Surface Modification of Aluminum Nitride to Fabricate Thermally Conductive poly(Butylene Succinate) Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
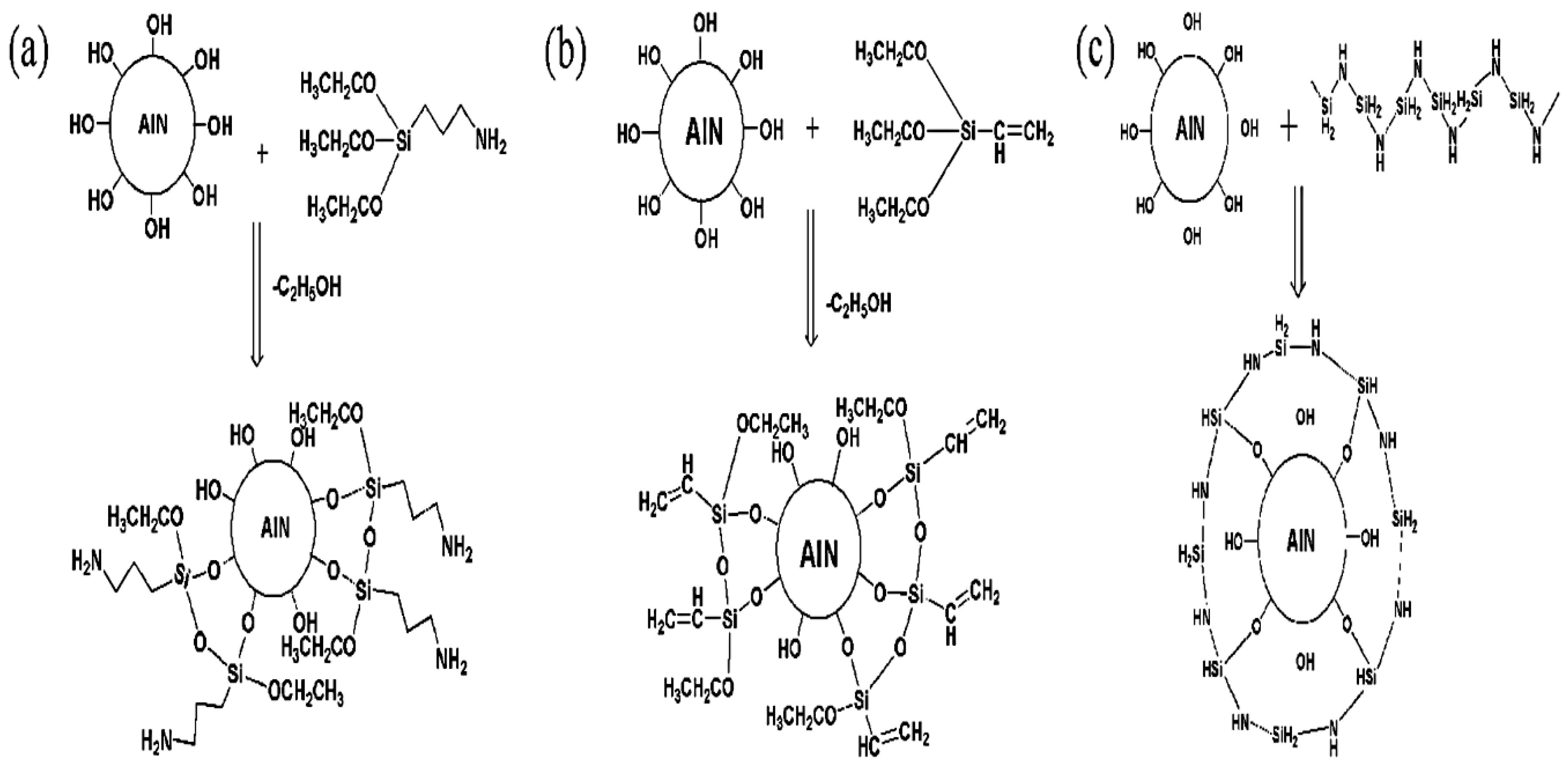
2.2.1. Nanoparticle Treatment
2.2.2. Composite Fabrication
2.3. Characterizations
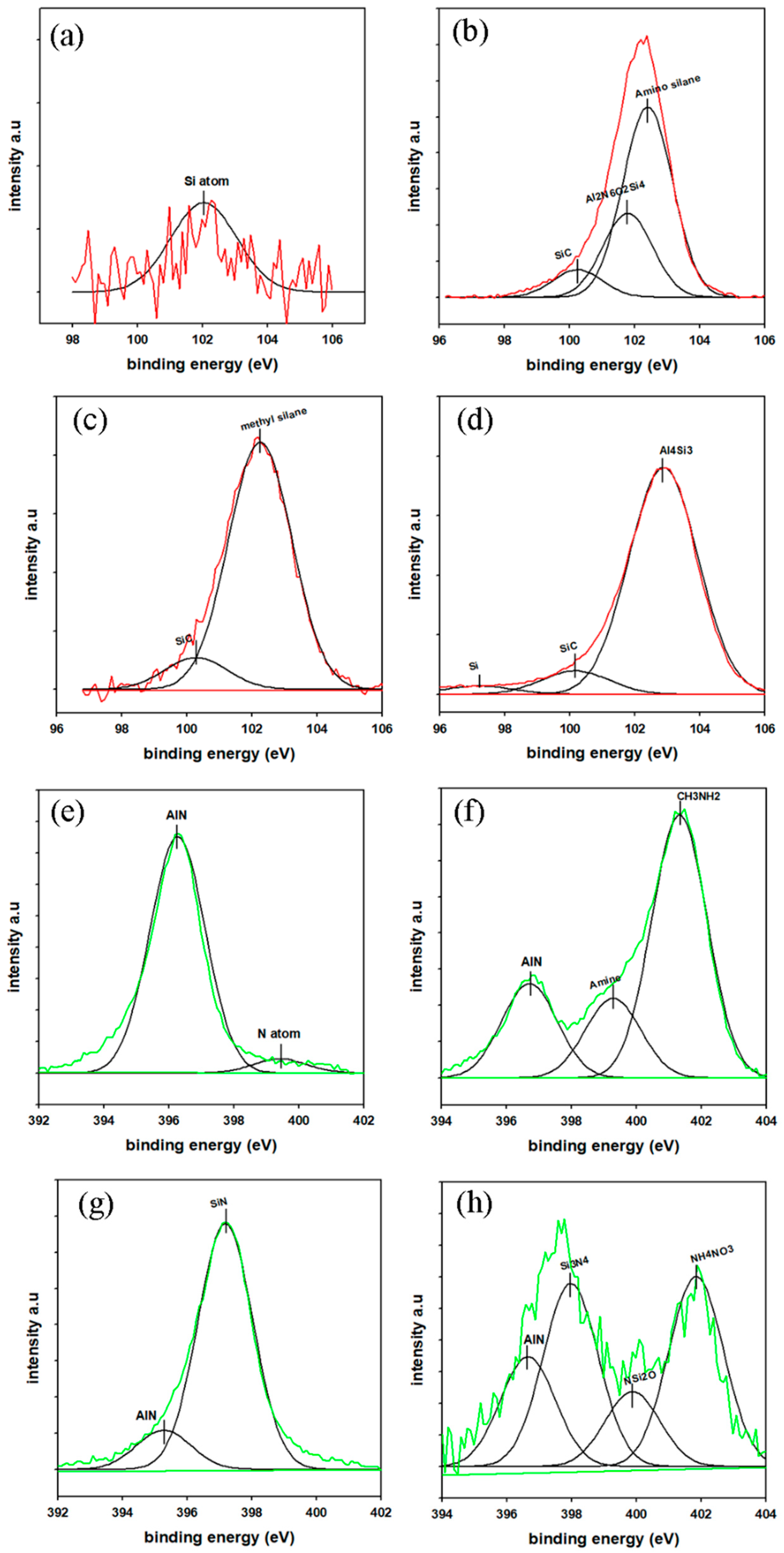
2.3.1. Nanoparticle Characterization
2.3.2. Composite Characterization
3. Results and Discussion
3.1. Nanoparticle Analysis
3.2. Nanocomposite Analysis
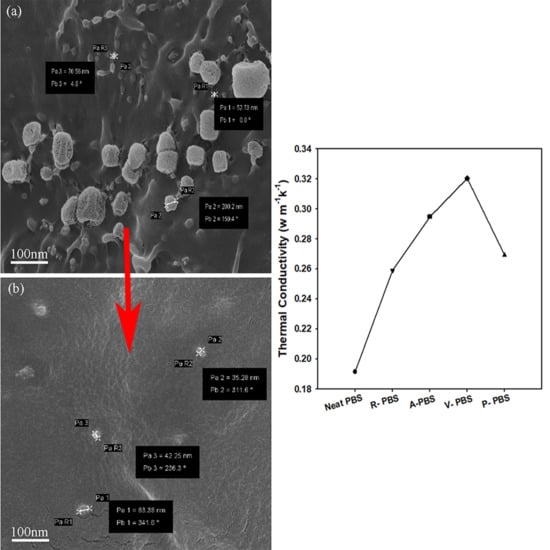
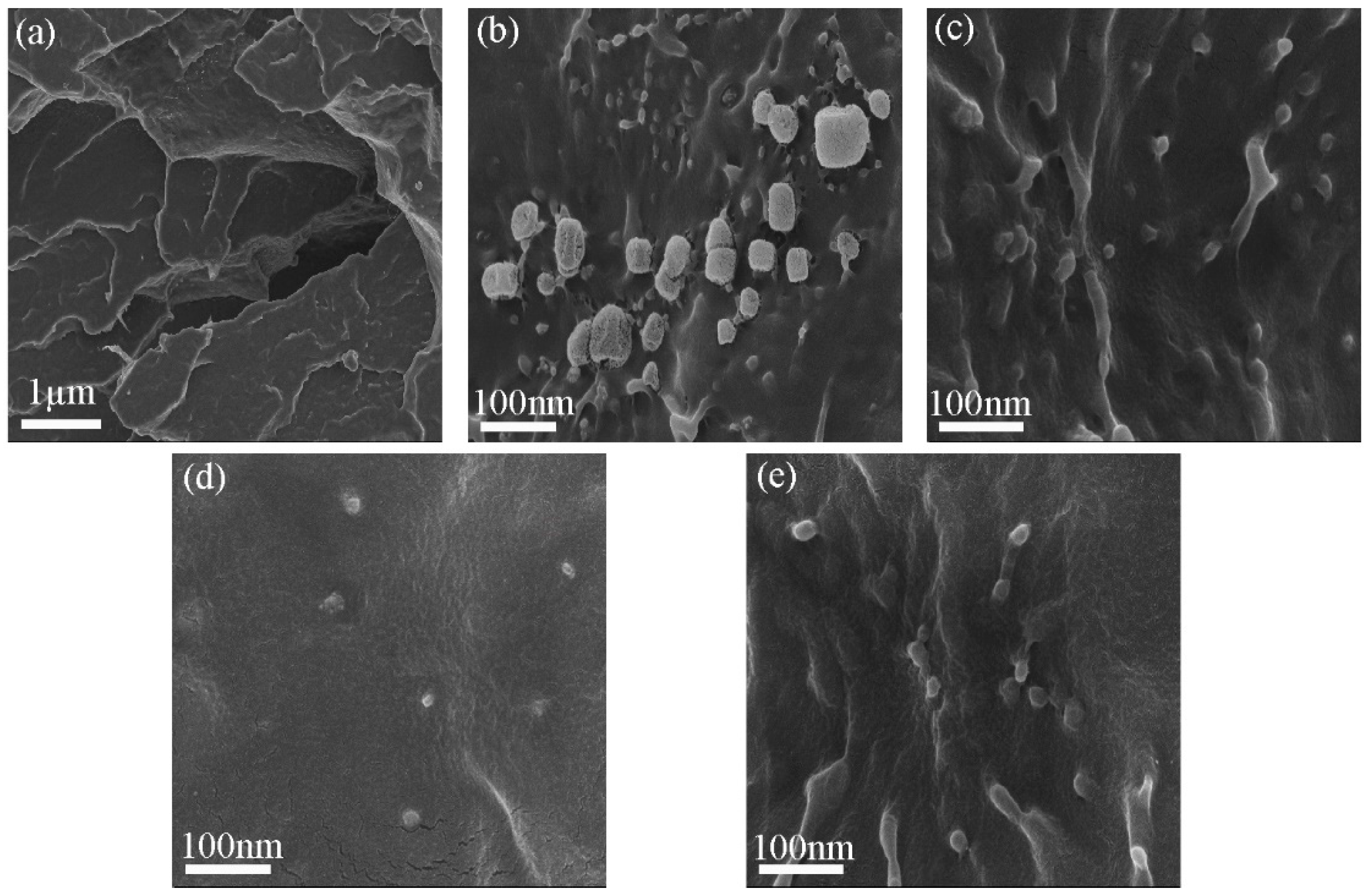
3.2.1. Morphology
3.2.2. Thermal Behaviors
3.2.3. Thermogravimetry
3.2.4. Thermal Conductivity
3.2.5. Tensile Property
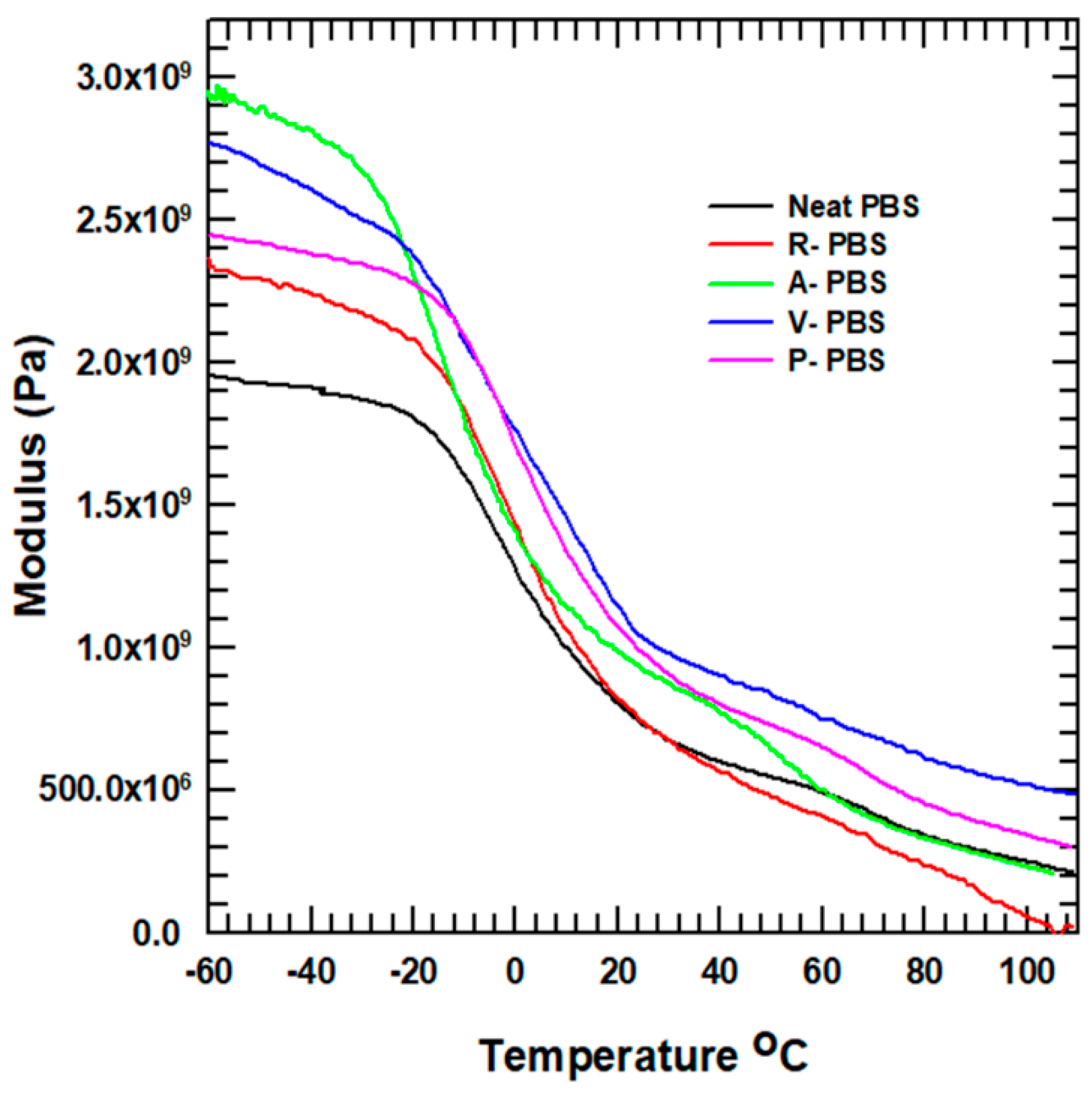
3.2.6. Dynamic Mechanical Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Liu, Q.; Shi, J.; Ye, H.; Zhou, Q. Distinctive Tensile Properties of the Blends of poly(l-lactic acid) (PLLA) and poly(butylene succinate) (PBS). J. Polym. Environ. 2018, 26, 1737–1744. [Google Scholar] [CrossRef]
- Lee, J.M.; Mohd Ishak, Z.A.; Mat Taib, R.; Law, T.T.; Ahmad Thirmizir, M.Z. Mechanical, Thermal and Water Absorption Properties of Kenaf-Fiber-Based Polypropylene and Poly(Butylene Succinate) Composites. J. Polym. Environ. 2013, 21, 293–302. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Polybutylene succinate/cellulose nanocrystals: Role of phthalic anhydride in squeeze oriented bionanocomposites. Carbohydr. Polym. 2018, 196, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Georgousopoulou, I.; Vouyiouka, S.; Dole, P.; Papaspyrides, C.D. Thermo-mechanical degradation and stabilization of poly(butylene succinate). Polym. Degrad. Stab. 2016, 128, 182–192. [Google Scholar] [CrossRef]
- Huang, J.; Lu, X.; Zhang, N.; Yang, L.; Yan, M.; Liu, H.; Zhang, G.; Ji, Q. Study on the Properties of Nano-TiO2/Polybutylene Succinate Composites Prepared by Vane Extruder. Polym. Polym. Compos. 2014, 16, 101–113. [Google Scholar] [CrossRef]
- Hassan, E.; Wei, Y.; Jiao, H.; Yu, M. Dynamic Mechanical properties and Thermal stability of Poly(lactic acid) and poly(butylene succinate) blends composites. J. Fiber Bioeng. Inform. 2013, 6, 85–94. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Shih, Y.F.; Chen, L.S.; Jeng, R.J. Preparation and properties of biodegradable PBS/multi-walled carbon nanotube nanocomposites. Polymer 2008, 49, 4602–4611. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. Organomodification of montmorillonite and its effects on the properties of poly(butylene succinate) nanocomposites. Polym. Eng. Sci. 2013, 53, 1947–1957. [Google Scholar] [CrossRef]
