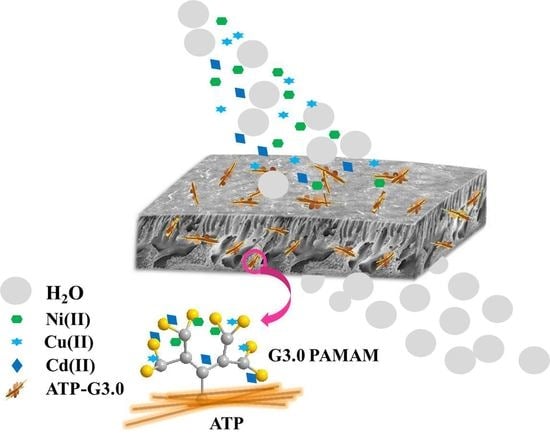
Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization
2.3.1. General Characterization
2.3.2. Heavy Metal Ion Adsorption
3. Results
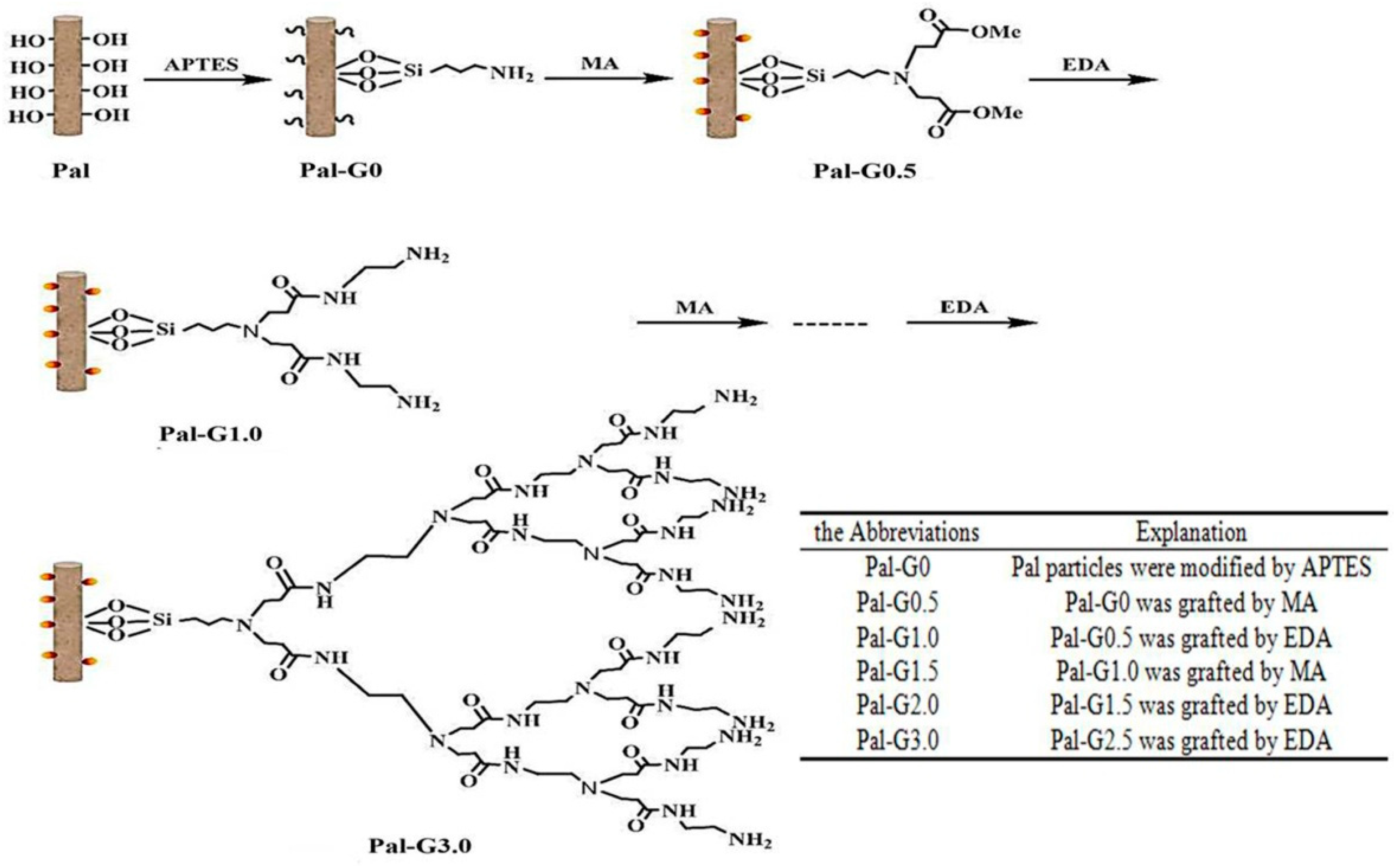
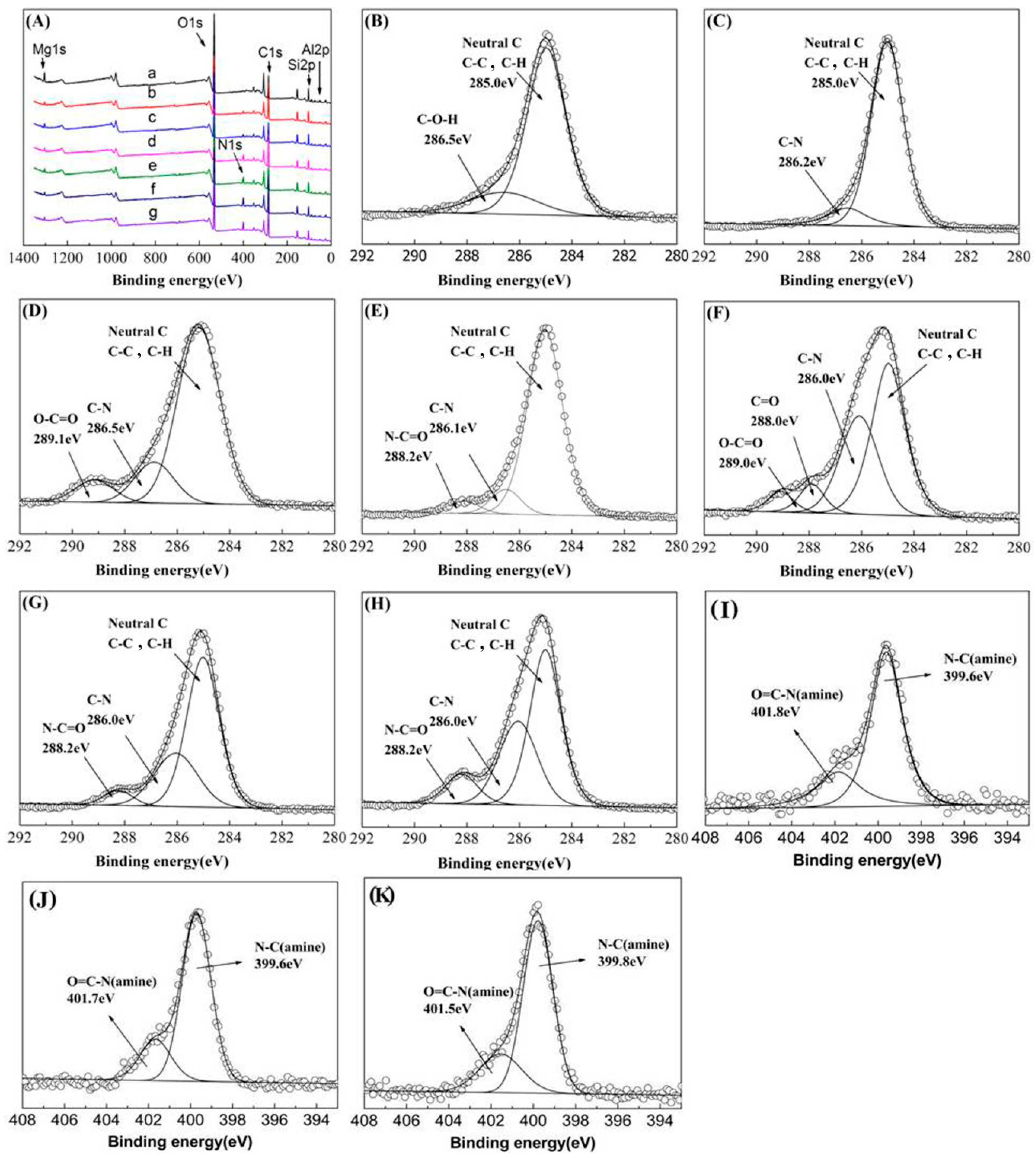
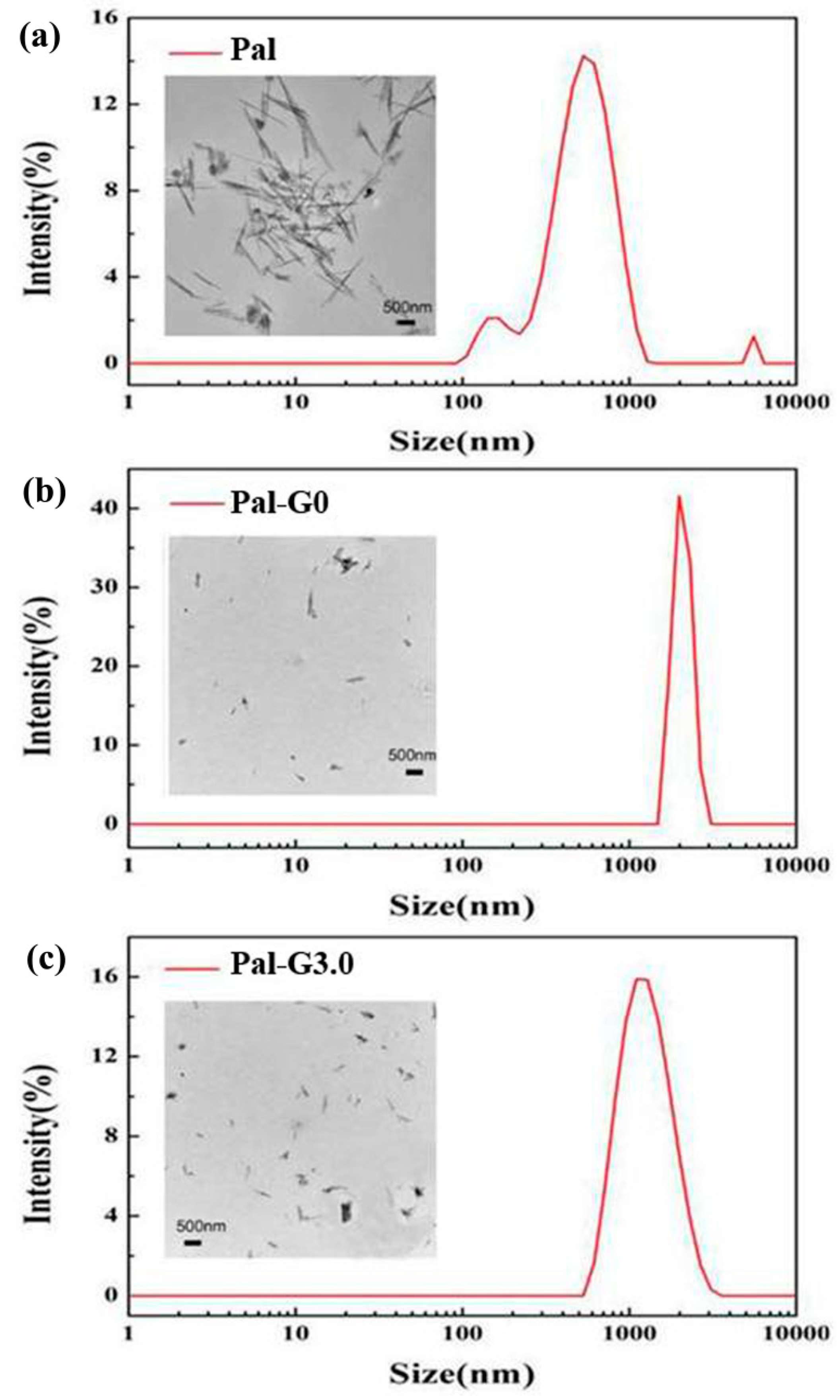
3.1. Characterization of the PAMAM-Pal
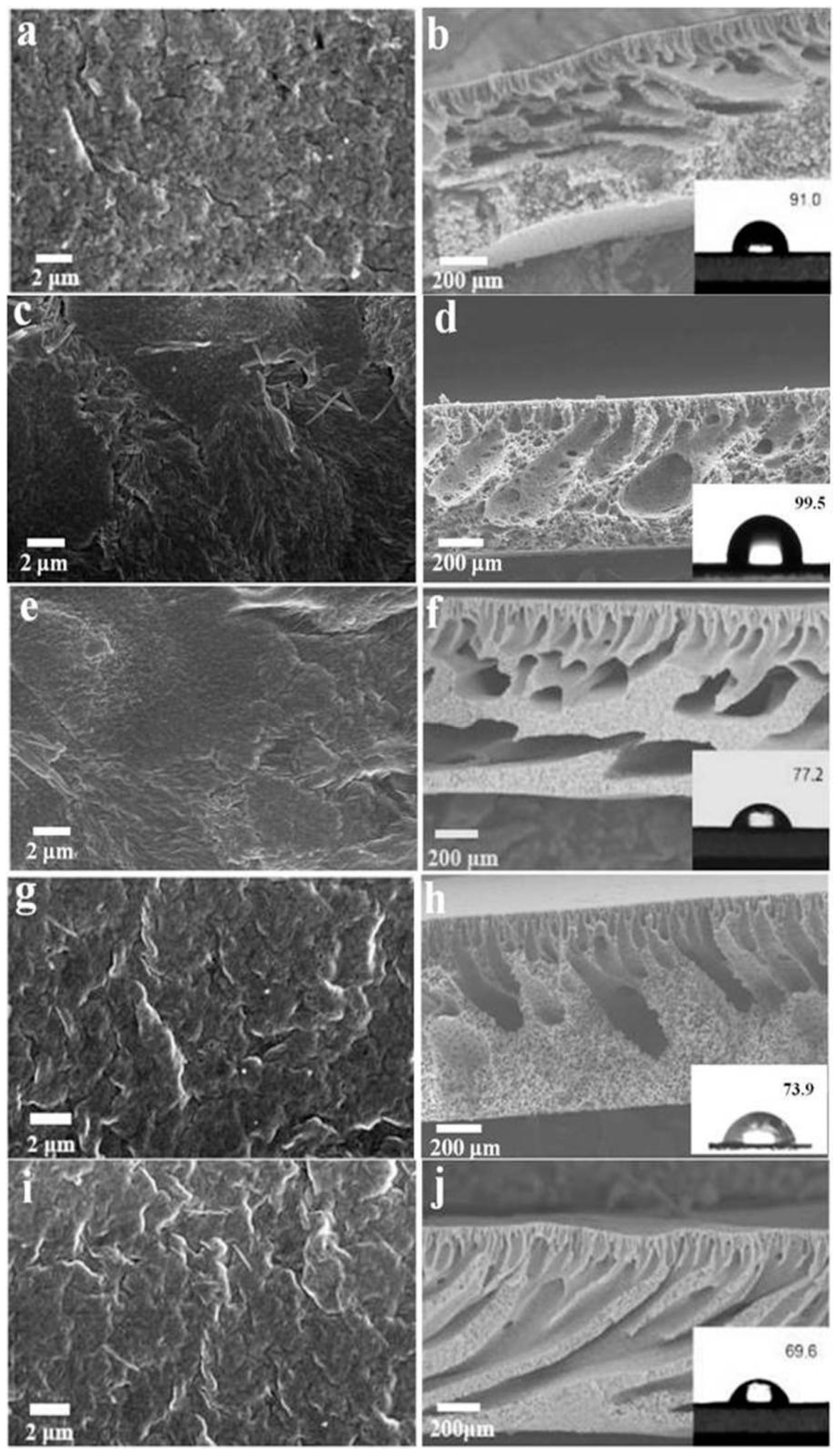
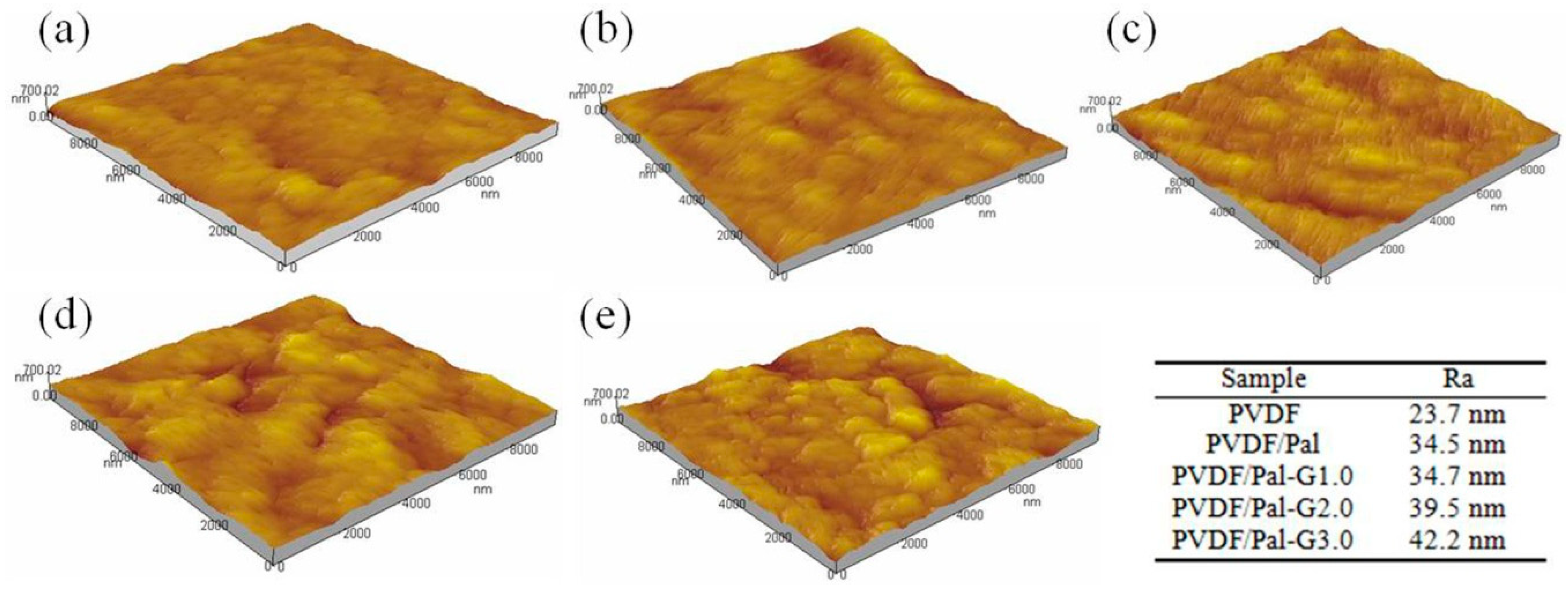
3.2. Membrane Structure and Performance
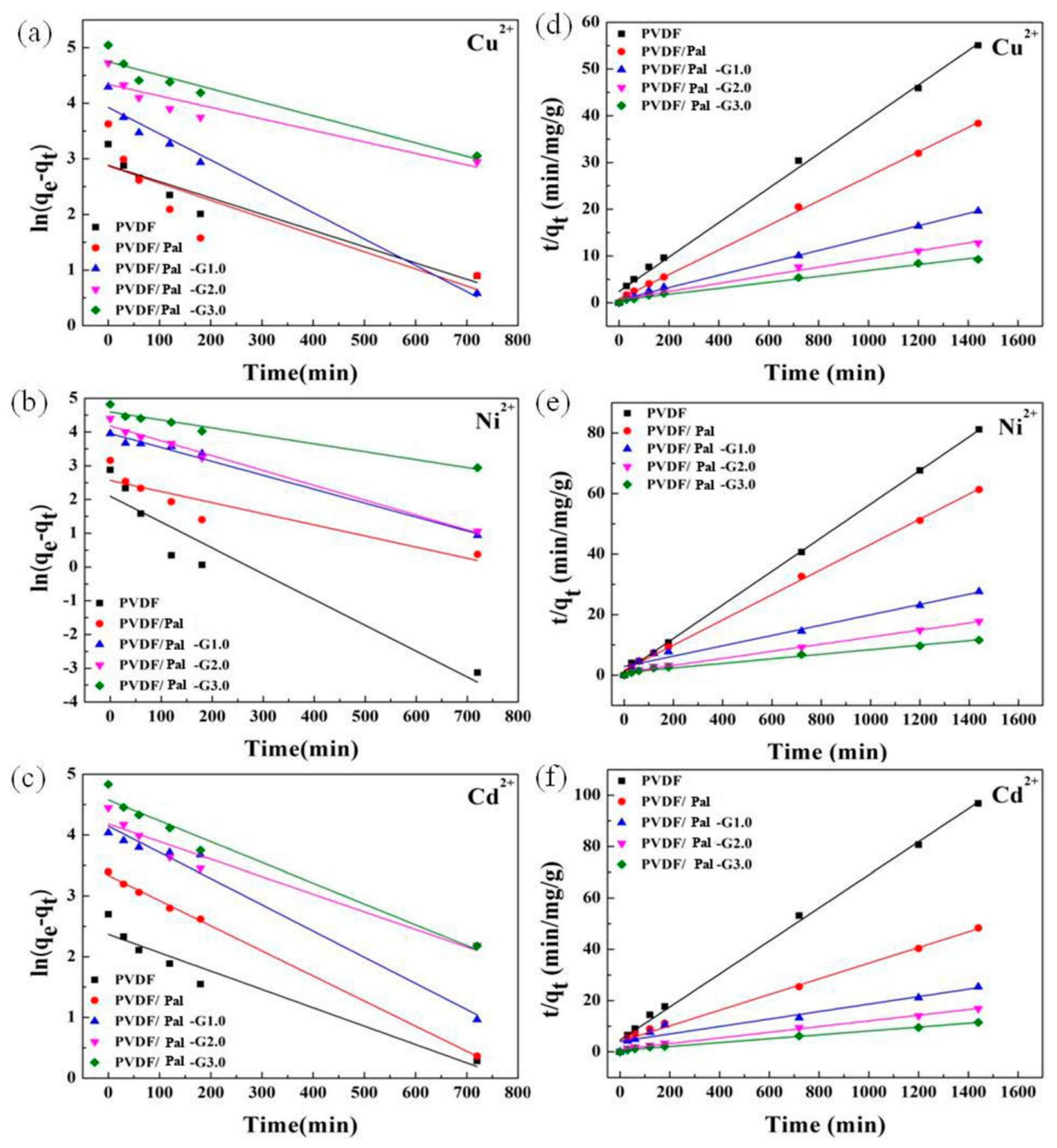
3.3. Heavy Metal Ions Adsorption and Adsorption Kinetic Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Rashdi, B.; Somerfield, C.; Hilal, N. Heavy Metals Removal Using Adsorption and Nanofiltration Techniques. Sep. Purif. Rev. 2011, 40, 209–259. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J.; Loizidou, M. Use of ultrafiltration membranes and aluminosilicate minerals for nickel removal from industrial wastewater. J. Membr. Sci. 2010, 360, 234–249. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Han, G.; Chung, T.-S. Novel thin-film composite nanofiltration membranes consisting of a zwitterionic co-polymer for selenium and arsenic removal. J. Membr. Sci. 2018, 555, 299–306. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Camarillo, R.; Pérez, Á.; Cañizares, P.; de Lucas, A. Removal of heavy metal ions by polymer enhanced ultrafiltration. Desalination 2012, 286, 193–199. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Ghyselbrecht, K.; Santos, R.M.; Meesschaert, B.; Martens, J.A. Synthesis of zeolitic-type adsorbent material from municipal solid waste incinerator bottom ash and its application in heavy metal adsorption. Catal. Today 2012, 190, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Kryvoruchko, A.P.; Atamanenko, I.D.; Yurlova, L.Y. Concentration/purification of Co(II) ions by reverse osmosis and ultrafiltration combined with sorption on clay mineral montmorillonite and cation-exchange resin KU-2-8n. J. Membr. Sci. 2004, 228, 77–81. [Google Scholar] [CrossRef]
- Xiang, L.; Pan, Y.; Zeng, G.; Jiang, J.; Chen, J.; Wang, C. Preparation of poly(ether-block-amide)/attapulgite mixed matrix membranes for CO2/N2 separation. J. Membr. Sci. 2016, 500, 66–75. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of metal ions by clays and inorganic solids. RSC Adv. 2014, 4, 28537–28586. [Google Scholar] [CrossRef]
- Wu, M.; Ma, T.; Su, Y.; Wu, H.; You, X.; Jiang, Z.; Kasher, R. Fabrication of composite nanofiltration membrane by incorporating attapulgite nanorods during interfacial polymerization for high water flux and antifouling property. J. Membr. Sci. 2017, 544, 79–87. [Google Scholar] [CrossRef]
- Pan, D.; Fan, Q.; Fan, F.; Tang, Y.; Zhang, Y.; Wu, W. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Sep. Purif. Technol. 2017, 177, 86–93. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, P.; Wang, A. Facile approach to magnetic attapulgite-Fe3O4/polystyrene tri-component nanocomposite. Mater. Lett. 2012, 85, 11–13. [Google Scholar] [CrossRef]
- Deng, Y.; Gao, Z.; Liu, B.; Hu, X.; Wei, Z.; Sun, C. Selective removal of lead from aqueous solutions by ethylenediamine-modified attapulgite. Chem. Eng. J. 2013, 223, 91–98. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, B.; Li, H.; Zhang, W.; Lv, L.; Wu, J. Selective removal of Cu (II) ions by using cation-exchange resin-supported polyethyleneimine (PEI) nanoclusters. Environ. Sci. Technol. 