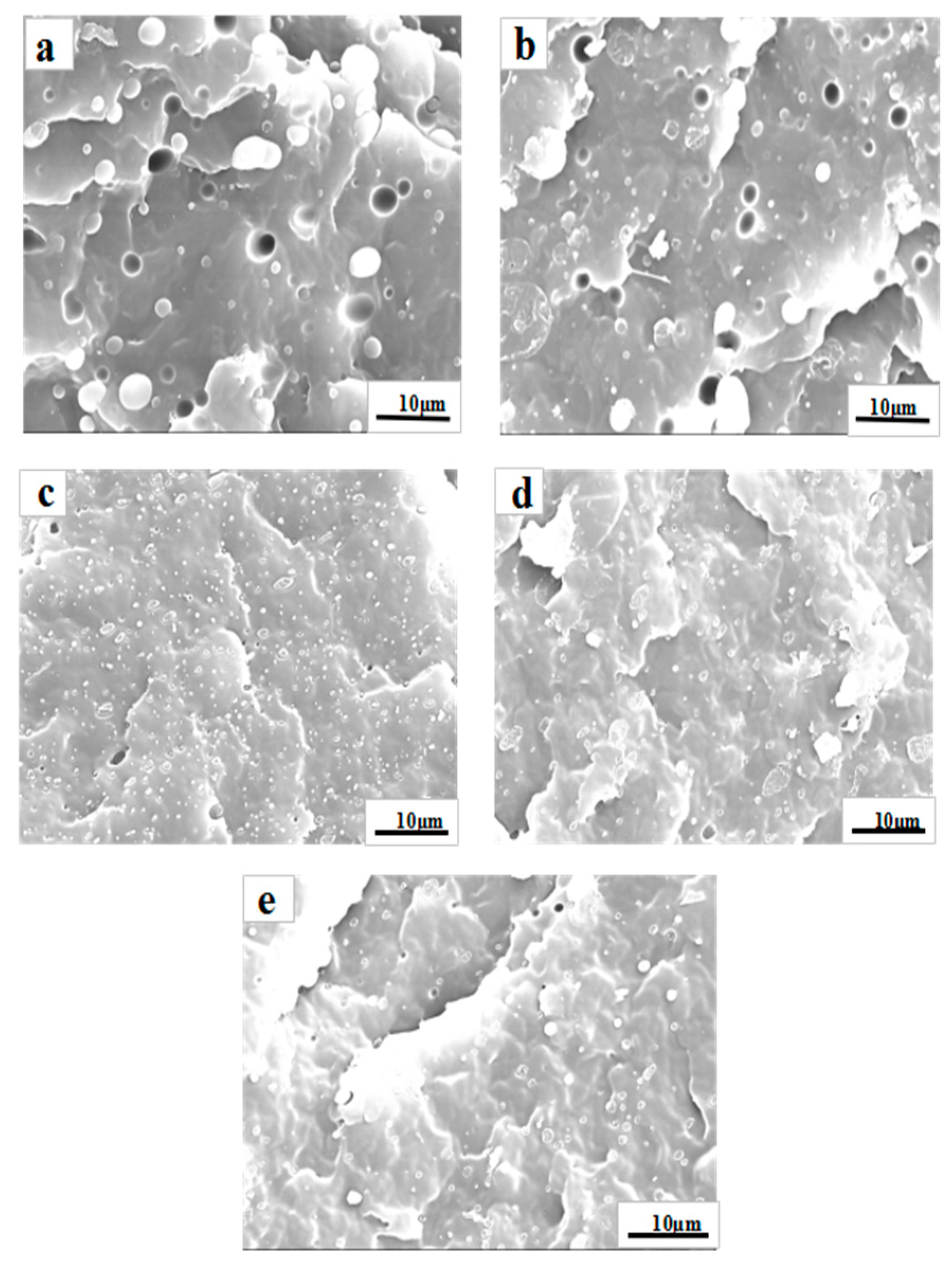
3.3. Morphology Observation
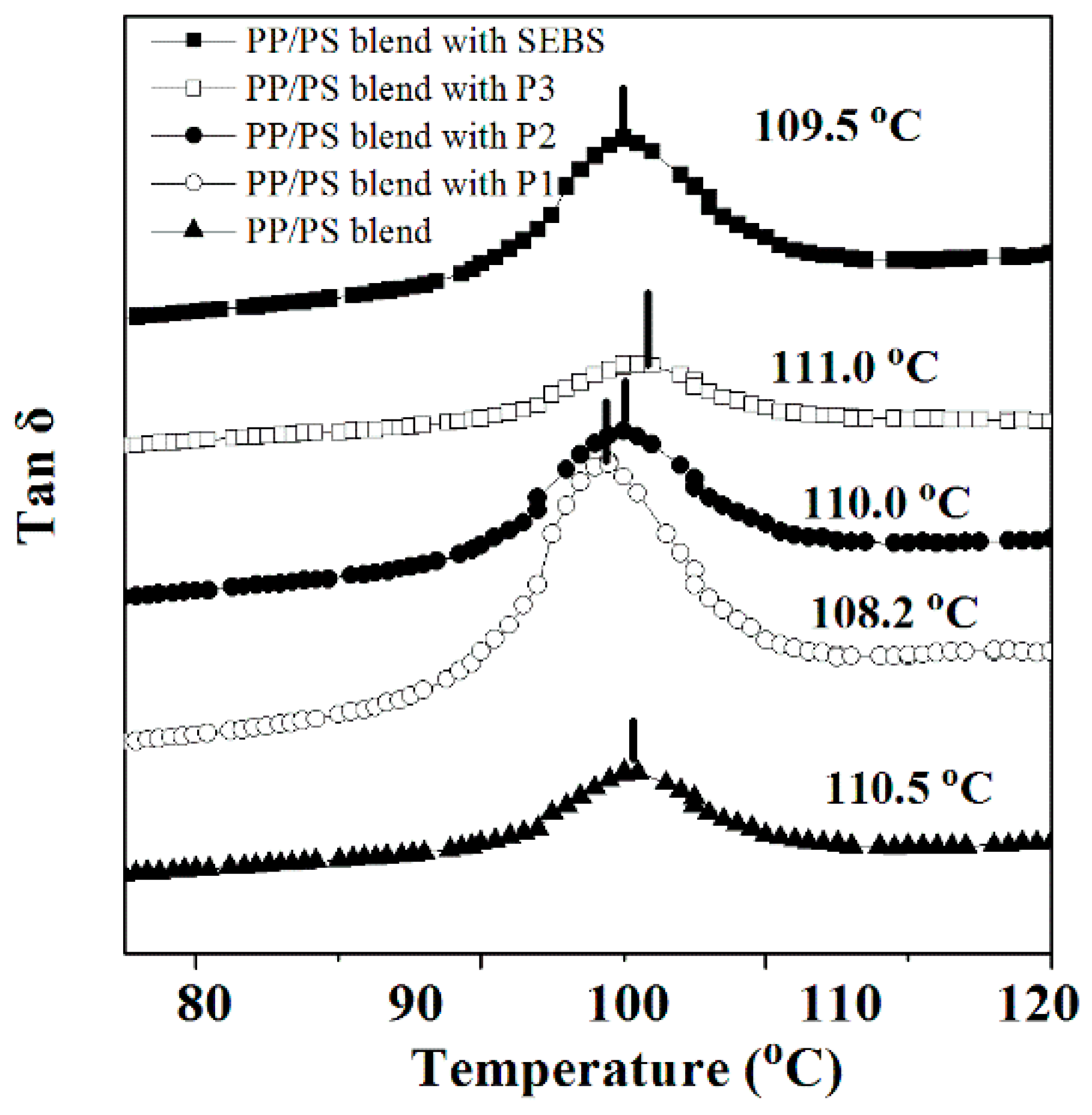
3.4. Dynamic Mechanical Analysis
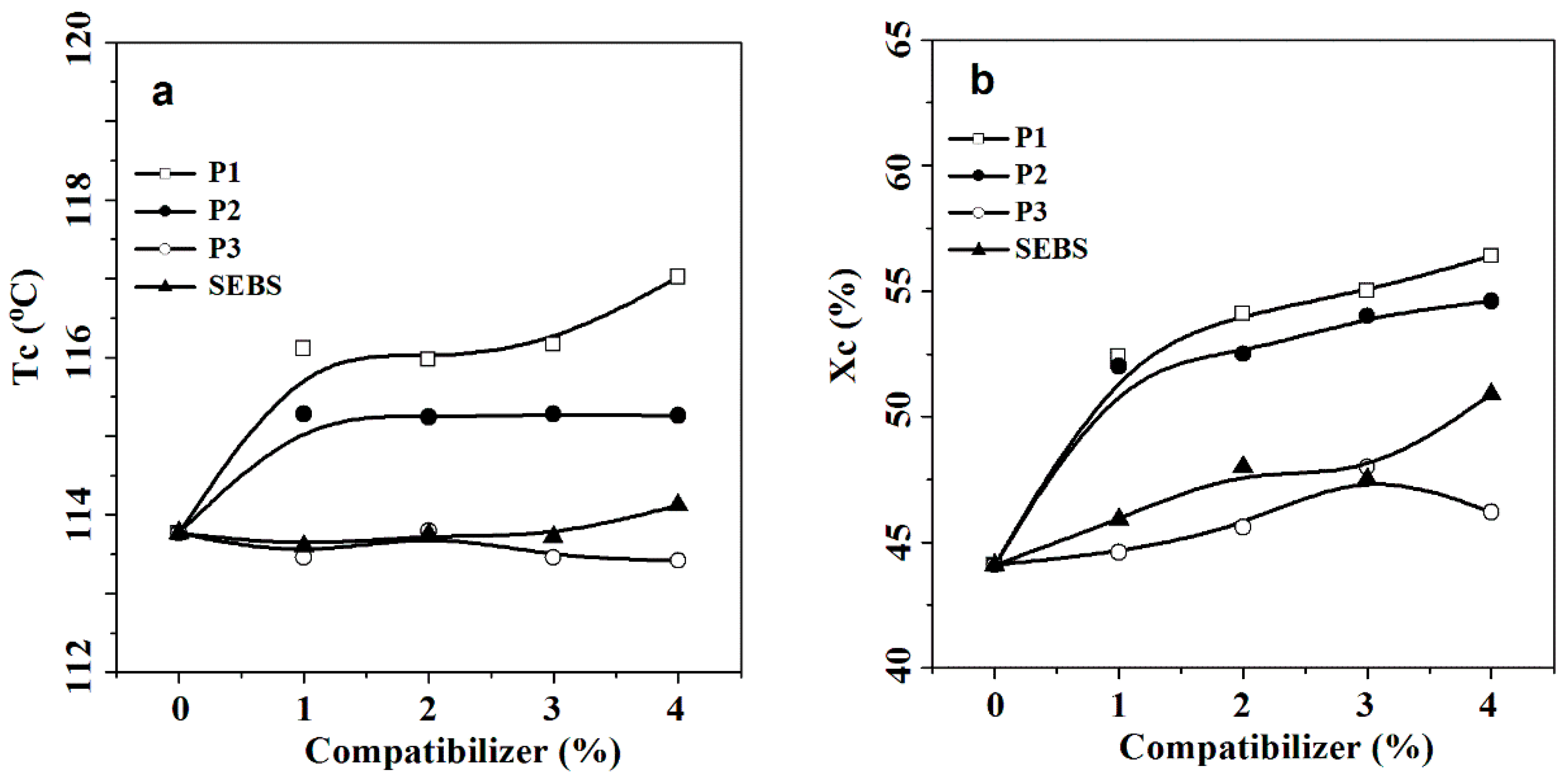
3.5. Thermoanalysis
3.6. Rheology Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sclavons, M.; Franquinet, P.; Carlier, V.; Verfaillie, G.; Fallais, I. Quantification of the maleic anhydride grafted onto polypropylene by chemical and viscosimetric titrations, and FTIR spectroscopy. Polymer 2000, 41, 1989–1999. [Google Scholar] [CrossRef]
- Li, J.G.; Li, H.Y.; Wu, C.H.; Ke, Y.C.; Wang, D.J.; Li, Q.; Zhang, L.Y.; Hu, Y.L. Morphologies, crystallinity and dynamic mechanical characterizations of polypropylene/polystyrene blends compatibilized with PP-g-PS copolymer: Effect of the side chain length. Eur. Polym. J. 2009, 45, 2619–2628. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Dong, B.; Zhang, H.X.; Hu, Y.M.; Zhang, X.Q. Morphology and properties of PP in-reactor alloys prepared with a MgCl2/TiCl4/diisobutyl phthalate/phosphate tris-methylphenyl ester catalyst system. Chem. Res. Chin. Univ. 2018, 34, 145–150. [Google Scholar] [CrossRef]
- Contreras-López, D.; Saldívar-Guerra, E.; Luna-Bárcenas, G. Copolymerization of isoprene with polar vinyl monomers: Reactivity ratios, characterization and thermal properties. Eur. Polym. J. 2013, 49, 1760–1772. [Google Scholar] [CrossRef]
- Kiryukhin, D.P.; Barkalov, I.M. Radiation polymerization of alkyl methacrylates in polymer–monomer compositions. Polym. Adv. Technol. 2015, 7, 287–294. [Google Scholar] [CrossRef]
