Recent Progress in Thermoelectric Materials Based on Conjugated Polymers
Abstract
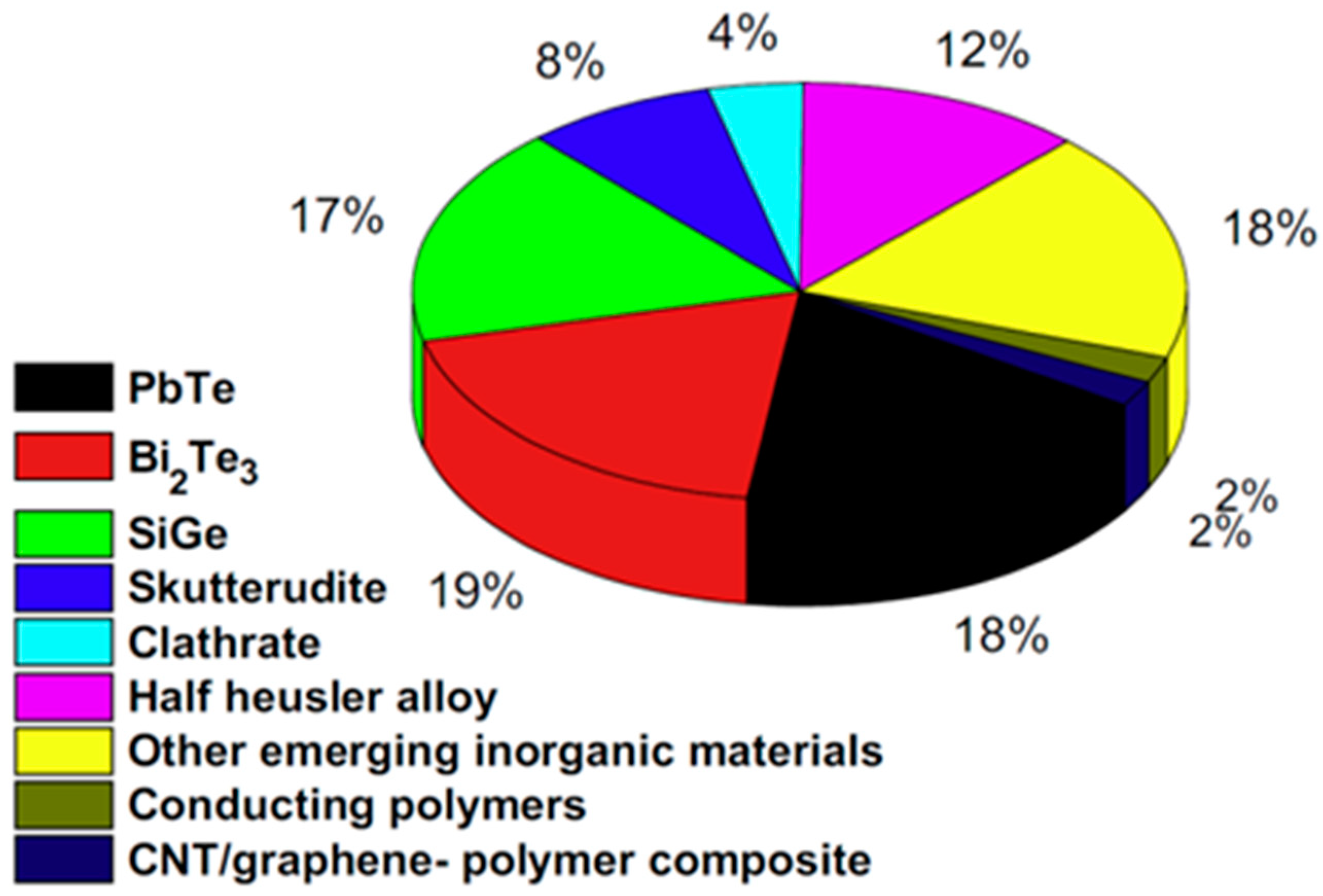
1. Introduction
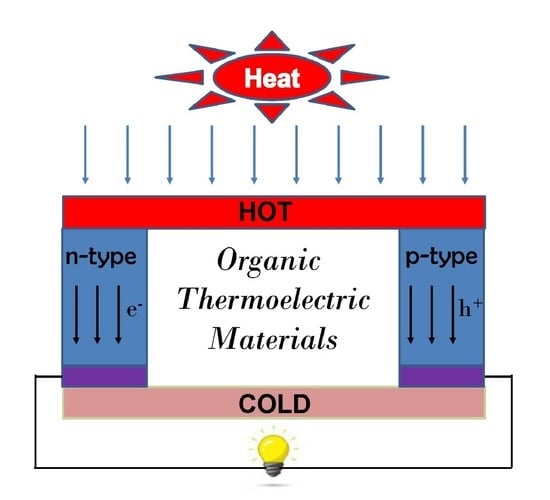
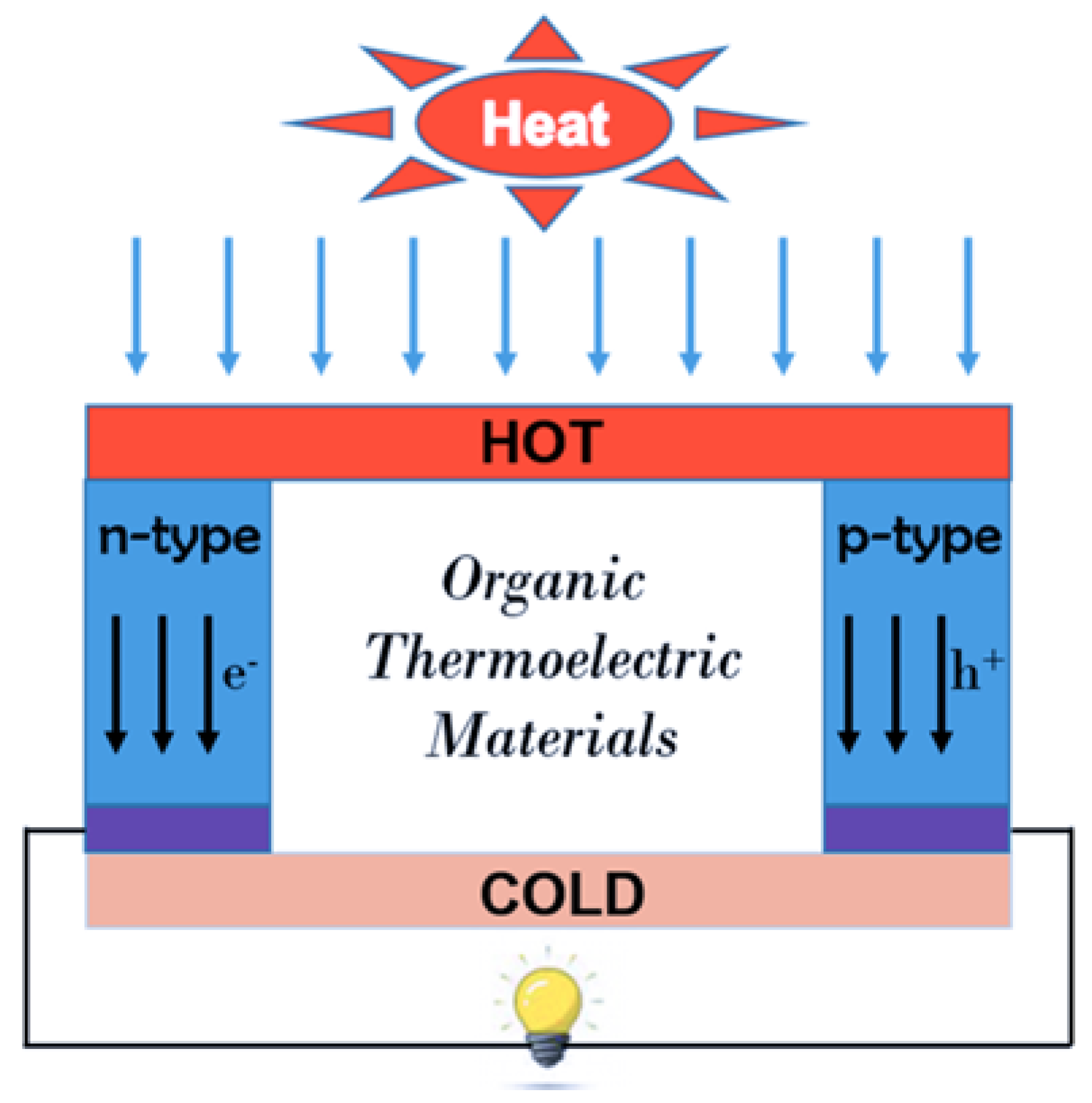
2. Theory of TE Devices
- T1: Hot side temperature;
- T2: Cold side temperature;
- k: Thermal Conductivity;
- RL: Load resistance;
- R: Internal resistance of the semiconductor.