2010, 44, 3508–3513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Zhang, D.-Y.; Liu, X.-Q.; Shi, L.-Y.; Sun, L.-B. A tandem demetalization–desilication strategy to enhance the porosity of attapulgite for adsorption and catalysis. Chem. Eng. Sci. 2016, 141, 184–194. [Google Scholar] [CrossRef]
- Bu, J.; Li, R.; Quah, C.W.; Carpenter, K.J. Propagation of PAMAM Dendrons on Silica Gel: A Study on the Reaction Kinetics. Macromolecules 2004, 37, 6687–6694. [Google Scholar] [CrossRef]
- Kaneko, Y.; Imai, Y.; Shirai, K.; Yamauchi, T.; Tsubokawa, N. Preparation and properties of hyperbranched poly(amidoamine) grafted onto a colloidal silica surface. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 212–218. [Google Scholar] [CrossRef]
- Yoo, H.; Kwak, S.-Y. Surface functionalization of PTFE membranes with hyperbranched poly(amidoamine) for the removal of Cu2+ ions from aqueous solution. J. Membr. Sci. 2013, 448, 125–134. [Google Scholar] [CrossRef]
- Yantasee, W.; Lin, Y.; Fryxell, G.E.; Alford, K.L.; Busche, B.J.; Johnson, C.D. Selective Removal of Copper(II) from Aqueous Solutions Using Fine-Grained Activated Carbon Functionalized with Amine. Ind. Eng. Chem. Res. 2004, 43, 2759–2764. [Google Scholar] [CrossRef]
- Cao, J.S.; Wang, C.; Fang, F.; Lin, J.X. Removal of heavy metal Cu(II) in simulated aquaculture wastewater by modified palygorskite. Environ. Pollut. 2016, 219, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211–212, 216–223. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Deng, B. Enhanced mercury ion adsorption by amine-modified activated carbon. J. Hazard. Mater. 2009, 166, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-L.; Ang, M.B.M.Y.; Hung, H.