- Krentsel, B.A.; Semenido, G.Y.; Il’Ina, D.Y. Degradation of chlorine-containing polymers—II. mechanism of dehydrochlorination of chlorinated propylene. Polym. Sci. USSR 1963, 4, 1239–1244. [Google Scholar] [CrossRef]
- Sun, Y.J.; Willemse, R.J.G.; Liu, T.M.; Baker, W.E. In situ, compatibilization of polyolefin and polystyrene using Friedel–Crafts alkylation through reactive extrusion. Polymer 1998, 39, 2201–2208. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.Q.; Feng, Z.L.; Huang, B.T. Effect of ethylene-propylene copolymer with residual PE crystallinity on mechanical-properties and morphology of PP/HDPE blends. J. Appl. Polym. Sci. 2010, 58, 551–557. [Google Scholar] [CrossRef]
- Halimatudahliana, H.I.; Nasir, M. The effect of various compatibilizers on mechanical properties of polystyrene/polypropylene blend. Polym. Test. 2002, 21, 163–170. [Google Scholar] [CrossRef]
- Caporaso, L.; Iudici, N.; Oliva, L. Synthesis of well-defined polypropylene-graft-polystyrene and relationship between structure and the ability to compatibilize the polymeric blends. Macromolecules 2005, 38, 4894–4900. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Dubey, V.K.; Sisanth, K.S.; Jose, S.; Zachariah, A.K. Tailoring of interface of polypropylene/polystyrene/carbon nanofibre composites by polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene. Polym. Test. 2016, 51, 131–141. [Google Scholar] [CrossRef]
