Detecting Glucose Levels in Blood Plasma and Artificial Tear by Au(I) Complex on the Carbopol Polymer: A Microfluidic Paper-Based Method
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Materials
2.2. The Synthesis of (AuC2C6H4OMe)(Ph2(C6H4)3PPh2) (Complex B5)
2.3. Standard Glucose Detection
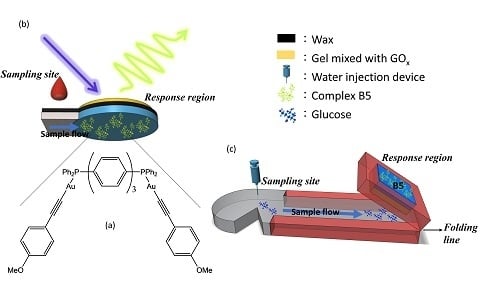
2.4. Fabrication of μPADs
2.5. Determination of Blood Glucose
2.6. Statistical Analysis
2.7. Determination of Tear Glucose
3. Results and Discussion
3.1. Synthesis and Structural Description of B5
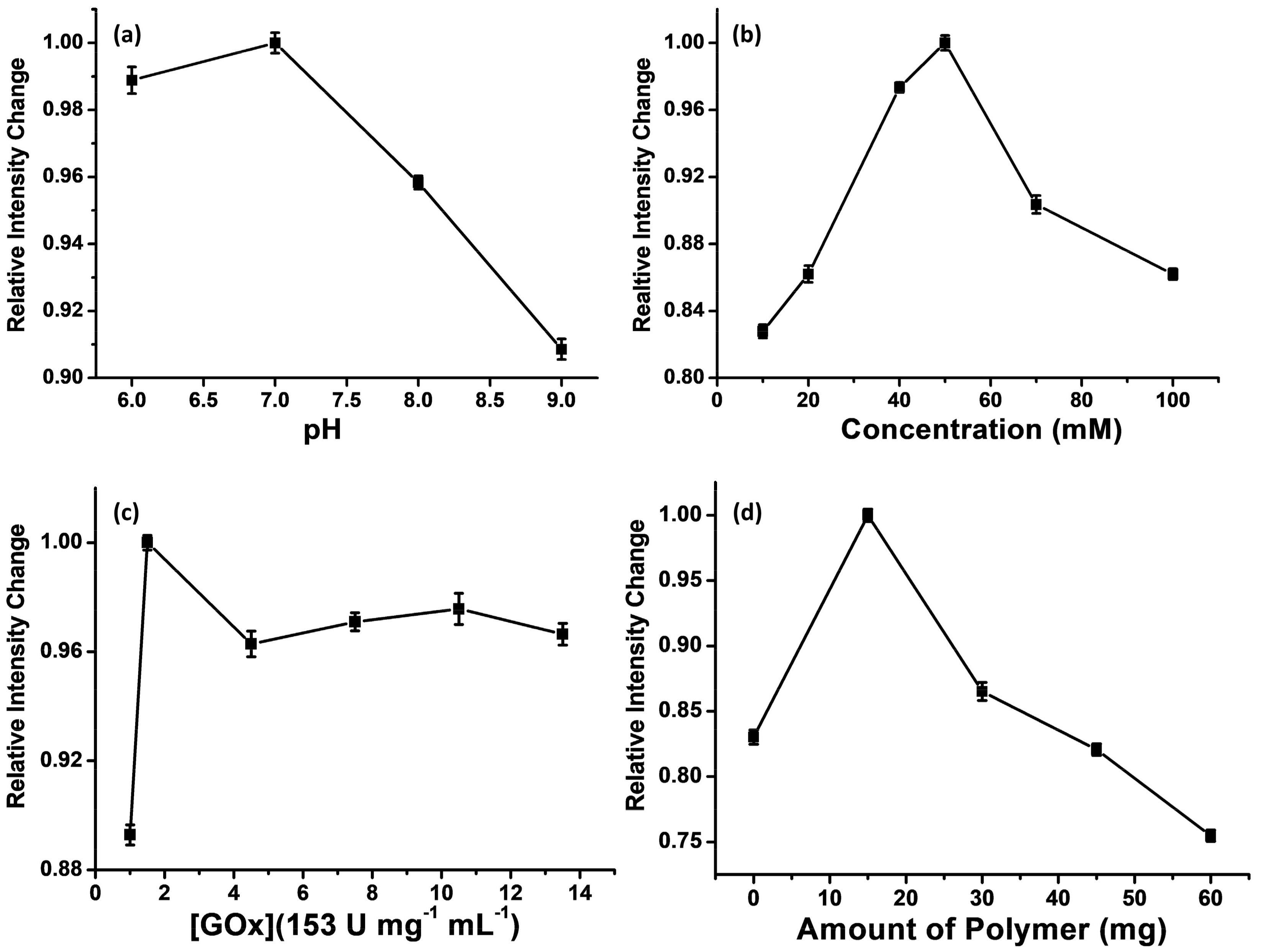
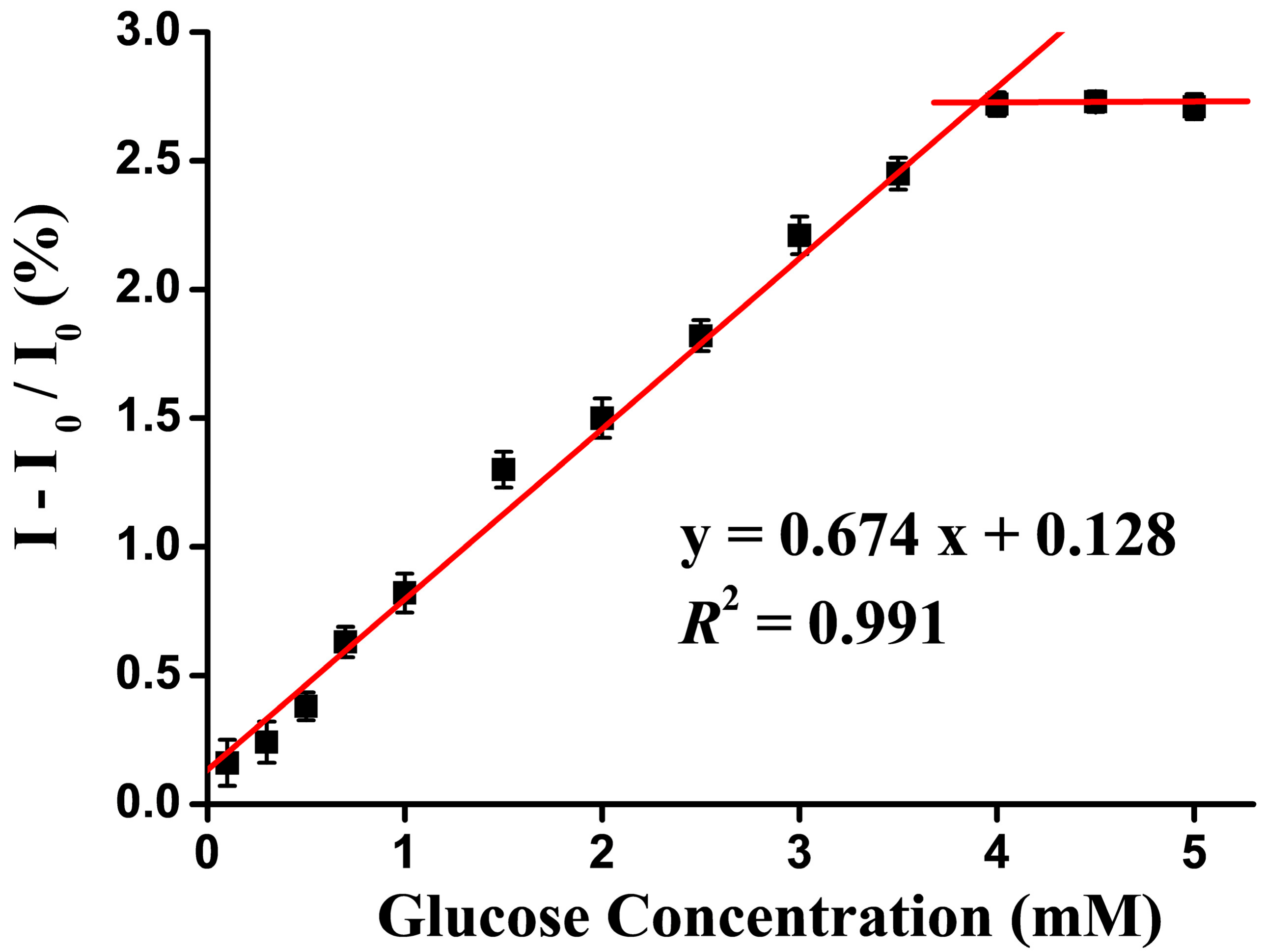
3.2. Optimization of the Sensing Method
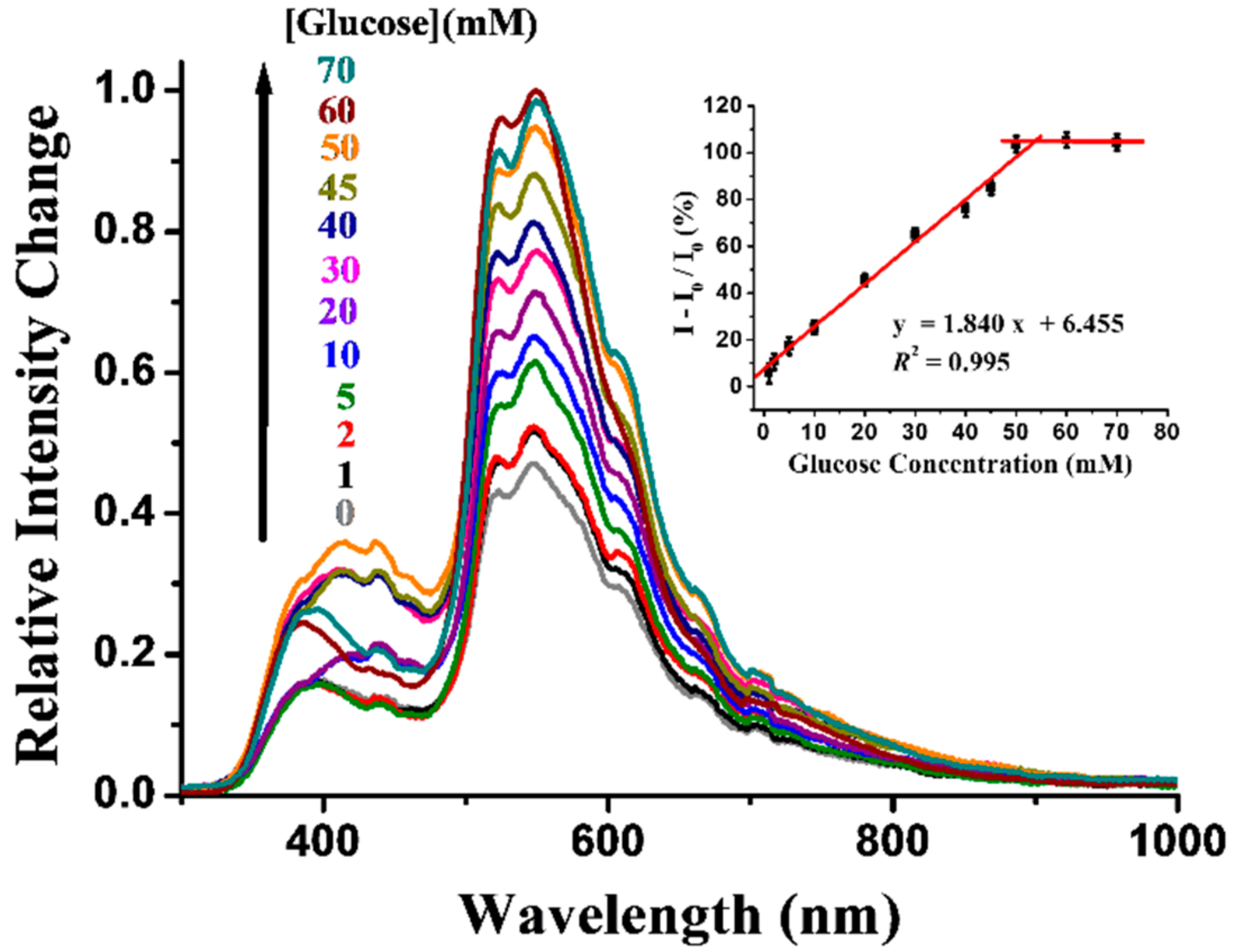
3.3. Optical Properties of Sensor
3.4. Interference Study
3.5. Storage Stability
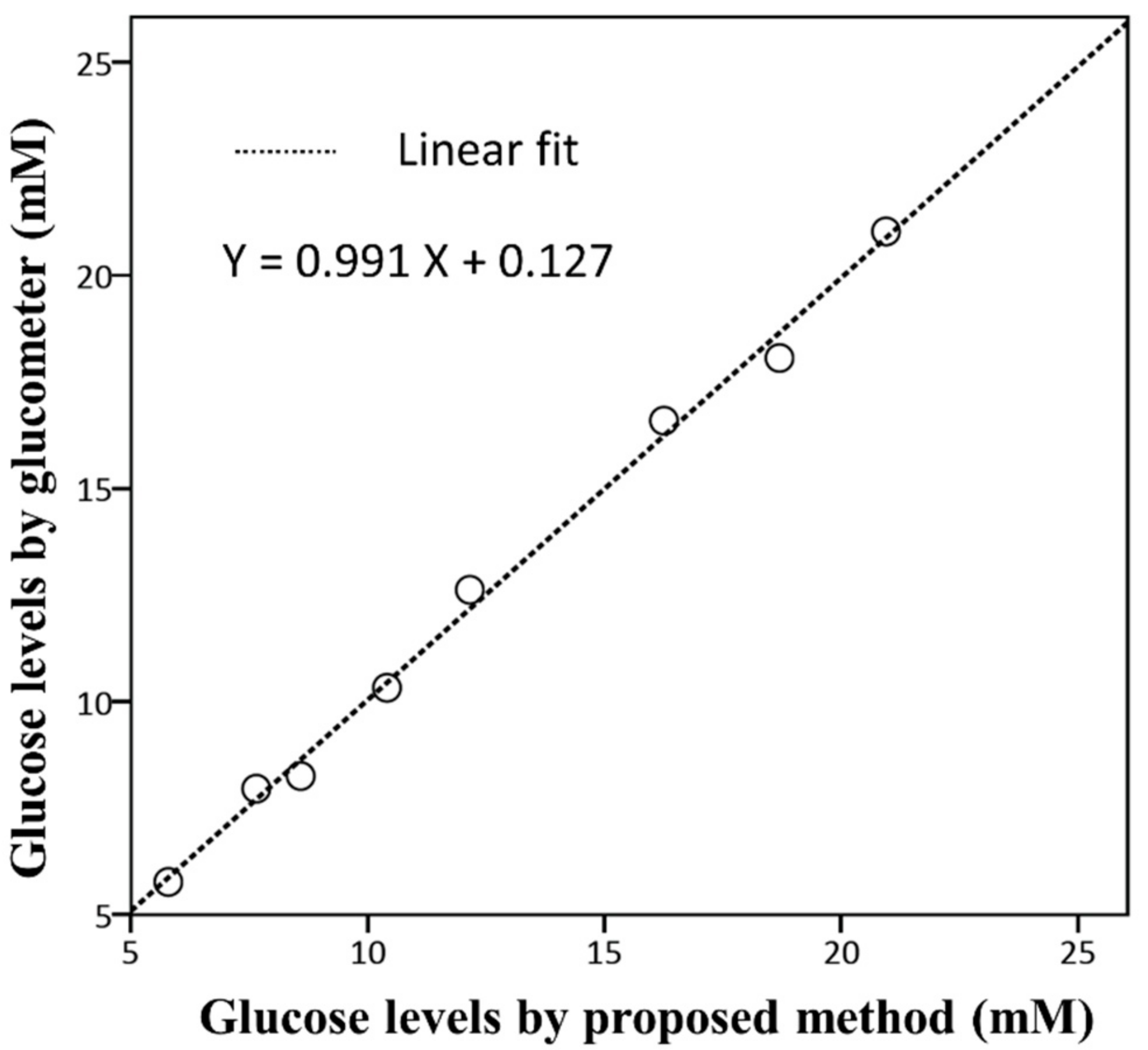
3.6. Determination of Human Plasma Glucose
3.7. μPADs for Tear Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Kang, X.; Pan, D. Gram-scale synthesis of hydrophilic PEI-coated AgInS2 quantum dots and its application in hydrogen peroxide/glucose detection and cell imaging. Inorg. Chem. 2017, 56, 6122–6130. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeong, S.; Noh, H. Quantitative determination of tear glucose using paper based microfluidic devices. Korean Chem. Soc. 2015, 59, 88–92. [Google Scholar] [CrossRef]