- S: Seebeck Coefficient;
- σ: Conductivity;
- T: Temperature;
- k: Thermal Conductivity.
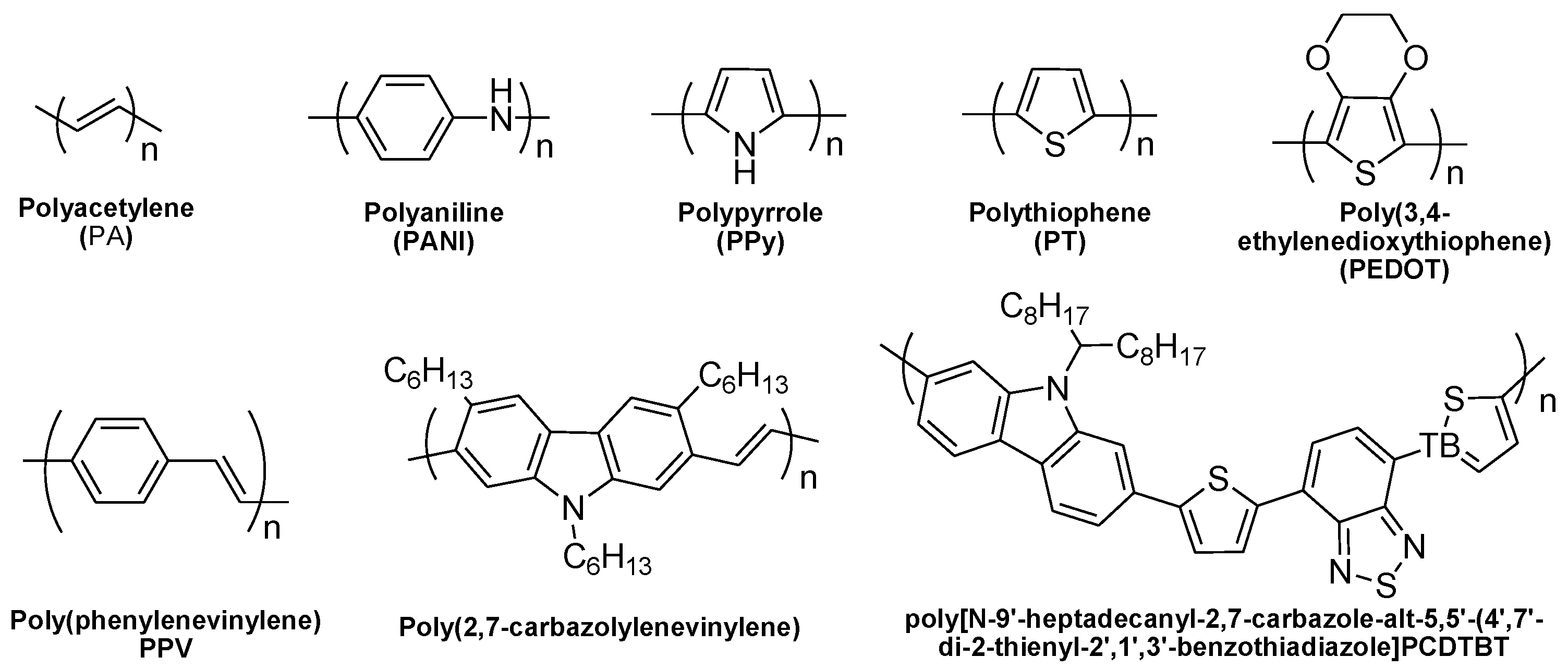
3. p-Type TE-Conducting Polymers
3.1. Polyacetylene (PA)
3.2. Polyaniline (PANI)
3.3. Polypyrrole
3.4. Polythiophene-Based Derivatives
3.5. Other p-Type TE Polymers
4. TE Properties of n-Type Polymers
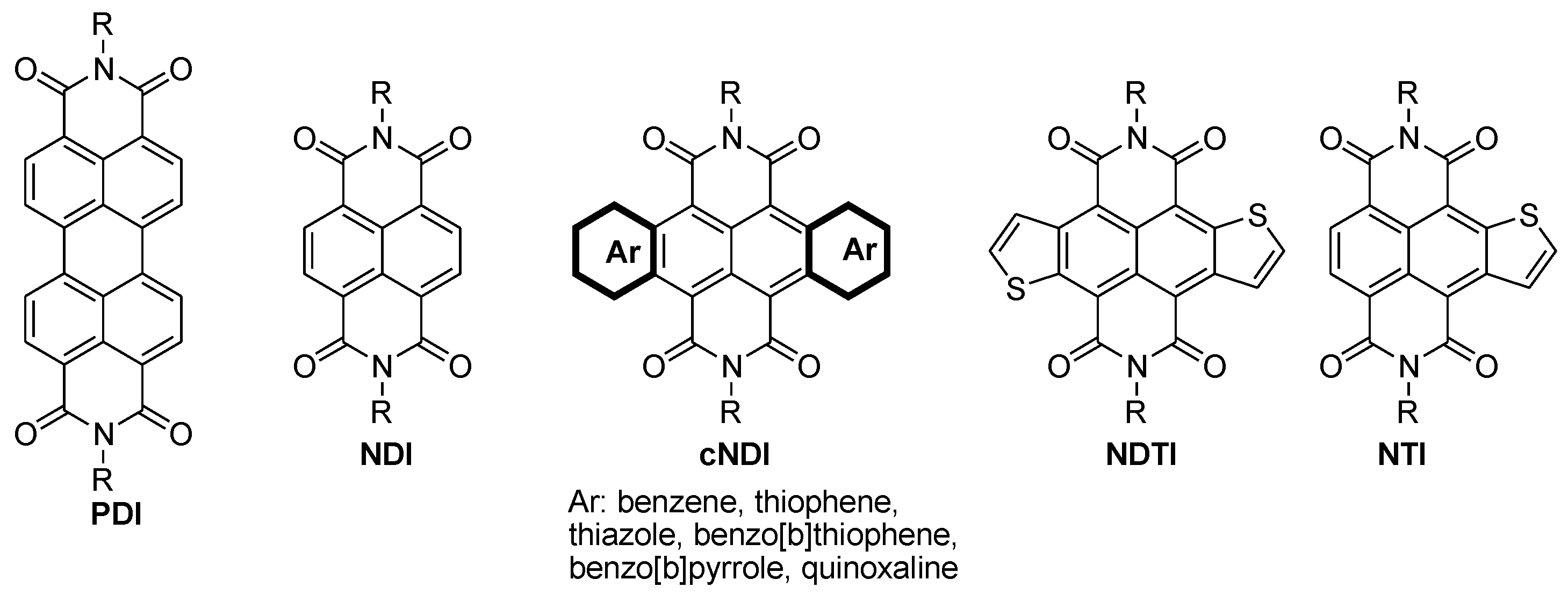
4.1. Building Units for n-Type Polymers
4.2. Poly(nickel-ethylenetetrathiolate) (KxNiett)
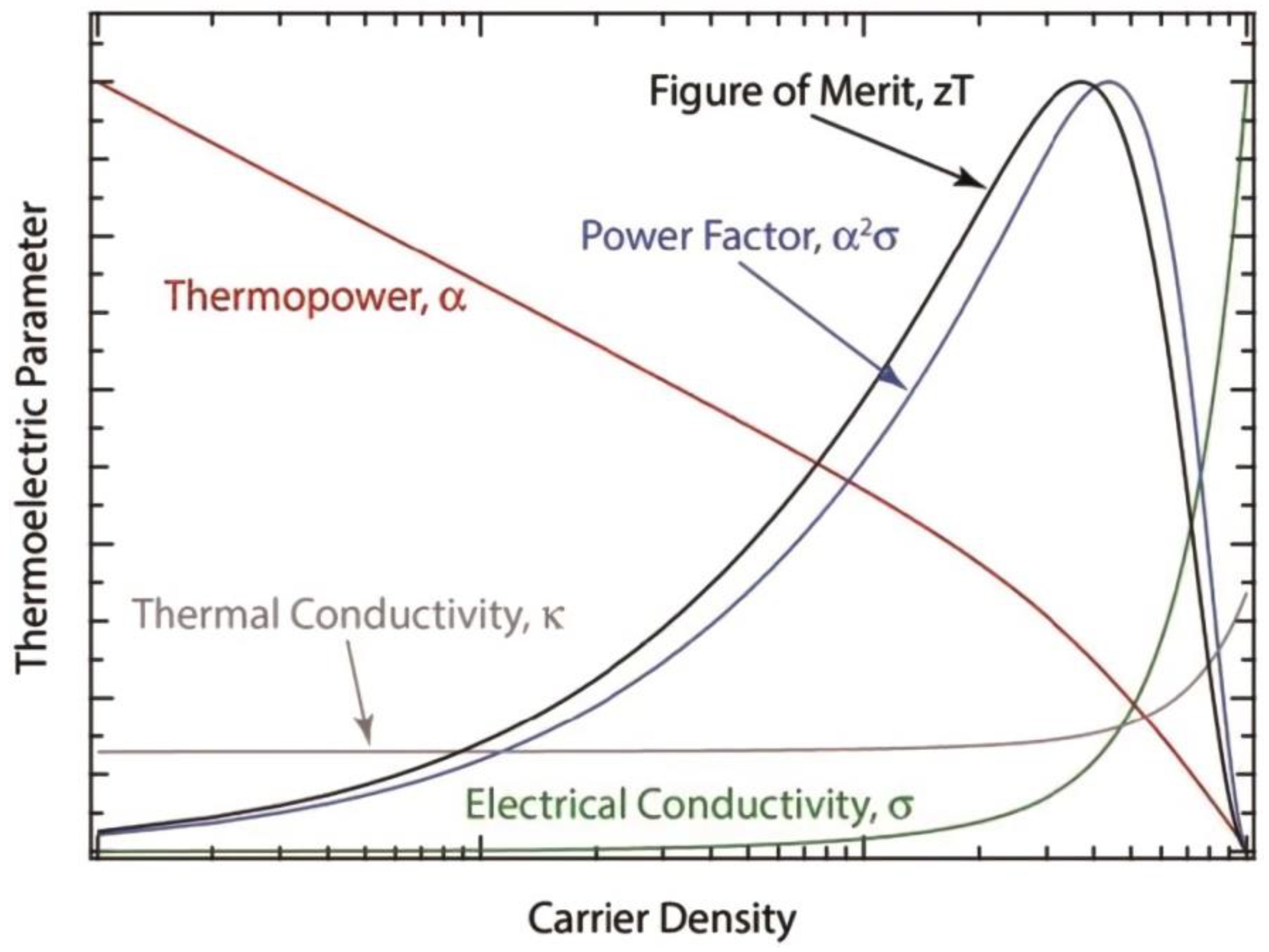
5. Strategies for Enhancing the ZT of Organic TE Materials
5.1. Molecular Structure Modification
5.2. Nanocomposites of Organic/Inorganic Hybridization
5.3. Organic Polymer Doped with Fillers
6. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Goldsmid, H.J.; Douglas, R.W.D. The use of semiconductors in thermoelectric refrigeration. Br. J. Appl. Phys. 1954, 5, 386. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Di, C.A.; Zhu, D. Metal-organic complexes-towards promising organic thermoelectric materials. Synth. Met. 2017, 225, 22–30. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials for Sustainable Energy; Nature Publishing Group: London, UK, 2008; Volume 7, p. 105. [Google Scholar]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R.; Silvola, E.; Colpitts, T.; O’quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Recent Progress on PEDOT-Based Thermoelectric Materials. Materials 2015, 8, 732–750. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429. [Google Scholar] [CrossRef]
- Asano, H.; Sakura, N.; Oshima, K.; Shiraishi, Y.; Toshima, N. Development of ethenetetrathiolate hybrid thermoelectric materials consisting of cellulose acetate and semiconductor nanomaterials. Jpn. J. Appl. Phys. 2016, 55, 02BB02. [Google Scholar] [CrossRef]
- Gayner, C.; Kar, K.K. Recent advances in thermoelectric materials. Prog. Mater. Sci. 2016, 83, 330–382. [Google Scholar] [CrossRef]
- Bubnova, O. Thermoelectric Properties of Conducting Polymer. Ph.D. Thesis, Linköping University, Norrköping, Sweden, 2013. [Google Scholar]
- Sherman, B.; Heikes, R.R.; Ure, R.W., Jr. Calculation of Efficiency of Thermoelectric Devices. J. Appl. Phys. 1960, 31, 1–16. [Google Scholar] [CrossRef]
- Ma, Q.; Fang, H.; Zhang, M. Theoretical analysis and design optimization of thermoelectric generator. Appl. Therm. Eng. 2017, 127, 758–764. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Wu, L. The influence of Thomson effect on the maximum power output and maximum efficiency of a thermoelectric generator. J. Appl. Phys. 1996, 79, 8823–8828. [Google Scholar] [CrossRef]
- Ruciński, A.; Rusowicz, A. Thermoelectric Generation of Current—Theoretical and Experimental Analysis. Arch. Thermodyn. 2017, 38, 3–13. [Google Scholar] [CrossRef]
- Fleurial, J.-P. Thermoelectric Power generation materials: Technology and application opportunities. JOM 2009, 61, 79–85. [Google Scholar] [CrossRef]
- Seebeck, T.J. Magnetic polarization of metals and minerals. Abh. Dtsch. Akad. Wiss. Berl. 1822, 265, 289–346. [Google Scholar]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.; Lee, H.; Wang, D.; Ren, Z.; Fleurial, J.-P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 35. [Google Scholar] [CrossRef]
- Chen, X.P.; Jiang, J.K.; Liang, Q.H.; Yang, N.; Ye, H.Y.; Cai, M.; Shen, L.; Yang, D.G.; Ren, T.L. First-principles study of the effect of functional groups on polyaniline backbone. Sci. Rep. 2015, 5, 16907. [Google Scholar] [CrossRef]