- Kurokawa, N.; Kimura, S.; Hotta, A. Mechanical properties of poly(butylene succinate) composites with aligned cellulose-acetate nanofibers. J. Appl. Polym. Sci. 2017, 45429, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B. Microbial Succinic Acid, Its Polymer poly(butylene succinate), and Applications. In Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14. [Google Scholar]
- Mincheva, R.; Delangre, A.; Raquez, J.M.; Narayan, R.; Dubois, P. Biobased polyesters with composition-dependent thermomechanical properties: Synthesis and characterization of poly(butylene succinate-co-butylene azelate). Biomacromolecules 2013, 14, 890–899. [Google Scholar] [CrossRef]
- Sorrentino, A.; Vittoria, V. Potential perspectives of for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Weerasunthorn, S.; Potiyaraj, P. Mechanical property improvement of poly(butylene succinate) by reinforcing with modified fumed silica. Adv. Mater. Res. 2014, 1025–1026, 215–220. [Google Scholar] [CrossRef]
- Lule, Z.; Ju, H.; Kim, J. Effect of surface-modified Al2O3 on the thermomechanical properties of polybutylene succinate/Al2O3 composites. Ceram. Int. 2018, 44, 13530–13537. [Google Scholar] [CrossRef]
- Rajeshwari, P.; Dey, T.K. Thermochimica Acta Finite element modelling and experimental investigation on effective thermal conductivity of AlN (nano) particles reinforced HDPE polymer nanocomposites. Thermochim. Acta 2016, 638, 103–112. [Google Scholar] [CrossRef]
- Rajeshwari, P.; Dey, T.K. Novel HDPE nanocomposites containing aluminum nitride (nano) particles: Micro-structural and nano-mechanical properties correlation. Mater. Chem. Phys. 2017, 190, 175–186. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Composites: Part A Effects of aluminium nitride inclusions on thermal and electrical properties of epoxy and polypropylene: An experimental investigation. Compos. Part A 2014, 63, 51–58. [Google Scholar] [CrossRef]
- Ma, A.; Gu, J.; Chen, W. Thermal conductivity Polypropylene/Aluminum Nitride Composites. In Advanced Materials Research; Trans Tech Publications: Zürich, Switzerland, 2011; pp. 1577–1580. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Zhang, J.; Yang, Z. Thermal Conductivity and Mechanical Properties of Aluminum Nitride Filled Linear Low-Density Polyethylene Composites. Polym. Eng. Sci. 2009, 49, 1030–1034. [Google Scholar] [CrossRef]