- Hu, J.-W.; Lin, W.-C.; Hsiao, S.-Y.; Wu, Y.-H.; Chen, H.-W.; Chen, K.-Y. An indanedione-based chemodosimeter for selective naked-eye and fluorogenic detection of cyanide. Sens. Actuators B Chem. 2016, 233, 510–519. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Lopes, F.M.; Coltro, W.K.T. Paper-based colorimetric biosensor for tear glucose measurements. Micromachines 2017, 8, 104. [Google Scholar] [CrossRef]
- Yoshihara, T.; Murayama, S.; Tobita, S. Ratiometric molecular probes based on dual emission of a blue fluorescent coumarin and a red phosphorescent cationic iridium(III) complex for intracellular oxygen sensing. Sensors 2015, 5, 13503–13521. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zho, W.; Lam, J.W.Y.; Peng, Q.; Ma, H.; Liang, G.; Shuai, Z.; Tang, B.Z. White light emission from a single organic molecule with dual phosphorescence at room temperature. Nat. Commun. 2017, 8, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Hu, R.; Wang, S.; Guo, X.; Yang, G. Luminescent properties of benzothiazole derivatives and their application in white light emission. RSC Adv. 2017, 7, 4196–4202. [Google Scholar] [CrossRef]
- Kondrasenko, I.; Chung, K.-Y.; Chen, Y.-T.; Koivistoinen, J.; Grachova, E.V.; Karttunen, A.J.; Chou, P.-T.; Koshevoy, I.O. Harnessing fluorescence versus phosphorescence ratio via ancillary ligand fine-tuned MLCT contribution. J. Phys. Chem. C 2016, 120, 12196–12206. [Google Scholar] [CrossRef]
- Pan, Z.-B.; Wang, Y.-C.; Chakkaradhari, G.; Zhu, J.F.; He, R.-Y.; Liu, Y.-C.; Hsu, C.-H.; Koshevoy, I.O.; Chou, P.-T.; Pan, S.-W.; et al. A silver metal complex as a luminescent probe for enzymatic sensing of glucose in blood plasma and urine. Dalton Trans. 2018, 47, 8346–8355. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.J.; Ramnial, T.; Yu, H.-Z.; Leznoff, D.B. Polymorphism of Zn[Au(CN)2]2 and its luminescent sensory response to NH3 vapor. J. Am. Chem. Soc. 2018, 130, 10662–10673. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Takaki, S.; Komuro, N.; Suzuki, K.; Citterio, D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 2014, 139, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, W.; Ding, X. A review on microfluidic paper-based analytical devices for glucose detection. Sensors 2016, 16, 2086–2102. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Dong, T.; Hanke, U.; Hoivik, N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors 2014, 14, 15458–15479. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.E.; Parkin, C. Gold(I) alkynyls and their co-ordination complexes. J. Chem. Soc. 1962, 3220–3226. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Karttunen, A.J.; Tunik, S.P.; Haukka, M.; Selivanov, S.I.; Melnikov, A.S.; Serdobintsev, P.Y.; Khodorkovskiy, M.A.; Pakkanen, T.A. Supramoleculer luminescent gold(I)-copper(I) complexes: Self-assembly of the AuxCuy clusters inside the [Au3(diphosphine)3]3+ triangles. Inorg. Chem. 2008, 47, 9487–9488. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-H.; Park, M.; Jeoung, K.-H. Colorimetric Schirmer strip for tear glucose detection. BioChip J. 2017, 11, 294–299. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Chen, C.; Shen, J.-H.; Zhao, X.-L.; Qian, S.-H.; Zhu, Z.-G. A gelated colloidal crystal attached lens for noninvasive continuous monitoring of tear glucose. Polymers 2017, 9, 125–136. [Google Scholar] [CrossRef]
- Milanović, M.; Krstonošić, V.; Dokić, L.; Hadnađev, M.; Hadnađev, T.D. Insight into the interaction between carbopol® 940 and ionic/nonionic surfactant. J. Surfactants Deterg. 2015, 18, 505–516. [Google Scholar] [CrossRef]
