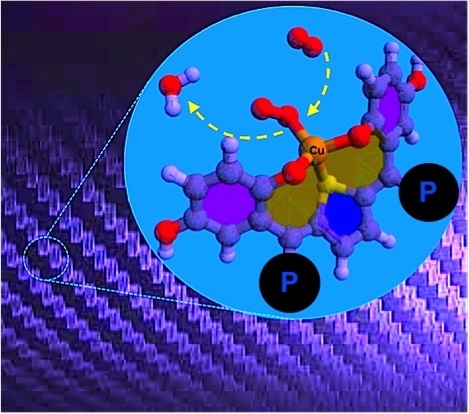
Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.3. Metallopolymer Synthesis
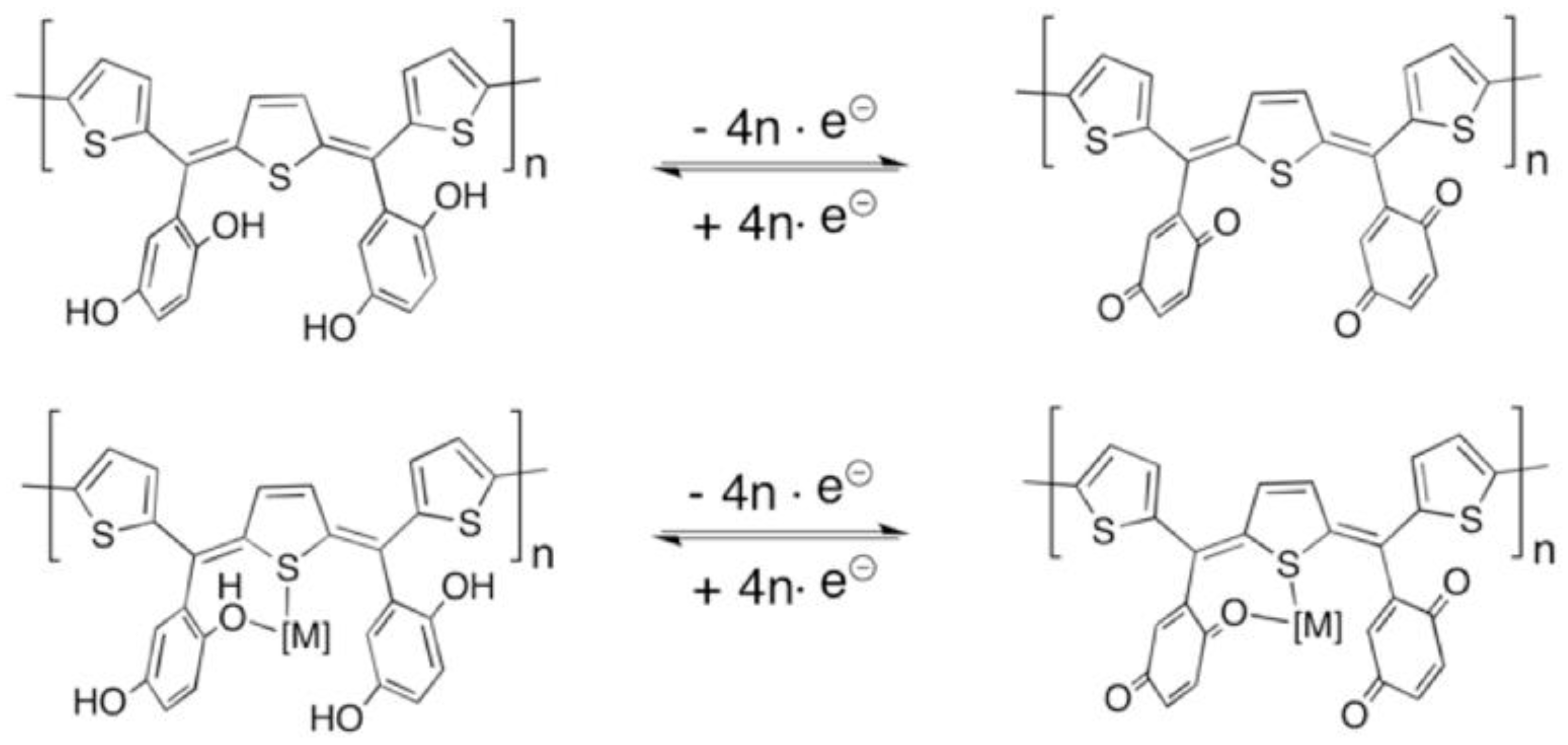
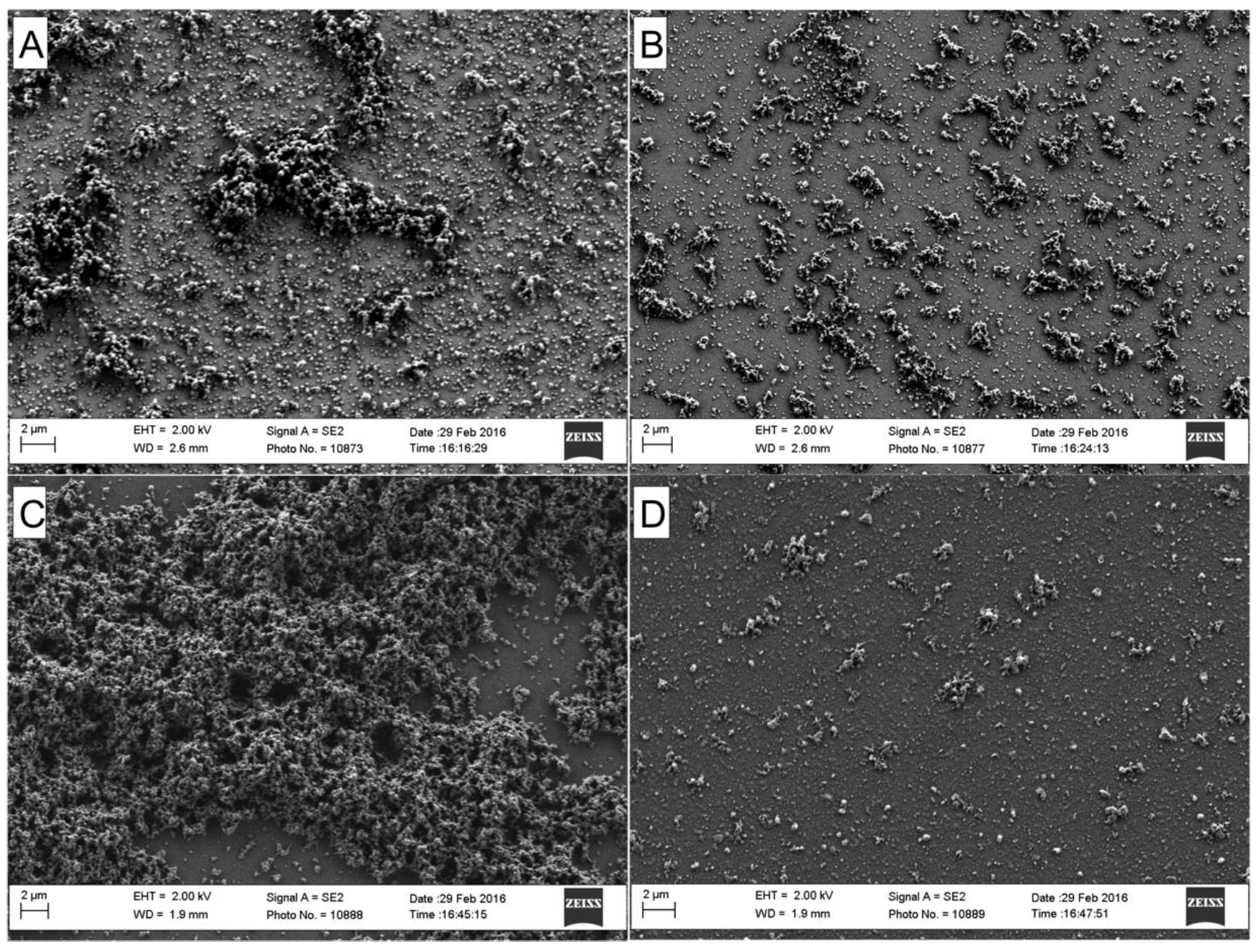
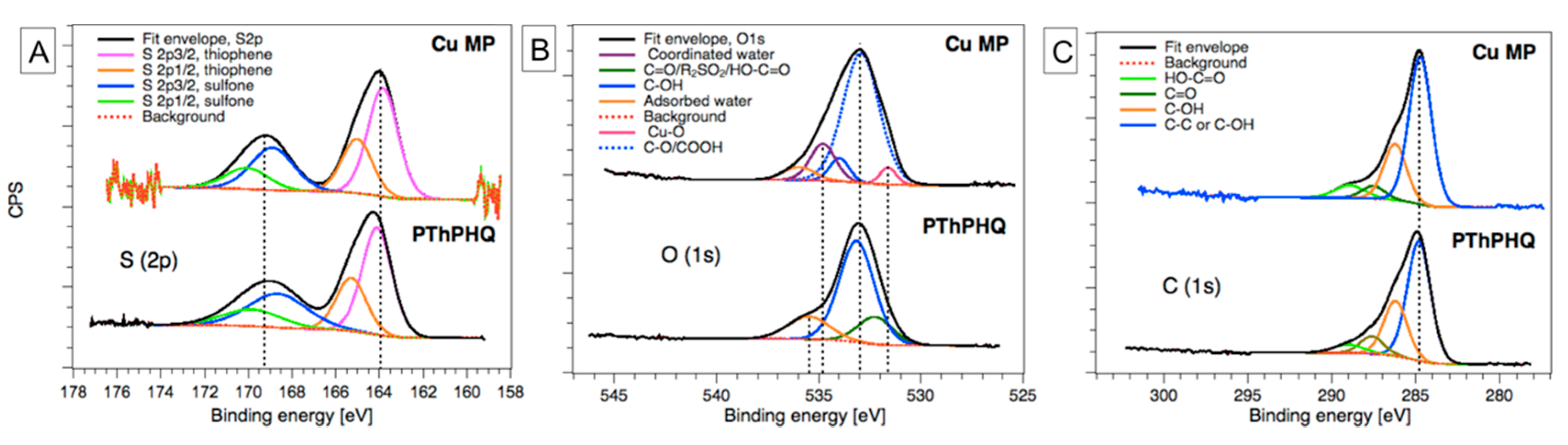
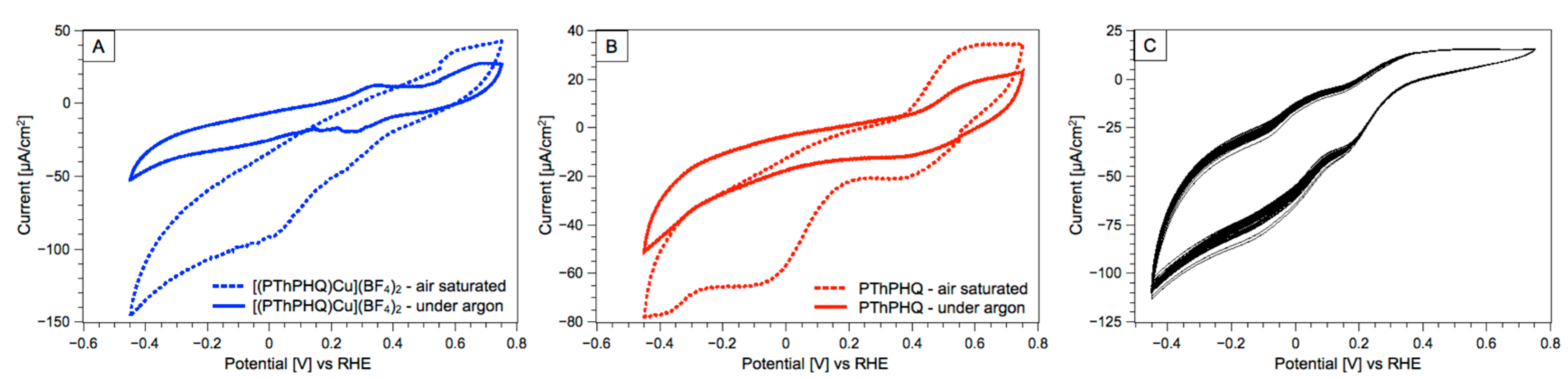
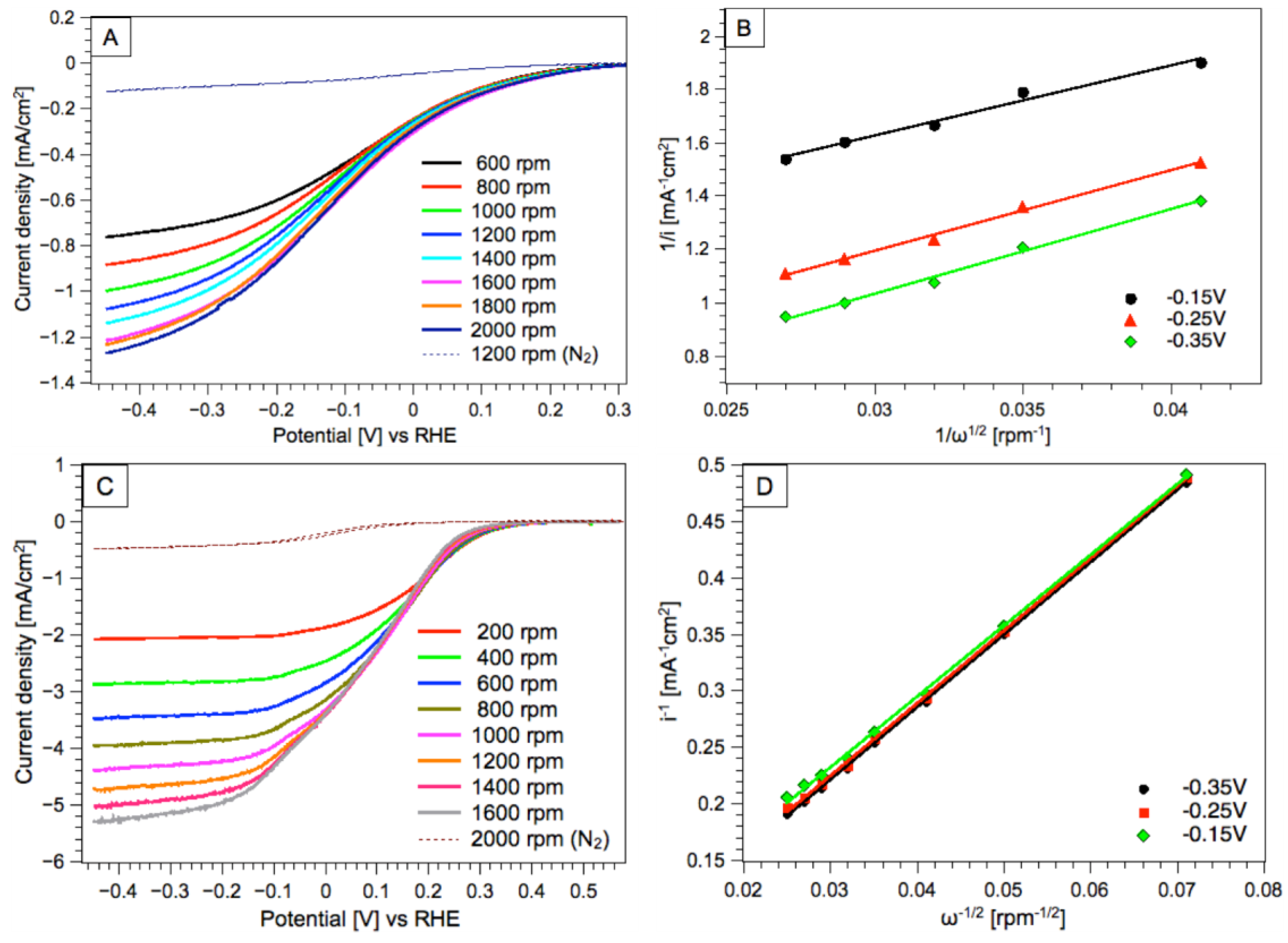
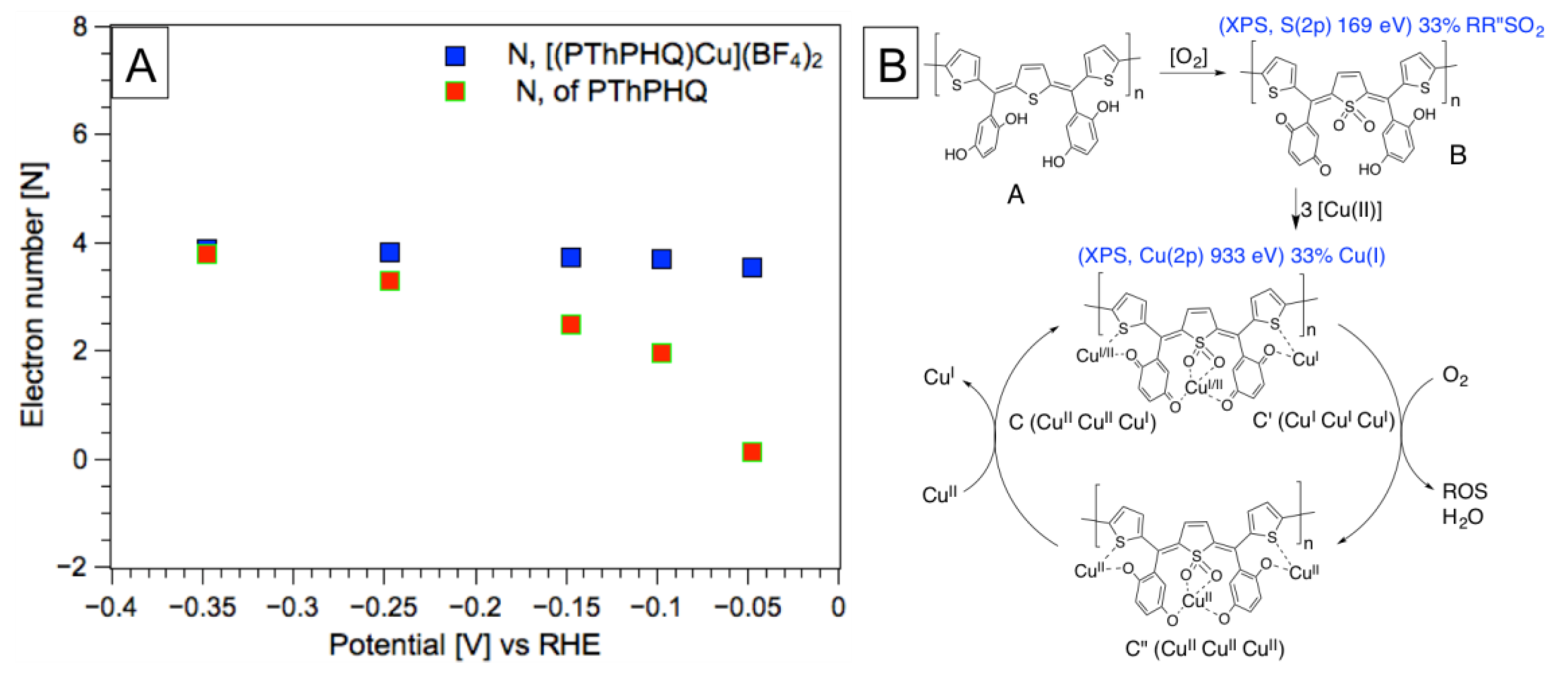
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, C.S.; Zhang, Y. Hexaazatrinaphthylene-Based Porous Organic Polymers as Organic Cathode Materials for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1772–1779. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; Hua, W.; Wang, J.; Nan, D.; Wei, C. Scale-up production of high-tap-density carbon/MnOx/carbon nanotube microcomposites for Li-ion batteries with ultrahigh volumetric capacity. Chem. Eng. J. 2018, 354, 220–227. [Google Scholar] [CrossRef]
- Wang, J.-G.; Sun, H.; Liu, H.; Jin, D.; Liu, X.; Li, X.; Kang, F. Triaxial Nanocables of Conducting Polypyrrole@SnS2@Carbon Nanofiber Enabling Significantly Enhanced Li-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 13581–13587. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Li, D.; Sun, S.; Peng, Z.; Komarneni, S.; Yang, D. Direct Interfacial Growth of MnO2 Nanostructure on Hierarchically Porous Carbon for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 633–641. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, H.; Liu, H.; Hua, W.; Shao, M. Interfacial Constructing Flexible V2O5 @Polypyrrole Core–Shell Nanowire Membrane with Superior Supercapacitive Performance. ACS Appl. Mater. Interfaces 2018, 10, 18816–18823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Facile Synthesis of Three-Dimensional Ternary ZnCo2O4/Reduced Graphene Oxide/NiO Composite Film on Nickel Foam for Next Generation Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2017, 5, 241–251. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Garg, R.; Elmas, S.; Nann, T.; Andersson, M.R. Deposition Methods of Graphene as Electrode Material for Organic Solar Cells. Adv. Energy Mater. 2017, 7, 1601393. [Google Scholar] [CrossRef]
- Lin, X.-X.; Wang, A.-J.; Fang, K.-M.; Yuan, J.; Feng, J.-J. One-Pot Seedless Aqueous Synthesis of Reduced Graphene Oxide (rGO)-Supported Core–Shell Pt@Pd Nanoflowers as Advanced Catalysts for Oxygen Reduction and Hydrogen Evolution. ACS Sustain. Chem. Eng. 2017, 5, 8675–8683. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.; Chang, C.-H.; Wu, K.; Tung, K.-L.; Lue, S. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef]