- Mao, R.; Kong, B.D.; Kim, K.W.; Jayasekera, T.; Calzolari, A.; Buongiorno Nardelli, M. Phonon engineering in nanostructures: Controlling interfacial thermal resistance in multilayer-graphene/dielectric heterojunctions. Appl. Phys. Lett. 2012, 101, 113111. [Google Scholar] [CrossRef]
- Landry, E.S.; McGaughey, A.J. Thermal boundary resistance predictions from molecular dynamics simulations and theoretical calculations. Phys. Rev. B 2009, 80, 165304. [Google Scholar] [CrossRef]
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A Review on Organic Polymer-Based Thermoelectric Materials. J. Polym. Environ. 2017, 25, 1208–1218. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. B 1931, 37, 405426. [Google Scholar] [CrossRef]
- Arnas, O.A.; Miller, D.L. On an irreversible thermodynamic analysis of thermoelectric devices. In Proceedings of the 2nd International Conference on Thermoelectric Energy Conversion, Arlington, VA, USA, 22–24 March 1978; pp. 36–40. [Google Scholar]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the Doping Method on Conductivity and Thermopower in Semiconducting Polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic Thermoelectric Materials: Emerging Green Energy Materials Converting Heat to Electricity Directly and Efficiently. Adv. Mater. 2014, 26, 6829. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Glaudell, A.M.; Peterson, K.A.; Thomas, E.M.; O’Hara, K.A.; Lim, E.; Chabinyc, M.L. Morphology controls the thermoelectric power factor of a doped semiconducting polymer. Sci. Adv. 2017, 3, e1700434. [Google Scholar] [CrossRef]
- Mateeva, N.; Niculescu, H.; Schlenoff, J.; Testardi, L.R. Correlation of Seebeck coefficient and electric conductivity in polyaniline and polypyrrole. J. Appl. Phys. 1998, 83, 3111. [Google Scholar] [CrossRef]
- Kemp, N.T.; Kaiser, A.B.; Liu, C.J.; Chapman, B.; Mercier, O.; Carr, A.M.; Trodahl, H.J.; Buckley, R.G.; Partridge, A.C.; Lee, J.Y.; et al. Thermoelectric Power and Conductivity of Different Types of Polypyrrole. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 953. [Google Scholar] [CrossRef]
- Park, Y.W.; Han, W.K.; Choi, C.H.; Shirakawa, H. Metallic nature of heavily doped polyacetylene derivatives: Thermopower. Phys. Rev. B 1984, 30, 5847. [Google Scholar] [CrossRef]
- Lévesque, I.; Bertrand, P.O.; Blouin, N.; Leclerc, M.; Zecchin, S.; Zotti, G.; Ratcliffe, C.I.; Klug, D.D.; Gao, X.; Gao, F.; et al. Synthesis and Thermoelectric Properties of Polycarbazole, Polyindolocarbazole, and Polydiindolocarbazole Derivatives. Chem. Mater. 2007, 19, 2128. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914. [Google Scholar] [CrossRef]
- Yu, C.; Kim, Y.S.; Kim, D.; Grunlan, J.C. Thermoelectric Behavior of Segregated-Network Polymer Nanocomposites. Nano Lett. 2008, 8, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Hiroshige, Y.; Ookawa, M.; Toshima, N. High thermoelectric performance of poly(2,5-dimethoxyphenylenevinylene) and its derivatives. Synth. Met. 2006, 156, 1341–1347. [Google Scholar] [CrossRef]