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless steel electrode for sensitive luminol electrochemiluminescent detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Liu, H.; Wang, M.; Su, X. A boronic acid based glucose assay based on the suppression of the inner filter effect of gold nanoparticles on the orange fluorescence of graphene oxide quantum dots. Microchim. Acta 2017, 184, 1463–1470. [Google Scholar] [CrossRef]
- Ma, J.-L.; Yin, B.-C.; Wu, X.; Ye, B.-C. Simple and cost-effective glucose detection based on carbon nanodots supported on silver nanoparticles. Anal. Chem. 2017, 89, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Toncelli, C.; Innocenti Malini, R.; Jankowska, D.; Spano, F.; Cölfen, H.; Maniura-Weber, K.; Rossi, R.M.; Boesel, L.F. Optical glucose sensing using ethanolamine–polyborate complexes. J. Mater. Chem. B 2018, 6, 816–823. [Google Scholar] [CrossRef]
- Sharma, S.P.; Anjankar, A.P.; Kale, A. Comparison of glucose levels using glucometer and GOD-POD Method in diabetic patients. Int. J. Clin. Biochem. Res. 2017, 4, 6–10. [Google Scholar]

| Compounds | Linear Range (mM) | LOD (mM) | Response Time (s) | Ref. |
|---|---|---|---|---|
| Gel-encapsulated B5 | 1.0–50.0 (plasma) | 0.9 | 0.3 | Our work |
| 0.1–2.0 (tear) | 0.08 | 0.3 | ||
| Ag3 on paper [a] | 1.0–35.0 | 0.09 | 10 | Our previous work [9] |