- Brian, C.H. Steele Angelika Heinzel Materials for fuel cell technologies. Nat. Mater. 2001, 414, 345–351. [Google Scholar]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Yu, E. Development of direct methanol alkaline fuel cells using anion exchange membranes. J. Power Sources 2004, 137, 248–256. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of Platinum Oxygen-Reduction Electrocatalysts Using Gold Clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Kondo, T.; Takahashi, Y.; Bando, Y.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activities for Pt/Au(hkl) Bimetallic Surfaces Prepared by Molecular Beam Epitaxy. J. Electrochem. Soc. 2013, 160, F898–F904. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Fu, G.; Duchesne, P.N.; Gu, L.; Zheng, Y.; Weng, X.; Chen, M.; Zhang, P.; Pao, C.-W.; et al. Interfacial Effects in Iron-Nickel Hydroxide-Platinum Nanoparticles Enhance Catalytic Oxidation. Science 2014, 344, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced Platinum Alloy Electrocatalysts for the Oxygen Reduction Reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Gong, K.; Yu, P.; Su, L.; Xiong, S.; Mao, L. Polymer-Assisted Synthesis of Manganese Dioxide/Carbon Nanotube Nanocomposite with Excellent Electrocatalytic Activity toward Reduction of Oxygen. J. Phys. Chem. C 2007, 111, 1882–1887. [Google Scholar] [CrossRef]
- Donne, S.W. Redox Processes at the Manganese Dioxide Electrode. J. Electrochem. Soc. 1997, 144, 2954. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Kuwabata, S.; Girishkumar, G.; Kamat, P. Single-Wall Carbon Nanotubes Supported Platinum Nanoparticles with Improved Electrocatalytic Activity for Oxygen Reduction Reaction. Langmuir 2006, 22, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Guangli, C.; Brinda, B.L.; Ellen, R.F.; Charles, L. Martin Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349. [Google Scholar] [CrossRef]
- Coleman, E.J.; Chowdhury, M.H.; Co, A.C. Insights into the Oxygen Reduction Reaction Activity of Pt/C and PtCu/C Catalysts. ACS Catal. 2015, 5, 1245–1253. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.-L.; Boero, M.; Hou, Z.; Terakura, K.; Cheng, W. Indirect Four-Electron Oxygen Reduction Reaction on Carbon Materials Catalysts in Acidic Solutions. ACS Catal. 2017, 7, 7908–7916. [Google Scholar] [CrossRef]