- Wakim, S.; Aïch, B.R.; Tao, Y.; Leclerc, M. Charge transport, photovoltaic, and thermoelectric properties of poly(2,7-carbazole) and poly(indolo 3,2-b carbazole) derivatives. Polym. Rev. 2008, 48, 432–462. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Hu, X.; Boughton, R.I.; Zhao, S.; Li, Q.; Jiang, M. Structure and electronic transport properties of polyaniline=NaFe4P12 composite. Chem. Phys. Lett. 2002, 352, 185–190. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer–inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, Z.; Xu, W.; Zhu, D. A three-in-one improvement in thermoelectric properties of polyaniline brought by nanostructures. Synth. Met. 2010, 160, 2371–2376. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Li, H.; Yan, Y.; Zhang, Q. Synthesis and thermoelectric properties of hydrochloric acid-doped polyaniline. Synth. Met. 2010, 160, 1153–1158. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Şenkal, B.F.; Saraç, A. Electrical conductivity, thermoelectric power, and optical properties of organosoluble polyaniline organic semiconductor. J. Electron. Mater. 2008, 37, 930–934. [Google Scholar] [CrossRef]
- Toshima, N. Conductive polymers as a new type of thermoelectric material. Macromol. Symp. 2002, 186, 81–86. [Google Scholar] [CrossRef]
- Yoon, C.O.; Reghu, M.; Moses, D.; Cao, Y.; Heeger, A.J. Thermoelectricpower of doped polyaniline near the metal-insulator-transition. Synth. Met. 1995, 69, 273–274. [Google Scholar] [CrossRef]
- Wang, Z.H.; Scherr, E.M.; MacDiarmid, A.G.; Epstein, A.J. Transport and EPR studies of polyaniline-a quasi-one-dimensional conductor with 3-dimensional metallic states. Phys. Rev. B 1992, 45, 4190. [Google Scholar] [CrossRef]
- Park, Y.W.; Yoon, C.O.; Lee, C.H.; Shirakawa, H.; Suezaki, Y.; Akagi, K. Conductivity and thermoelectric-power of the newly processed polyacetylene. Synth. Met. 1989, 28, D27–D34. [Google Scholar] [CrossRef]
- Kaneko, H.; Ishiguro, T.; Takahashi, A.; Tsukamoto, J. Magnetoresistance and thermoelectric-power studies of metal-nonmetal transition in iodine-doped polyacetylene. Synth. Met. 1993, 57, 4900–4905. [Google Scholar] [CrossRef]
- Pukacki, W.; Płocharski, J.; Roth, S. Anisotropy of thermoelectricpower of stretch-oriented new polyacetylene. Synth. Met. 1994, 62, 253–256. [Google Scholar] [CrossRef]
- Park, E.B.; Yoo, J.S.; Choi, H.J.; Park, J.Y.; Park, Y.W.; Akagi, K.; Shirakawa, H. Positive thermoelectric-power of alkali-metal-doped polyacetylene. Synth. Met. 1995, 69, 61–64. [Google Scholar] [CrossRef]
- Yoon, C.O.; Na, B.C.; Park, Y.W.; Shirakawa, H.; Akagi, K. Thermoelectricpower and conductivity of the stretch-oriented polyacetylene film doped with MOCl5. Synth. Met. 1991, 41, 125–128. [Google Scholar] [CrossRef]
- Choi, E.S.; Suh, D.S.; Kim, G.T.; Kim, D.C.; Park, Y.W. Magneto thermoelectric power of the doped polyacetylene. Synth. Met. 1999, 101, 375–376. [Google Scholar] [CrossRef]
- Gao, X.; Uehara, K.; Klug, D.D.; Patchkovskii, S.; John, S.T.; Tritt, T.M. Theoretical studies on the thermopower of semiconductors and low-band-gap crystalline polymers. Phys. Rev. B 2005, 72, 125202. [Google Scholar] [CrossRef]
- Hiraishi, K.; Masuhara, A.; Nakanishi, H.; Oikawa, H.; Shinohara, Y. Evaluation of thermoelectric properties of polythiophene films synthesized by electrolytic polymerization. Jpn. J. Appl. Phys. 2009, 48, 071501. [Google Scholar] [CrossRef]
- Yue, R.; Chen, S.; Lu, B.; Liu, C.; Xu, J. Facile electrosynthesis and thermoelectric performance of electroactive free-standing polythieno[3,2-b]thiophene films. J. Solid State Electrochem. 2011, 15, 539–548. [Google Scholar] [CrossRef]
- Bao, L.; Cong, L.; Shan, L.; Jing, X.; Feng, J.; Yu, L.; Zhuo, Z. Thermoelectric performances of free-standing polythiophene and poly(3-methylthiophene) nanofilms. Chin. Phys. Lett. 2010, 27, 057201. [Google Scholar]