- Kim, K.; Ju, H.; Kim, J. Pyrolysis behavior of polysilazane and polysilazane-coated-boron nitride for high thermal conductive composite. Compos. Sci. Technol. 2017, 141, 1–7. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. Mechanical properties and structure development in poly(butylene succinate)/organo-montmorillonite nanocomposites under uniaxial cold rolling. Express Polym. Lett. 2011, 5, 93–103. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Wu, Y.; Gao, X.; Wang, J.; He, W.; Silberschmidt, V.V.; Xu, H. Effects of Surface-Functionalized Aluminum Nitride on Thermal, Electrical, and Mechanical Behaviors of Polyarylene Ether Nitrile-Based Composites. Polym. Compos. 2016, 37, 3033–3041. [Google Scholar] [CrossRef]
- Choudhury, M.; Mohanty, S.; Nayak, S.K. Effect of Surface Modification of Aluminum Nitride on Electrical and Thermal Characterizations of Thermosetting Polymeric Nanocomposites. Polym. Compos. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Ramdani, N.; Derradji, M.; Wang, J.; Liu, W.; Ramdani, N.; Derradji, M.; Wang, J.; Liu, W.; Mokhnache, E. Effects of aluminium nitride silane-treatment on the mechanical and thermal properties of polybenzoxazine matrix Effects of aluminium nitride silane-treatment on the mechanical and thermal properties of polybenzoxazine matrix. Rubber Compos. 2016, 45, 72–80. [Google Scholar] [CrossRef]