- Mitani, K.; Ogata, T.; Iwasaki, M. Chlorine distribution and structure-property relationship of chlorinated polypropylene. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 1653–1669. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Wu, J.R.; Li, L.; Chen, X.H.; Lai, G.Q.; Jiang, J.X.; Liu, Y.X.; Xu, L.W. Efficient Lewis acid-assisted Bronsted acid (LBA) catalysis in the iron-catalyzed Friedel-Crafts alkylation reaction of indoles. Cent. Eur. J. Chem. 2010, 8, 669–673. [Google Scholar] [CrossRef]
- Touhtouh, S.; Becquart, F.; Pillon, C.; Mohamed, T. Effect of Compatibilization on Poly-ε-Caprolactone Grafting onto Poly(ethylene-co-vinyl alcohol). Polymers 2011, 3, 1734–1749. [Google Scholar] [CrossRef]
- Jun, S.; Ryoo, R. Aluminum Impregnation into Mesoporous Silica Molecular Sieves for Catalytic Application to Friedel–Crafts Alkylation. J. Catal. 2000, 195, 237–243. [Google Scholar] [CrossRef]
- Xu, G.X.; Lin, S.G. Diblock copolymer compatibilizers for blends of isotactic polystyrene and isotactic polypropylene. Polymer 1996, 37, 421–427. [Google Scholar] [CrossRef]
- Dong, L.L.; Peng, H.L.; Wang, S.Q.; Zhang, Z.; Li, J.H.; Ai, F.R.; Zhao, Q.; Luo, M.; Xiong, H.; Chen, L.X. Thermally and magnetically dual-responsive mesoporous silica nanospheres: Preparation, characterization, and properties for the controlled release of sophoridine. J. Appl. Polym. Sci. 2014, 131, 178–184. [Google Scholar] [CrossRef]
- Chiu, W.Y.; Fang, S.J. Mechanical properties and morphology of crosslinked PP/PE blends and PP/PE/propylene–ethylene copolymer blends. J. Appl. Polym. Sci. 2010, 30, 1473–1489. [Google Scholar] [CrossRef]