- Kemp, N.T.; Kaiser, A.B.; Trodahl, H.J.; Chapman, B.; Buckley, R.G.; Partridge, A.C.; Foot, P.J. Effect of ammonia on the temperature-dependent conductivity and thermopower of polypyrrole. J. Polym. Sci. B Polym. Phys. 2006, 44, 1331–1338. [Google Scholar] [CrossRef]
- Aïch, R.B.; Blouin, N.; Bouchard, A.; Leclerc, M. Electrical and Thermoelectric Properties of Poly(2,7-Carbazole) Derivatives. Chem. Mater. 2009, 21, 751. [Google Scholar] [CrossRef]
- Chang, K.C.; Jeng, M.S.; Yang, C.C.; Chou, Y.W.; Wu, S.K.; Thomas, M.A.; Peng, Y.C. The Thermoelectric Performance of Poly(3,4-ethylenedi oxythiophene)/Poly(4-styrenesulfonate) Thin Films. J. Electron. Mater. 2009, 38, 1182–1188. [Google Scholar] [CrossRef]
- Scholdt, M.; Do, H.; Lang, J.; Gall, A.; Colsmann, A.; Lemmer, U.; Koenig, J.D.; Winkler, M.; Boettner, H. Organic Semiconductors for Thermoelectric Applications. J. Electron. Mater. 2010, 39, 1589–1592. [Google Scholar] [CrossRef]
- Feng, J.; Jing, X.; Bao, L.; Yu, X.; Rong, H.; Lai, L. Thermoelectric performance of poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate). Chin. Phys. Lett. 2008, 25, 2202–2205. [Google Scholar] [CrossRef]
- Moses, D.; Chen, J.; Denenstein, A.; Kaveh, M.; Chung, T.C.; Heeger, A.J.; MacDiarmid, A.G.; Park, Y.W. Inter-Soliton Electron Hopping Transport in Trans-(CH)x. Solid State Commun. 1981, 40, 1007–1010. [Google Scholar] [CrossRef]
- Zuzok, R.; Kaiser, A.B.; Pukacki, W.; Roth, S. Thermoelectric power and conductivity of iodine-doped "new" polyacetylene. J. Chem. Phys. 1991, 95, 1270. [Google Scholar] [CrossRef]
- Park, Y.W.; Yoon, C.O.; Na, B.C.; Shirakawa, H.; Akagi, K. Metallic Properties of Transition Metal Halides Dped Polyacetylene: The Solution Liquid State. Synth. Met. 1991, 41, 27–32. [Google Scholar] [CrossRef]
- Pukacki, W. Anisotropy of thermoelectric power of stretch-oriented new polyacetylene. Synth. Met. 1994, 62, 253. [Google Scholar] [CrossRef]
- Yoon, C.O.; Reghu, M.; Moses, D.; Heeger, A.J.; Cao, Y.; Chen, T.A.; Wu, X.; Rieke, R.D. Hopping transport in doped conducting polymers in the insulating regime near the metal-insulator boundary: Polypyrrole, polyaniline and poly alkylthiophenes. Synth. Met. 1995, 75, 229. [Google Scholar] [CrossRef]
- Dubey, N.; Leclerc, M. Conducting Polymers: Efficient Thermoelectric Materials. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 467. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Xu, W.; Zhang, Q. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Pei, W.B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Choi, K.; Grunlan, J.C.; Yu, C. Improved Thermoelectric Behavior of Nanotube-Filled Polymer Composites with Poly(3,4-ethylenedioxythiophene) Poly(styrenesulfonate). ACS Nano 2010, 4, 513–523. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719. [Google Scholar] [CrossRef]
- Culebras, M.; Gómez, C.M.; Cantarero, A. Enhanced thermoelectric performance of PEDOT with different counter-ions optimized by chemical reduction. J. Mater. Chem. A 2014, 2, 10109–10115. [Google Scholar] [CrossRef]
- Jonas, F.; Heywang, G.; Werner, S. Novel Polythiophenes, Process for Their Preparation, and Their Use. Bayer AG German Patent DE 3813589A1, 22 April 1988. [Google Scholar]
- Kim, J.Y.; Kwon, M.H.; Min, Y.K.; Kwon, S.; Ihm, D.W. Self-Assembly and Crystalline Growth of Poly(3,4-ethylenedioxythiophene) Nanofilms. Adv. Mater. 2007, 19, 3501–3506. [Google Scholar] [CrossRef]
- Li, H.; DeCoster, M.E.; Ireland, R.M.; Song, J.; Hopkins, P.E.; Katz, H.E. Modification of the Poly(bisdodecylquaterthiophene) Structure for High and Predominantly Nonionic Conductivity with Matched Dopants. J. Am. Chem. Soc. 2017, 139, 11149–11157. [Google Scholar] [CrossRef]