- Tang, H.; Sukachonmakul, T.; Tai, M.; Hsiang, Y. Applied Surface Science Surface modification of aluminum nitride by polysilazane and its polymer-derived amorphous silicon oxycarbide ceramic for the enhancement of thermal conductivity in silicone rubber composite. Appl. Surf. Sci. 2014, 292, 928–936. [Google Scholar]
- Kim, K.; Ryu, S.; Kim, J. Melt-processable aggregated boron nitride particle via polysilazane coating for thermal conductive composite. Ceram. Int. 2017, 43, 2441–2447. [Google Scholar] [CrossRef]
- Chiu, H.; Sukachonmakul, T.; Wang, C.; Wattanakul, K.; Guo, M.; Wang, Y. Fabrication and characterization of silicon-based ceramic/aluminum nitride as thermally conductive hybrid fi ller in silicone rubber composite. Mater. Chem. Phys. 2014, 147, 11–16. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. Compatibility of biodegradable poly(lactic acid) (PLA) and poly(butylene succinate) (PBS) blends for packaging application. Korea Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Althues, H.; Henle, J.; Kaskel, S. Functional inorganic nanofillers for transparent polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef]
- Yoo, E.S.; Im, S.S. Melting Behavior of poly(butylene succinate) during Heating Scan by DSC. J. Polym. Sci. Part B Polym. Phys. 1999, 1357–1366. [Google Scholar] [CrossRef]
- Ning, N.; Deng, H.; Luo, F.; Wang, K.; Zhang, Q.; Chen, F.; Fu, Q. Composites: Part B Effect of whiskers nucleation ability and shearing function on the interfacial crystal morphology of polyethylene (PE)/raw whiskers composites. Compos. Part B 2011, 42, 631–637. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(l-lactic acid)/TiO2 Nanocomposites: Thermal Properties and Degradation. J. Appl. Polym. Sci. 2011, 123, 2187–2193. [Google Scholar] [CrossRef]
- Lee, G.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites lled with hybrid ller. Mater. Sci. 2006, 37, 727–734. [Google Scholar]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Friedrich, K. Improvement of tensile properties of nano-SiO2/PP composites in relation to percolation mechanism. Polymer 2001, 42, 3301–3304. [Google Scholar] [CrossRef]
- Someya, Y.; Nakazato, T.; Teramoto, N.; Shibata, M. Thermal and Mechanical Properties of poly(butylene succinate) Nanocomposites with Various Organo-Modified Montmorillonites. Polymer 2003. [Google Scholar] [CrossRef]

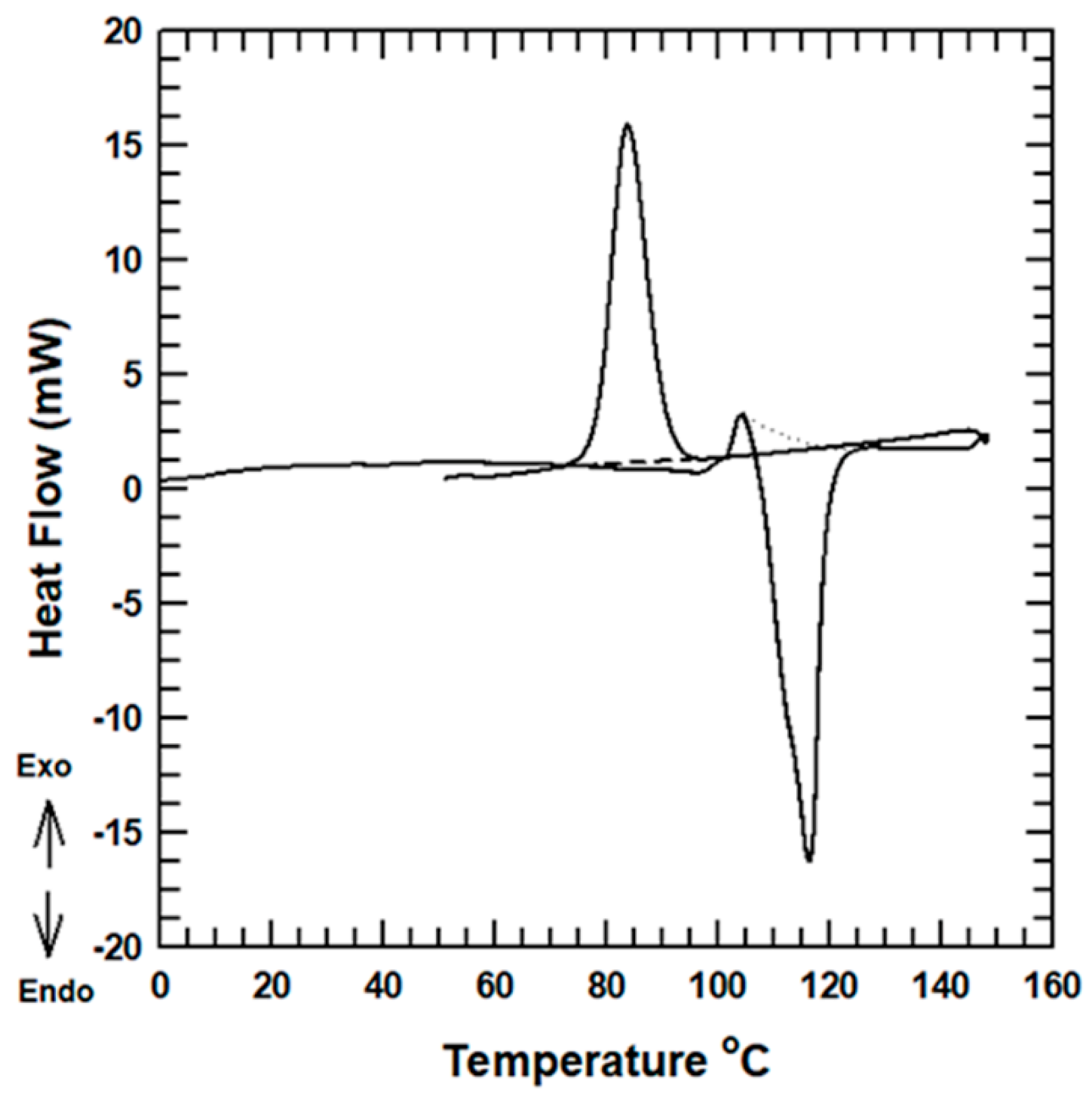