-C.; Huang, S.-H.; An, Q.-F.; Lee, K.-R.; Lai, J.-Y. Bio-inspired deposition of polydopamine on PVDF followed by interfacial cross-linking with trimesoyl chloride as means of preparing composite membranes for isopropanol dehydration. J. Membr. Sci. 2018, 557, 58–66. [Google Scholar] [CrossRef]
- Wu, H.; Mansouri, J.; Chen, V. Silica nanoparticles as carriers of antifouling ligands for PVDF ultrafiltration membranes. J. Membr. Sci. 2013, 433, 135–151. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Characterization of surface-modified porous PVDF hollow fibers for refinery wastewater treatment using microscopic observation. Desalination 2011, 283, 206–213. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, A.; Zhang, Y.; Li, M.; Li, K.; Zhao, Y.; Xing, W. Novel polyamidoamine dendrimer-functionalized palygorskite adsorbents with high adsorption capacity for Pb2+ and reactive dyes. Appl. Clay. Sci. 2015, 107, 220–229. [Google Scholar] [CrossRef]
- Salehi, E.; Madaeni, S.S.; Heidary, F. Dynamic adsorption of Ni(II) and Cd(II) ions from water using 8-hydroxyquinoline ligand immobilized PVDF membrane: Isotherms, thermodynamics and kinetics. Sep. Purif. Technol. 2012, 94, 1–8. [Google Scholar] [CrossRef]
- Ricordel, S.; Taha, S.; Cisse, I.; Dorange, G. Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling. Sep. Purif. Technol. 2001, 24, 389–401. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. J. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, D.; Tan, D.; Yuan, P.; Chen, M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1052–1057. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Ozturk, O.; Black, T.J.; Pizzolato, K.; Williams, C.T.; Parsons, F.W.; Ratliff, J.S.; Gao, J.; Murphy, C.J.; Xie, H.; Ploehn, H.J.; et al. Thermal Decomposition of Generation-4 Polyamidoamine Dendrimer Films: Decomposition Catalyzed by Dendrimer-Encapsulated Pt Particles. Langmuir ACS J. Surfaces Colloids 2005, 21, 3998–4006. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, N.; Prasad, P.; Shirbhate, S.; Sehrawat, S.; Lochab, B. Stable Dispersions of Covalently Tethered Polymer Improved Graphene Oxide Nanoconjugates as an Effective Vector for siRNA Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14577–14593. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Fang, S.; Zhan, J.; Hu, Y.; Wu, J. Preparation and characterization of γ-irradiated crosslinked silicone rubber/organoattapulgite composites. Polym. Compos. 2010, 31, 405–410. [Google Scholar] [CrossRef]
- Kotte, M.R.; Kuvarega, A.T.; Cho, M.; Mamba, B.B.; Diallo, M.S. Mixed Matrix PVDF Membranes With in Situ Synthesized PAMAM Dendrimer-Like Particles: A New Class of Sorbents for Cu(II) Recovery from Aqueous Solutions by Ultrafiltration. Environ. Sci. Technol. 2015, 49, 9431–9442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, M.A.; Schmidt, E. Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Zhu, W.-P.; Gao, J.; Sun, S.-P.; Zhang, S.; Chung, T.-S. Poly(amidoamine) dendrimer (PAMAM) grafted on thin film composite (TFC) nanofiltration (NF) hollow fiber membranes for heavy metal removal. J. Membr. Sci. 2015, 487, 117–126. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Liu, S.; Jiang, J.; Cao, C.; Yao, J. Simple fabrication of easy handling millimeter-sized porous attapulgite/polymer beads for heavy metal removal. J. Colloid Interface Sci. 2017, 502, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, H.; Wang, A. Adsorption characteristics of Cd(II) from aqueous solution onto activated palygorskite. Sep. Purif. Technol. 