- Gong, X.; Liu, S.; Ouyang, C.; Strasser, P.; Yang, R. Nitrogen- and Phosphorus-Doped Biocarbon with Enhanced Electrocatalytic Activity for Oxygen Reduction. ACS Catal. 2015, 5, 920–927. [Google Scholar] [CrossRef]
- Halime, Z.; Kotani, H.; Li, Y.; Fukuzumi, S.; Karlin, K.D. Homogeneous catalytic O2 reduction to water by a cytochrome c oxidase model with trapping of intermediates and mechanistic insights. Proc. Natl. Acad. Sci. USA 2011, 108, 13990–13994. [Google Scholar] [CrossRef] [PubMed]
- Collman, J.P.; Devaraj, N.K.; Decreau, R.A.; Yang, Y.; Yan, Y.-L.; Ebina, W.; Eberspacher, T.A.; Chidsey, C.E.D. A Cytochrome c Oxidase Model Catalyzes Oxygen to Water Reduction Under Rate-Limiting Electron Flux. Science 2007, 315, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Parimi, N.S.; Umasankar, Y.; Atanassov, P.; Ramasamy, R.P. Kinetic and Mechanistic Parameters of Laccase Catalyzed Direct Electrochemical Oxygen Reduction Reaction. ACS Catal. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Winther-Jensen, B.; Winther-Jensen, O.; Forsyth, M.; MacFarlane, D.R. High Rates of Oxygen Reduction over a Vapor Phase-Polymerized PEDOT Electrode. Science 2008, 321, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Barsukov, V.; Chivikov, S. The “capacitor” concept of the current-producing process mechanism in polyaniline-type conducting polymers. Electrochim. Acta 1996, 41, 1773–1779. [Google Scholar] [CrossRef]
- Khomenko, V.G.; Barsukov, V.Z.; Katashinskii, A.S. The catalytic activity of conducting polymers toward oxygen reduction. Electrochim. Acta 2005, 50, 1675–1683. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating Oxygen Reduction Active Sites in Pyrolyzed Metal–Nitrogen Coordinated Non-Precious-Metal Electrocatalyst Systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Bouwkamp-Wijnoltz, A.L.; Visscher, W.; van Veen, J.A.R.; Boellaard, E.; van der Kraan, A.M.; Tang, S.C. On Active-Site Heterogeneity in Pyrolyzed Carbon-Supported Iron Porphyrin Catalysts for the Electrochemical Reduction of Oxygen: An In Situ Mössbauer Study. J. Phys. Chem. B 2002, 106, 12993–13001. [Google Scholar] [CrossRef]