| Sample | Melting Pt. (°C) | Crystallization Pt. (°C) | ∆Hm | ∆HC | XC (%) |
|---|---|---|---|---|---|
| Neat PBS | 116.395 | 83.9 | 96.46 | 81.83 | 13.9 |
| R- PBS | 116.1 | 84.98 | 71.716 | 63.26 | 8.45 |
| A- PBS | 116.16 | 84.39 | 79.38 | 69.202 | 10.17 |
| V- PBS | 116.275 | 83.975 | 86.6 | 76.92 | 9.67 |
| P- PBS | 116.263 | 84.8 | 73.85 | 66.57 | 7.278 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lule, Z.; Kim, J. Surface Modification of Aluminum Nitride to Fabricate Thermally Conductive poly(Butylene Succinate) Nanocomposite. Polymers 2019, 11, 148. https://doi.org/10.3390/polym11010148
Lule Z, Kim J. Surface Modification of Aluminum Nitride to Fabricate Thermally Conductive poly(Butylene Succinate) Nanocomposite. Polymers. 2019; 11(1):148. https://doi.org/10.3390/polym11010148
Chicago/Turabian StyleLule, Zelalem, and Jooheon Kim. 2019. "Surface Modification of Aluminum Nitride to Fabricate Thermally Conductive poly(Butylene Succinate) Nanocomposite" Polymers 11, no. 1: 148. https://doi.org/10.3390/polym11010148
APA StyleLule, Z., & Kim, J. (2019). Surface Modification of Aluminum Nitride to Fabricate Thermally Conductive poly(Butylene Succinate) Nanocomposite. Polymers, 11(1), 148. https://doi.org/10.3390/polym11010148