2007, 55, 157–164. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Tao, W.W.; Zhang, G.F.; Lv, C.; Zhao, Y.P.; Chen, L. Adsorptive Removal of Ni(II) Ions from Aqueous Solution by Polyacrylic Acid/Attapulgite Composite Hydrogels. Key Eng. Mater. 2017, 727, 859–865. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tang, J.; Tan, L.; Wang, W.; Lu, H.; Guo, D. Synthesis and Adsorption of Ni(II) on Ni(II)-Imprinted Polyaniline Supported on Attapulgite Modified with 3-Methacryloxypropyltrimethoxysilane. Adsorpt. Sci. Technol. 2013, 31, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, X.; Ma, J.; Xia, P.; Zhao, J. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Mu, B.; Zheng, M.; Wang, A. One-Step Calcination of the Spent Bleaching Earth for the Efficient Removal of Heavy Metal Ions. ACS Sustain. Chem. Eng. 2015, 3, 1125–1135. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C. Chitosan-poly(vinyl alcohol)/attapulgite nanocomposites for copper(II) ions removal: pH dependence and adsorption mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 186–194. [Google Scholar] [CrossRef]
- Liu, G.; Li, Z.; Xu, L.; Xu, X.; Huang, Q.; Zeng, Y.; Wen, M. The dynamics and adsorption of Cd (II) onto hydroxyapatite attapulgite composites from aqueous solution. J. Sol-Gel Sci. Technol. 2018, 87, 269–284. [Google Scholar] [CrossRef]