- Boudreault, P.L.; Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s and related polymers. In Polyfluorenes; Ullrich, S., Dieter, N., Eds.; Springer: Berlin, Germany, 2008; pp. 99–124. [Google Scholar]
- Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s: Structure-Property Relationships. Acc. Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Fan, Z.; Cheng, H.; Guan, X.; Wang, C.; Sun, K.; Ouyang, J. Recent Development of Thermoelectric Polymers and Composites. Macromol. Rapid Commun. 2018, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Menke, T.; Ray, D.; Meiss, J.; Leo, K.; Riede, M. In-situ conductivity and Seebeck measurements of highly efficient n-dopants in fullerene C60. Appl. Phys. Lett. 2012, 100, 60. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.; Di, C.A.; Yan, T.W.; Zou, Y.; Zhou, X.; Zhu, D.; Wang, J.Y.; Pei, J. Toward High Performance n-Type Thermoelectric Materials by Rational Modification of BDPPV Backbones. J. Am. Chem. Soc. 2015, 137, 6979–6982. [Google Scholar] [CrossRef]
- Huang, D.; Yao, H.; Cui, Y.; Zou, Y.; Zhang, F.; Wang, C.; Shen, H.; Jin, W.; Zhu, J.; Diao, Y.; et al. Conjugated-Backbone Effect of Organic Small Molecules for n-Type Thermoelectric Materials with ZT over 0.2. J. Am. Chem. Soc. 2017, 139, 13013–13023. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Shi, K.; Wu, Y.; Lu, Z.Y.; Liu, H.Y.; Wang, J.Y.; Pei, J. Enhanced Molecular Packing of a Conjugated Polymer with High Organic Thermoelectric Power Factor. ACS Appl. Mater. Interfaces 2016, 8, 24737–24743. [Google Scholar] [CrossRef]
- Schlitz, R.A.; Brunetti, F.G.; Glaudell, A.M.; Miller, P.L.; Brady, M.A.; Takacs, C.J.; Hawker, C.J.; Chabinyc, M.L. Solubility-Limited Extrinsic n-Type Doping of a High Electron Mobility Polymer for Thermoelectric Applications. Adv. Mater. 2014, 26, 2825–2830. [Google Scholar] [CrossRef]
- Wang, Z.H.; Ichimura, K.; Dresselhaus, M.S.; Dresselhaus, G.; Lee, W.T.; Wang, K.A.; Eklund, P.C. Electronic transport properties of KxC70 thin films. Phys. Rev. B 1993, 48, 10657. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Ail, U.; Vagin, M.; Persson, P.O.; Andreasen, J.W.; Thiel, W.; Berggren, M.; Crispin, X.; Fazzi, D.; et al. Thermoelectric Properties of Solution-Processed n-Doped Ladder-Type Conducting Polymers. Adv. Mater. 2016, 28, 10764–10771. [Google Scholar] [CrossRef]
- Sun, Y.; Sheng, P.; Di, C.; Jiao, F.; Xu, W.; Qiu, D.; Zhu, D. Organic Thermoelectric Materials and Devices Based on p- and n-Type Poly(metal 1,1,2,2-ethenetetrathiolate)s. Adv. Mater. 2012, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qiu, L.; Tang, L.; Geng, H.; Wang, H.; Zhang, F.; Huang, D.; Xu, W.; Yue, P.; Guan, Y.S.; et al. Flexible n-Type High-Performance Thermoelectric Thin Films of Poly(nickel-ethylenetetrathiolate) Prepared by an Electrochemical Method. Adv. Mater. 2016, 28, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sheng, P.; Tu, Z.; Zhang, F.; Wang, J.; Geng, H.; Zou, Y.; Di, C.A.; Yi, Y.; Sun, Y.; et al. A two-dimensional pi-d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour. Nat. Commun. 2015, 6, 7408. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Thermoelectric properties of conducting polymers: The case of poly(3-hexylthiophene). Phys. Rev. B 2010, 82, 115454. [Google Scholar] [CrossRef]