- Tang, W.H.; Hu, X.Y.; Tang, J.; Jin, R.G. Toughening and compatibilization of polyphenylene sulfide/nylon 66 blends with SEBS and maleic anhydride grafted SEBS triblock copolymers. J. Appl. Polym. Sci. 2007, 106, 2648–2655. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, H.L.; Yao, Z.H.; Shi, D.A.; Ke, Z.; Yin, J.H. Morphology, structure, and properties of in situ compatibilized linear low-density polyethylene/polystyrene and linear low-density polyethylene/high-impact polystyrene blends. J. Polym. Sci. Part B Polym. Phys. 2010, 41, 1837–1849. [Google Scholar] [CrossRef]
- Stehling, F.C.; Huff, T.; Speed, C.S.; Wissler, G. Structure and properties of rubber-modified polypropylene impact blends. J. Appl. Polym. Sci. 2010, 26, 2693–2711. [Google Scholar] [CrossRef]
- Sheu, H.R.; El-Aasser, M.S.; Vanderhoff, J.W. Phase Separation in Polystyrene Latex Interpenetrating Polymer Networks. J. Polym. Sci. Part A Polym. Chem. 2010, 28, 629–651. [Google Scholar] [CrossRef]
- Chen, B.; Tao, T.; Li, X.L.; Xu, S.Q.; Zhang, X.Q. Morphology, Tensile Strength and Thermal Behavior of Isotactic Polypropylene/Syndiotactic Polystyrene Blends Compatibilized by SEBS Copolymers. Polym. J. 2004, 36, 284–293. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kikuchi, T.; Songeun, M.; Shimomura, T.; Ogino, K. Synthesis of Diblock Copolymer Consisting of Poly(4-butyltriphenylamine) and Morphological Control in Photovoltaic Applicati. Polymers 2011, 3, 1051–1064. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Williams, M.L. Tensile properties and morphology of blends of polyethylene and polypropylene. J. Appl. Polym. Sci. 2010, 25, 1703–1713. [Google Scholar] [CrossRef]