| Type 304 stainless steel electrode-based luminol [b] | 10−4–1.0 | 7.6 × 10−5 | N.A. [c] | [19] |
| GOQD/gold nanoparticles/APBA [d] | 2.5 × 10−3–7.5 × 10−2 | 6.5 × 10−4 | 2400 | [20] |
| AIS QDs [e] | 1.0 × 10−2–1.0 [f] | 9.0 ×10−4 | 1200 | [1] |
| C-dots/AgNPs | 2.0 × 10−3–0.1 | 1.39 × 10−3 | 300 | [21] |
| Ethanolamine-polyborate complexes | 0–8.0 | N.A. | 3000 | [22] |
| Sample Number | Glucose Added (mM) | Mean Glucose Levels [a] (mM) | R.S.D [b] (%) | Correlation r | p-value | |
|---|---|---|---|---|---|---|
| Glucometer [a] | Proposed Method [a] | |||||
| Group 1 | 0.999 | 0.001 | ||||
| 1 | 3 | 8.250 ± 0.06 | 8.588 ± 0.05 | 0.58 | ||
| 2 | 5 | 10.32 ± 0.24 | 10.41 ± 0.36 | 3.46 | ||
| 3 | 10 | 16.59 ± 0.08 | 16.26 ± 0.09 | 0.55 | ||
| 4 | 20 | 21.03 ± 0.12 | 20.95 ± 0.93 | 4.44 | ||
| Group 2 | 0.998 | 0.002 | ||||
| 5 | - | 5.764 ± 0.03 | 5.793 ± 0.19 | 3.28 | ||
| 6 | - | 7.953 ± 0.07 | 7.650 ± 0.12 | 1.57 | ||
| 7 | - | 12.62 ± 0.25 | 12.16 ± 0.09 | 0.74 | ||
| 8 | - | 18.06 ± 0.27 | 18.71 ± 0.51 | 2.73 | ||
| In artificial tear | 3.088 ± 0.06 | 3.186 ± 0.11 | 4.30 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.-J.; Pan, S.-W.; Yang, J.-H.; Chang, T.-L.; Lin, P.-Y.; Wu, C.-L.; Liu, W.-F.; Huang, X.-R.; Koshevoy, I.O.; Chou, P.