- Bigui, W.; Xiaofei, Z.; Xiabing, C. Facile Preparation of Magnetic Graphene Oxide and Attapulgite Composite Adsorbent for the Adsorption of Ni (II). IOP Conf. Ser. Earth Environ. Sci. 2017, 104, 012019. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, S.S.; Muthukrishnaraj, A.; Sivanesan, S.; Ravikumar, L. Novel hyperbranched polyurethane resins for the removal of heavy metal ions from aqueous solution. Process. Saf. Environ. 2016, 104, 11–23. [Google Scholar] [CrossRef]




| Adsorbents | C0 a (mg·L−1) | Q (mg/g) | Reference | ||
|---|---|---|---|---|---|
| Cu(II) | Ni(II) | Cd(II) | |||
| porous palygorskite (Pal)/polymer beads | 500 | 25.3 | -- | 32.7 | [43] |
| activated palygorskites | 500 | -- | -- | 52.99 | [44] |
| Polyacrylic Acid/palygorskite Composite Hydrogels | 200 | -- | 72.8 | -- | [45] |
| alginate-based palygorskite foams | 250 | 119 | -- | 160 | [46] |
| A novel Ni(II) ion-imprinted polymer | 100 | -- | 123.61 | -- | [47] |
| struvite/palygorskite | 100 | -- | -- | 121.14 | [48] |
| palygorskite/carbon nanocomposites | 1000 | 32.32 | -- | 46.72 | [49] |
| CTS-PVA/Pal | 210 | 35.79 | -- | -- | [50] |
| palygorskite hydroxyapatite composite | 120 | 126.45 | -- | 131.52 | [51] |
| a composite magnetic GO-Pal adsorbent | 100 | -- | 190.8 | -- | [52] |
| PVDF/hyperbranched-nano-palygorskite composite membrane | 200 | 155.19 | 124.28 | 125.55 | This work |
| Metalions | Samples | qe (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe1 (mg/g) | k1 (min−1) | r2 | qe2 (mg/g) | k2 (g/mg min) | r2 | |||
| Cu(II) | a | 24.8547 | 17.87316 | 0.0029 | 0.8834 | 27.1665 | 5.64 × 10−4 | 0.9964 |
| b | 37.5432 | 17.64072 | 0.0031 | 0.6403 | 38.1243 | 8.78 × 10−4 | 0.9989 | |
| c | 73.2668 | 50.61966 | 0.0047 | 0.9704 | 75.7002 | 2.89 × 10−4 | 0.9982 | |
| d | 112.7836 | 76.62627 | 0.0021 | 0.8211 | 115.2074 | 1.13 × 10−4 | 0.9912 | |