- Yan, L.C.; Gupta, N.; Pramana, S.S.; Aravindan, V.; Wee, G. Morphology, structure and electrochemical properties of single phase electrospun vanadium pentoxide nanofibers for lithium ion batteries. J. Power Sources 2011, 196, 6465–6472. [Google Scholar] [CrossRef]
- Halimatudahliana, A.; Ismail, H.; Nasir, M. Morphological studies of uncompatibilized and compatibilized polystyrene/polypropylene blend. Polym. Test. 2002, 21, 263–267. [Google Scholar] [CrossRef]
- Mazidi, M.M.; Aghjeh, M.K.R. Effects of blend composition and compatibilization on the melt rheology and phase morphology of binary and ternary PP/PA6/EPDM blends. Polym. Bull. 2015, 72, 1975–2000. [Google Scholar] [CrossRef]
- Levij, M.; Maurer, F.H.J. Morphology and rheological properties of polypropylene-linear low density polyethylene blends. Polym. Eng. Sci. 1988, 28, 670–678. [Google Scholar] [CrossRef]
- Han, C.D. Rheological properties of calcium carbonate-filled polypropylene melts. J. Appl. Polym. Sci. 2010, 18, 821–829. [Google Scholar] [CrossRef]
- Sugimoto, M.; Suzuki, Y.; Hyun, K.; Ahn, K.H.; Ushioda, T. Melt rheology of long-chain-branched polypropylenes. Rheol. Acta 2006, 46, 33–44. [Google Scholar] [CrossRef]
- Sharma, R.; Maiti, S.N. Effects of Crystallinity of PP and Flexibility of SEBS-g-MA Copolymer on the Mechanical Properties of PP/SEBS-g-MA Blends. Polym. Plast. Technol. Eng. 2014, 53, 229–238. [Google Scholar] [CrossRef]