-T.; et al. Detecting Glucose Levels in Blood Plasma and Artificial Tear by Au(I) Complex on the Carbopol Polymer: A Microfluidic Paper-Based Method. Polymers 2018, 10, 1001. https://doi.org/10.3390/polym10091001
Luo J-J, Pan S-W, Yang J-H, Chang T-L, Lin P-Y, Wu C-L, Liu W-F, Huang X-R, Koshevoy IO, Chou P-T, et al. Detecting Glucose Levels in Blood Plasma and Artificial Tear by Au(I) Complex on the Carbopol Polymer: A Microfluidic Paper-Based Method. Polymers. 2018; 10(9):1001. https://doi.org/10.3390/polym10091001
Chicago/Turabian StyleLuo, Jong-Jheng, Sheng-Wei Pan, Jia-Hui Yang, Tian-Lin Chang, Peng-Yi Lin, Chen-Liang Wu, Wei-Fang Liu, Xin-Ru Huang, Igor O. Koshevoy, Pi-Tai Chou, and et al. 2018. "Detecting Glucose Levels in Blood Plasma and Artificial Tear by Au(I) Complex on the Carbopol Polymer: A Microfluidic Paper-Based Method" Polymers 10, no. 9: 1001. https://doi.org/10.3390/polym10091001
APA StyleLuo, J.-J., Pan, S.-W., Yang, J.-H., Chang, T.-L., Lin, P.-Y., Wu, C.-L., Liu, W.-F., Huang, X.-R., Koshevoy, I. O., Chou, P.-T., & Ho, M.-L. (2018). Detecting Glucose Levels in Blood Plasma and Artificial Tear by Au(I) Complex on the Carbopol Polymer: A Microfluidic Paper-Based Method. Polymers, 10(9), 1001. https://doi.org/10.3390/polym10091001