| e | 155.1950 | 115.2980 | 0.0024 | 0.9188 | 157.4803 | 7.35 × 10−5 | 0.9900 | |
| Ni(II) | a | 12.9696 | 8.136825 | 0.0077 | 0.8915 | 18.0115 | 3.05 × 10−3 | 0.9995 |
| b | 23.4972 | 13.01952 | 0.0033 | 0.7964 | 24.0211 | 1.09 × 10−3 | 0.9986 | |
| c | 52.1184 | 52.18734 | 0.0041 | 0.9870 | 58.5480 | 1.02 × 10−4 | 0.9688 | |
| d | 81.2291 | 65.37566 | 0.0044 | 0.9861 | 85.3242 | 1.63 × 10−4 | 0.9957 | |
| e | 124.2819 | 98.64721 | 0.0024 | 0.9503 | 132.6260 | 6.54 × 10−5 | 0.9853 | |
| Cd(II) | a | 17.1907 | 10.69846 | 0.0030 | 0.9238 | 15.5400 | 8.91 × 10−4 | 0.9958 |
| b | 29.7817 | 28.11463 | 0.0041 | 0.9986 | 32.7118 | 2.32 × 10−4 | 0.9876 | |
| c | 56.7012 | 63.24145 | 0.0043 | 0.9749 | 68.6342 | 5.21 × 10−5 | 0.9208 | |
| d | 85.3710 | 65.50392 | 0.0029 | 0.9355 | 90.2527 | 1.22 × 10−4 | 0.9939 | |
| e | 125.5581 | 97.09792 | 0.0034 | 0.9658 | 132.4503 | 9.39 × 10−5 | 0.9948 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qin, Y.; Zhang, G.; Zhao, Y.; Lv, C.; Liu, X.; Chen, L. Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers 2019, 11, 156. https://doi.org/10.3390/polym11010156
Zhang X, Qin Y, Zhang G, Zhao Y, Lv C, Liu X, Chen L. Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers. 2019; 11(1):156. https://doi.org/10.3390/polym11010156
Chicago/Turabian StyleZhang, Xiaoye, Yingxi Qin, Guifang Zhang, Yiping Zhao, Chao Lv, Xingtian Liu, and Li Chen. 2019. "Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions" Polymers 11, no. 1: 156. https://doi.org/10.3390/polym11010156
APA StyleZhang, X., Qin, Y., Zhang, G., Zhao, Y., Lv, C., Liu, X., & Chen, L. (2019). Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers, 11(1), 156. https://doi.org/10.3390/polym11010156