- Tashiro, K.; Izuchi, M.; Kobayashi, M.; Stein, R.S. Cocrystallization and Phase Segregation of Polyethylene Blends between the D and H Species. 3. Blend Content Dependence of the Crystallization Behavior. Macromolecules 1994, 27, 1221–1227. [Google Scholar] [CrossRef]
- Xiao, W.C.; Wu, P.Y.; Feng, J.C.; Yao, R.Y. Influence of a Novel beta-Nucleating Agent on the Structure, Morphology, and Nonisothermal Crystallization Behavior of Isotactic Polypropylene. J. Appl. Polym. Sci. 2010, 111, 1076–1085. [Google Scholar] [CrossRef]
- Sharma, Y.N.; Patel, R.D.; Bhardwaj, I.S. The effect of elastomers on the crystallinity of polypropylene. Thermochim. Acta 1982, 54, 229–232. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Chua, J.O.; Gryte, C.C. Studies on the α and β forms of isotactic polypropylene by crystallization in a temperature gradient. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 641–656. [Google Scholar] [CrossRef]
- Binsbergen, F.L. Heterogeneous nucleation in the crystallization of polyolefins: Part 1. Chemical and physical nature of nucleating agents. Polymer 1970, 11, 253–267. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Hirano, C.; Suzuki, Y.; Hayakawa, T.; Kuo, S.W. Strong Screening Effect of Polyhedral Oligomeric Silsesquioxanes (POSS) Nanoparticles on Hydrogen Bonded Polymer Blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef]
- Diaz, M.F.; Barbosa, S.E. Polyethylene–polystyrene grafting reaction: Effects of polyethylene molecular weight. Polymer 2002, 43, 4851–4858. [Google Scholar] [CrossRef]
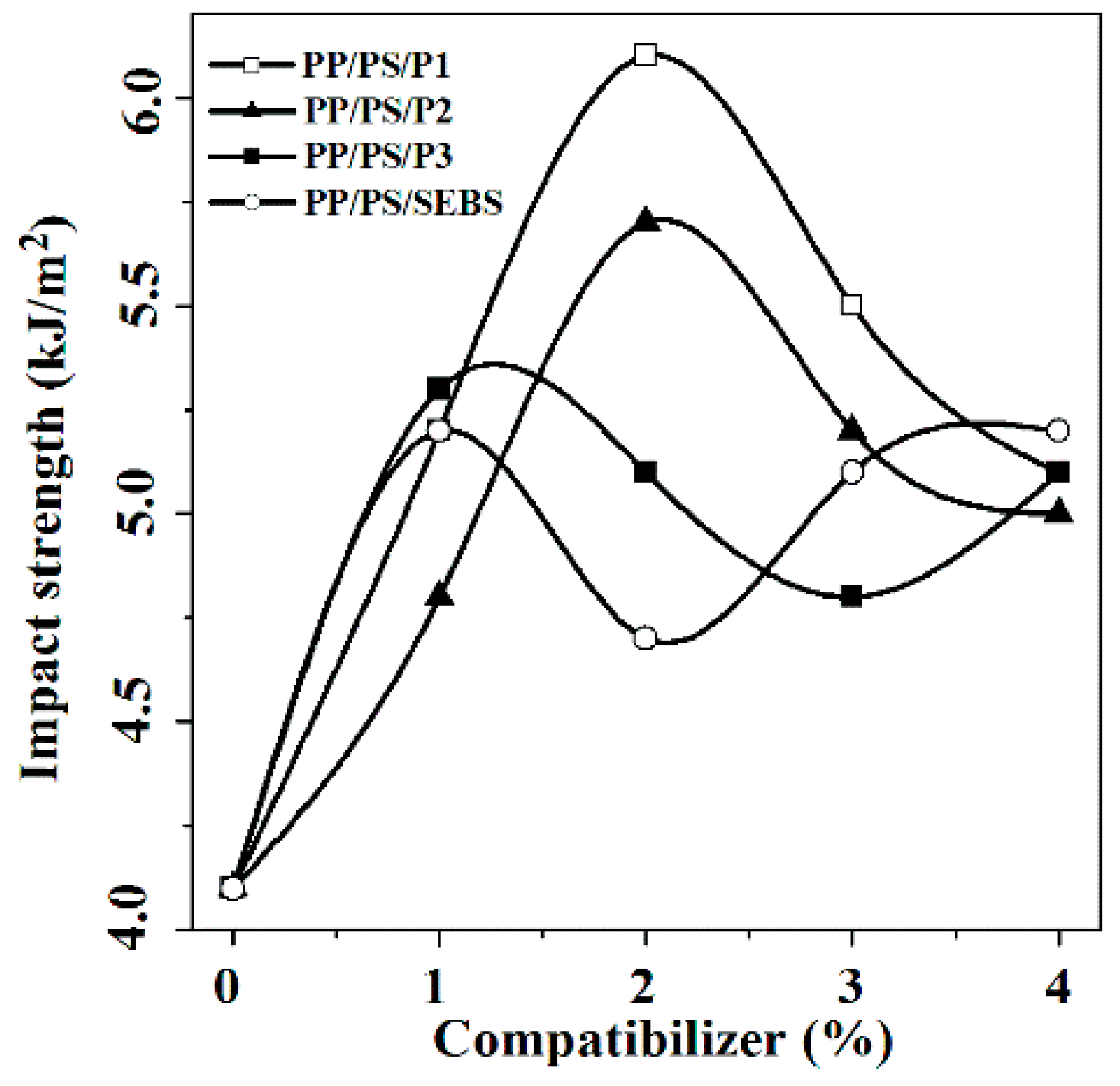

| Sample | Styrenic Unit | Aromatic Ring/100 C a | Mwb (104) | Cl Content c (mg/g) | Tmd (°C) |