- Schmidt, R.; Oh, J.H.; Sun, Y.S.; Deppisch, M.; Krause, A.M.; Radacki, K.; Braunschweig, H.; Könemann, M.; Erk, P.; Bao, Z.; et al. High-Performance Air-Stable n-Channel Organic Thin Film Transistors Based on Halogenated Perylene Bisimide Semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. [Google Scholar] [CrossRef] [PubMed]
- Nollau, A.; Pfeiffer, M.; Fritz, T.; Leo, K. Controlled n-type doping of a molecular organic semiconductor: Naphthalenetetracarboxylic dianhydride (NTCDA) doped with bis(ethylenedithio)-tetrathiafulvalene (BEDT-TTF). J. Appl. Phys. 2000, 87, 4340–4343. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Nakano, M.; Hu, J.Y.; Osaka, I.; Takimiya, K. Naphthodithiophenediimide (NDTI): Synthesis, structure, and applications. J. Am. Chem. Soc. 2013, 135, 11445–11448. [Google Scholar] [CrossRef] [PubMed]
- Banal, J.L.; Subbiah, J.; Graham, H.; Lee, J.K.; Ghiggino, K.P.; Wong, W.W. Electron deficient conjugated polymers based on benzotriazole. Polym. Chem. 2013, 4, 1077–1083. [Google Scholar] [CrossRef]
- Takimiya, K.; Nakano, M. Thiophene-Fused Naphthalene Diimides: New Building Blocks for Electron Deficient pi-Functional Materials. Bull. Chem. Soc. Jpn. 2018, 91, 121–140. [Google Scholar] [CrossRef]
- Gross, Y.M.; Trefz, D.; Tkachov, R.; Untilova, V.; Brinkmann, M.; Schulz, G.L.; Ludwigs, S. Tuning Aggregation by Regioregularity for High-Performance n-Type P(NDI2OD-T-2) Donor-Acceptor Copolymers. Macromolecules 2017, 50, 5353–5366. [Google Scholar] [CrossRef]
- Yang, C.Y.; Jin, W.L.; Wang, J.; Ding, Y.F.; Nong, S.; Shi, K.; Lu, Y.; Dai, Y.Z.; Zhuang, F.D.; Lei, T.; et al. Enhancing the n-Type Conductivity and Thermoelectric Performance of Donor-Acceptor Copolymers through Donor Engineering. Adv. Mater. 2018, 30, 1802850. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Lim, G.; Park, B.; Reichmanis, E. Control of Molecular Ordering, Alignment, and Charge Transport in Solution-Processed Conjugated Polymer Thin Films. Polym. Adv. Technol. 2017, 9, 212. [Google Scholar] [CrossRef]
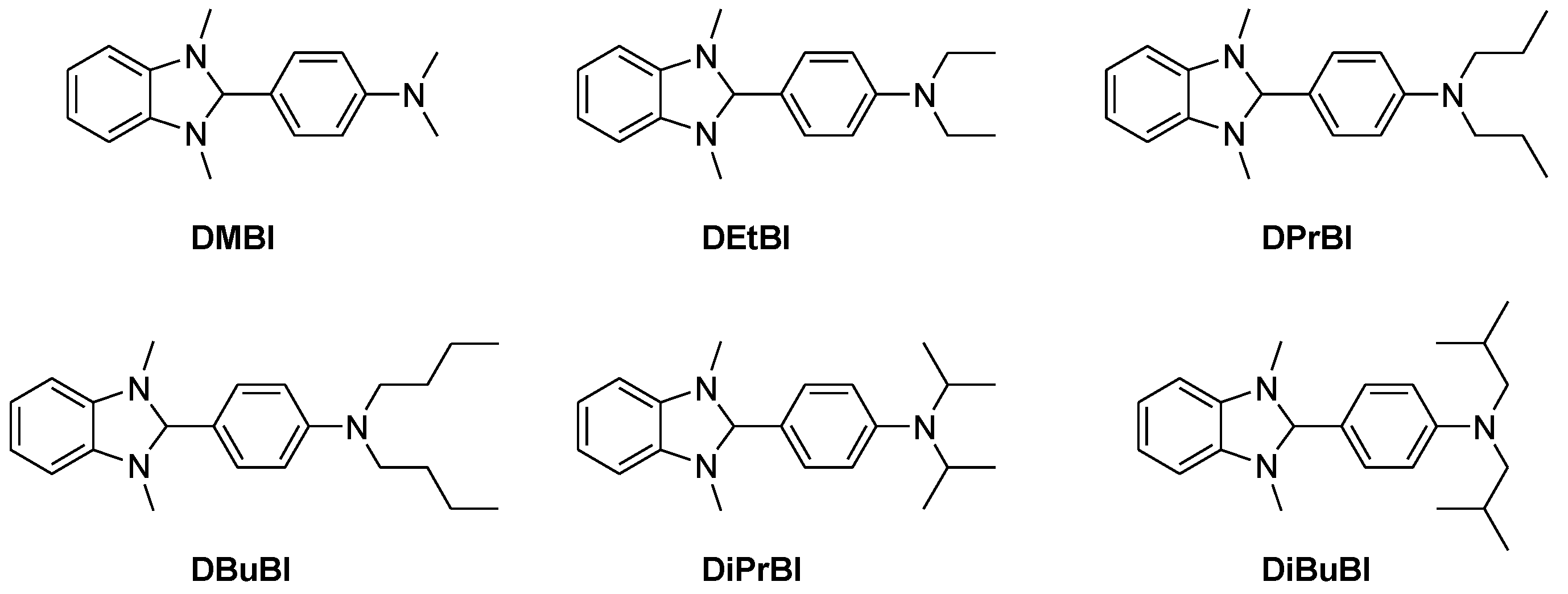
- Saglio, B.; Mura, M.; Massetti, M.; Scuratti, F.; Beretta, D.; Jiao, X.; McNeill, C.R.; Sommer, M.; Famulari, A.; Lanzani, G.; et al. N-Alkyl substituted 1H-benzimidazoles as improved n-type dopants for a naphthalene-diimide based copolymer. J. Mater. Chem. A 2018, 6, 15294–15302. [Google Scholar] [CrossRef]
- Tietze, M.L.; Benduhn, J.; Pahner, P.; Nell, B.; Schwarze, M.; Kleemann, H.; Krammer, M.; Zojer, K.; Vandewal, K.; Leo, K. Elementary steps in electrical doping of organic semiconductors. Nat. Commun. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Liu, J.; Ye, G.; Zee, B.V.; Dong, J.; Qiu, X.; Liu, Y.; Portale, G.; Chiechi, R.C.; Koster, L.J. N-Type Organic Thermoelectrics of Donor-Acceptor Copolymers: Improved Power Factor by Molecular Tailoring of the Density of States. Adv. Mater. 2018, 30, 1804290. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, L.; Alessandri, R.; Qiu, X.; Portale, G.; Dong, J.; Talsma, W.; Ye, G.; Sengrian, A.A.; Souza, P.C.; et al. Enhancing Molecular n-Type Doping of Donor-Acceptor Copolymers by Tailoring Side Chains. Adv. Mater. 2018, 30, 1704630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakano, M.; Michinobu, T.; Kiyota, Y.; Mori, T.; Takimiya, K. Naphthodithiophenediimide-Benzobisthiadiazole-Based Polymers: Versatile n-Type Materials for Field-Effect Transistors and Thermoelectric Devices. Macromolecules 2017, 50, 857–864. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, J.; Alessandri, R.; Qiu, X.; Koopmans, M.; Havenith, R.W.; Marrink, S.J.; Chiechi, R.C.; Koster, L.J.; Hummelen, J.C. Enhancing doping efficiency by improving host-dopant miscibility for fullerene-based n-type thermoelectrics. J. Mater. Chem. A 2017, 5, 21234–21241. [Google Scholar] [CrossRef]
- Shi, W.; Wu, G.; Hippalgaonkar, K.; Wang, J.S.; Xu, J.; Yang, S.W. Poly(nickel-ethylenetetrathiolate) and Its Analogs: Theoretical Prediction of High-Performance Doping-Free Thermoelectric Polymers. J. Am. Chem. Soc. 2018, 140, 13200–13204. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, M.A.; Sahamir, S.R.; Datta, R.S.; Long, B.D.; Sabri, M.; Faizul, M.; Mohd Said, S. A review on the fabrication of polymer-based thermoelectric materials and fabrication methods. Sci. World J. 2013, 2013, 713640. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Hayashi, T.; Kim, Y.A.; Muramatsu, H. Development and Application of Carbon Nanotubes. Jpn. J. Appl. Phys. 2006, 45, 4883. [Google Scholar] [CrossRef]
- He, M.; Qiu, F.; Lin, Z. Towards high-performance polymer-based thermoelectric materials. Energy Environ. Sci. 2013, 6, 1352–1361. [Google Scholar] [CrossRef]

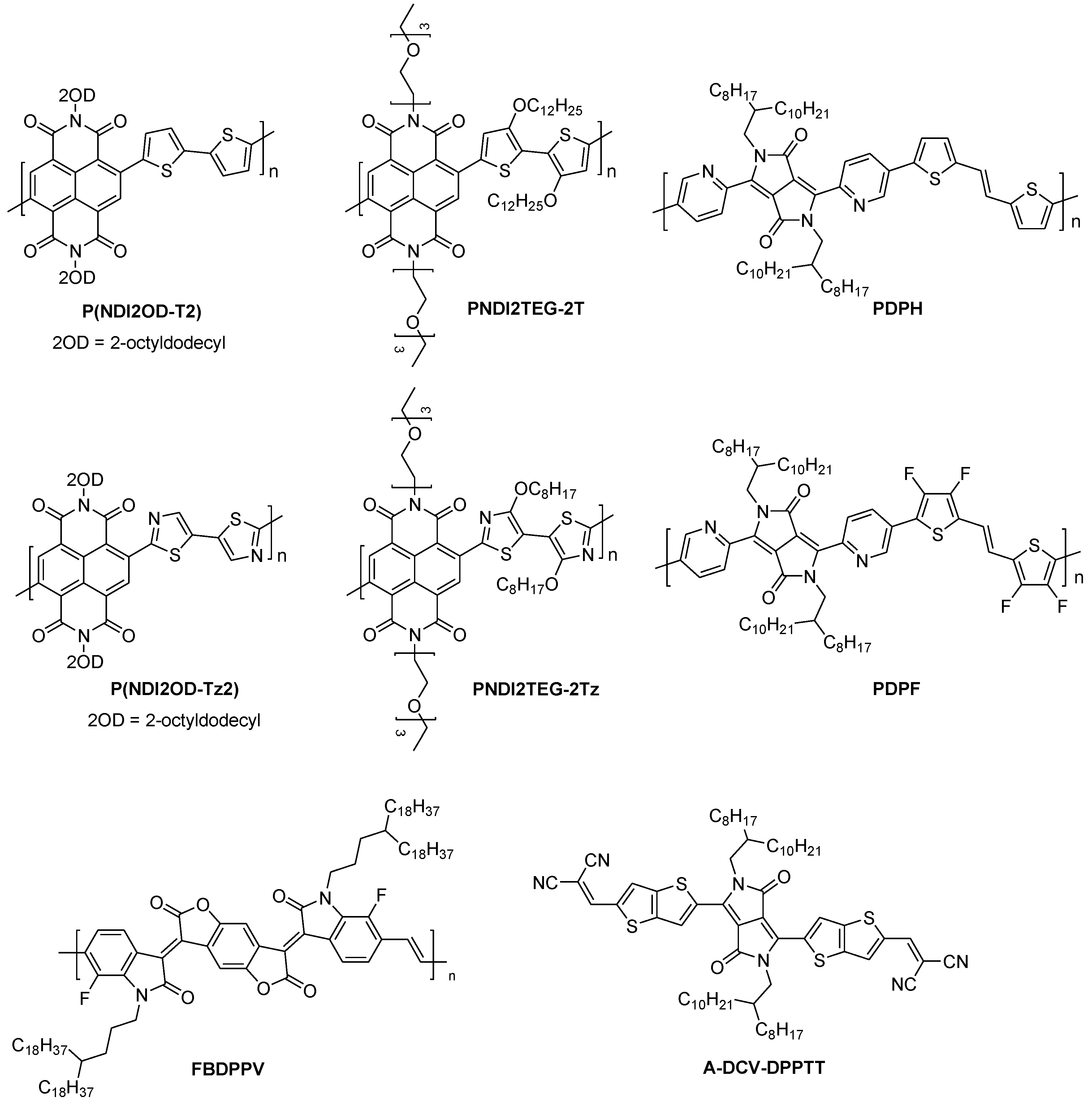
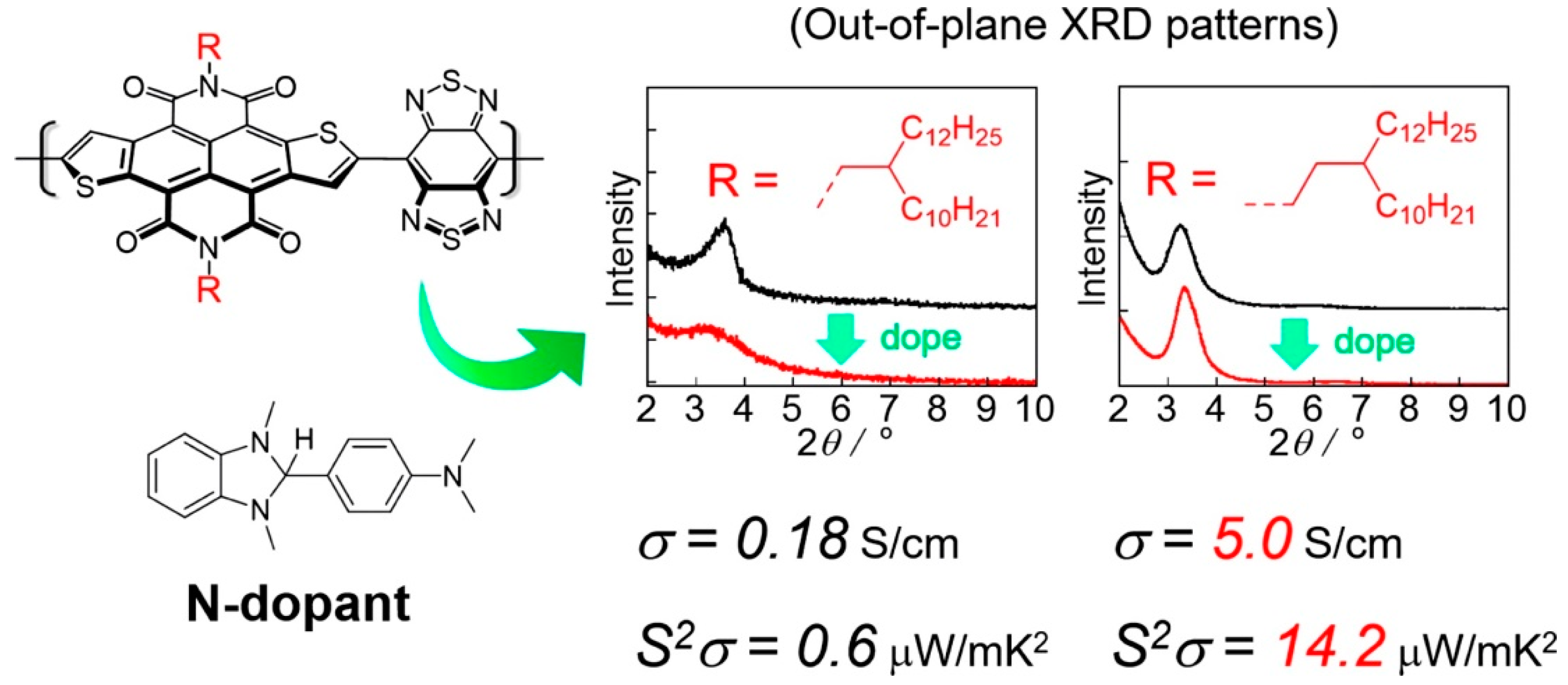
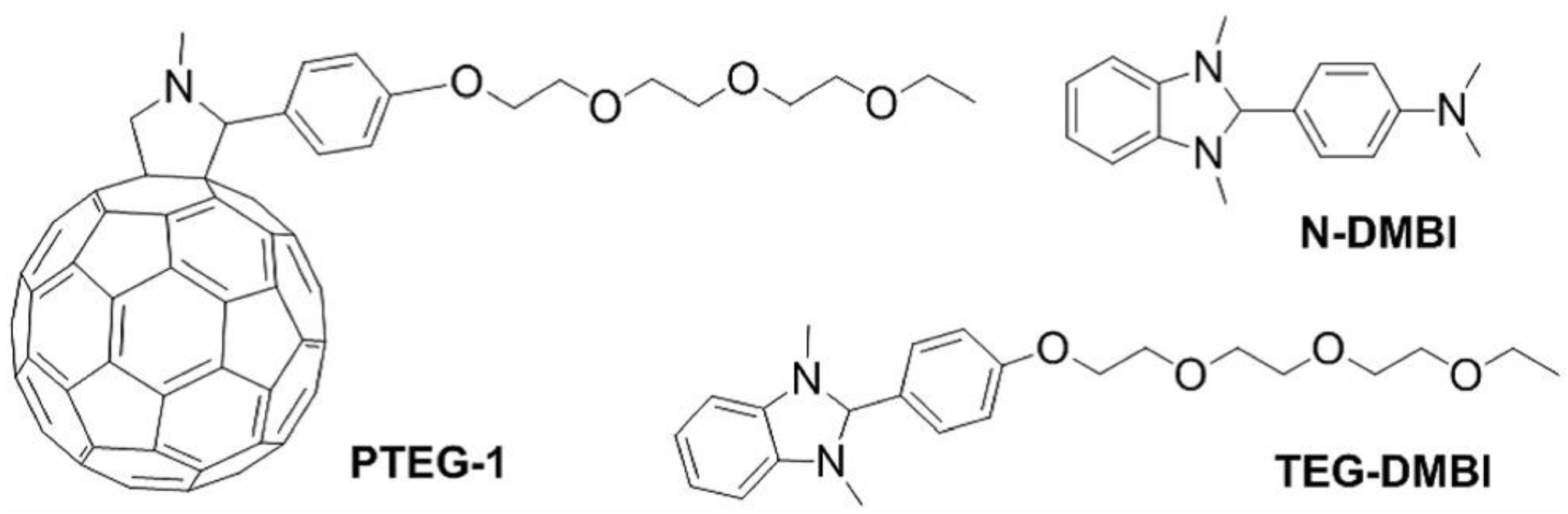
| Polymer | S μV/K | σ S/cm | k W/mK | ZTmax | PFmax μW/m K2 | Ref. |
|---|---|---|---|---|---|---|
| PANI | −16–225 | 10−7–320 | 0.02–0.542 | 1.1 × 10−2 at 423 K | [40,41,42,43,44,45] | |
| PA | −0.5–1077 | 1.53 × 10−3–2.85 × 104 | [46,47,48,49,50,51] | |||
| PT | 10–100 | 10−2–103 | 0.028–0.17 | 2.9 × 10−2 at 250 K | [52,53,54,55] | |
| PPy | −1–40 | 0–340 | 0.2 | 3 × 10−2 at 423 K | [31,56] | |
| PC | 4.9–600 | 4.0 × 10−5–5 × 10−2 | 19 | [33,57] | ||
| PEDOT:PSS | 8–888 | 0.06–945 | 0.34 | 1.0 × 10−2 at 300 K | [43,44,58,59,60] | |
| PEDOT-Tos | 40–780 | 6 × 10−2–300 | 0.37 | 0.25 at RT | [8] |
| Polymer | Dopant | S [μV·K−1] | σ [S·cm−1] | k [W·m−1·K−1] | PF [μW·m−1·K−2] | ZT | Ref |
|---|---|---|---|---|---|---|---|
| PA | Bu4N | −43.5 | 5 | - | 1 | - | [61] |
| C60 | Cr2(hpp)4 | −175 | 4 | 12 | [79] | ||
| FBDPPV (Figure 9) | DMBI | −141 | 14 | - | 28 | - | [80] |
| A-DCV-DPPTT (Figure 9) | 105 | 0.11 | [81] | ||||
| FBDPPV (Figure 9) | DMBI | −210 | 6.2 | - | 25.5 | - | [82] |
| P(NDIOD-T2) | N-DMBI | −850 | 0.008 | - | 0.6 | - | [83] |
| P(NDIOD-T2) | N-DPBI | −770 | 0.004 | - | 0.2 | - | [83] |
| KxC70 | −22.5 | 550 | 28 | [84] | |||
| BBL | TDAE | −101 | 0.42 | - | 0.43 | - | [85] |
| Poly[KxNi-ett] | N. A | −122 | 40 | −0.2 | 66 | −0.1 | [86] |
| KxNi-ett | N. A | −90 | 210 | 0.4–0.5 | 170 | 0.30 | [87] |
| CuBHT | N. A | −4 to −10 | 750–1580 | - | N. A. | - | [88] |
| P3HT | Doped with PF6 | 39 | 0.843 | 0.14 | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.-J.; Zhang, H.-L.; Zhang, Q. Recent Progress in Thermoelectric Materials Based on Conjugated Polymers. Polymers 2019, 11, 107. https://doi.org/10.3390/polym11010107
Yao C-J, Zhang H-L, Zhang Q. Recent Progress in Thermoelectric Materials Based on Conjugated Polymers. Polymers. 2019; 11(1):107. https://doi.org/10.3390/polym11010107
Chicago/Turabian StyleYao, Chang-Jiang, Hao-Li Zhang, and Qichun Zhang. 2019. "Recent Progress in Thermoelectric Materials Based on Conjugated Polymers" Polymers 11, no. 1: 107. https://doi.org/10.3390/polym11010107
APA StyleYao, C.-J., Zhang, H.-L., & Zhang, Q. (2019). Recent Progress in Thermoelectric Materials Based on Conjugated Polymers. Polymers, 11(1), 107. https://doi.org/10.3390/polym11010107