|---|---|---|---|---|---|
| CPP | - | - | 9.75 | 101 | 141.5 |
| P0 | benzene | 0.7 | 0.89 | 2.6 | 140.3 |
| P1 | methylbenzene | 3.5 | 4.50 | 1.6 | 144.4 |
| P2 | ethylbenzene | 3.6 | 3.89 | 2.2 | 144.7 |
| P3 | methoxyl-benzene | 4.0 | 7.31 | 1.3 | 151.7 |
| Blends | Maximum (µm) | Average (µm) |
|---|---|---|
| PP/PS | 4.97 | 2.06 |
| PP/PS/P1 | 2.11 | 0.68 |
| PP/PS/P2 | 2.38 | 0.84 |
| PP/PS/P3 | 2.62 | 0.83 |
| PP/PS/SEBS | 3.36 | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Liu, X.; Zhang, C.; Liu, H.; Hu, Y.; Zhang, X. Synthesis of Propylene-co-Styrenic Monomer Copolymers via Arylation of Chlorinated PP and Their Compatibilization for PP/PS Blend. Polymers 2019, 11, 157. https://doi.org/10.3390/polym11010157
Fu X, Liu X, Zhang C, Liu H, Hu Y, Zhang X. Synthesis of Propylene-co-Styrenic Monomer Copolymers via Arylation of Chlorinated PP and Their Compatibilization for PP/PS Blend. Polymers. 2019; 11(1):157. https://doi.org/10.3390/polym11010157
Chicago/Turabian StyleFu, Xiangming, Xijun Liu, Chunyu Zhang, Heng Liu, Yanming Hu, and Xuequan Zhang. 2019. "Synthesis of Propylene-co-Styrenic Monomer Copolymers via Arylation of Chlorinated PP and Their Compatibilization for PP/PS Blend" Polymers 11, no. 1: 157. https://doi.org/10.3390/polym11010157
APA StyleFu, X., Liu, X., Zhang, C., Liu, H., Hu, Y., & Zhang, X. (2019). Synthesis of Propylene-co-Styrenic Monomer Copolymers via Arylation of Chlorinated PP and Their Compatibilization for PP/PS Blend. Polymers, 11(1), 157. https://doi.org/10.3390/polym11010157