- Koslowski, U.I.; Abs-Wurmbach, I.; Fiechter, S.; Bogdanoff, P. Nature of the Catalytic Centers of Porphyrin-Based Electrocatalysts for the ORR: A Correlation of Kinetic Current Density with the Site Density of Fe−N 4 Centers. J. Phys. Chem. C 2008, 112, 15356–15366. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.-C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube–graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Genorio, B.; Strmcnik, D.; Subbaraman, R.; Tripkovic, D.; Karapetrov, G.; Stamenkovic, V.R.; Pejovnik, S.; Marković, N.M. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules. Nat. Mater. 2010, 9, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, X.; Jao, T.-C.; Weng, F.-B.; Su, A.; Chiang, Y.-C. Degradation analyses of Ru85Se15 catalyst layer in proton exchange membrane fuel cells. J. Power Sources 2012, 218, 79–87. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Kingsborough, R.P.; Swager, T.M. Electrocatalytic Conducting Polymers: Oxygen Reduction by a Polythiophene−Cobalt Salen Hybrid. Chem. Mater. 2000, 12, 872–874. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Nash, J.; Macdonald, T.J.; Jasieniak, M.; Griesser, H.J.; Nann, T. Photo-doping of plasma-deposited polyaniline (PAni). RSC Adv. 2016, 6, 70691–70699. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and Metallic Polymers: The Fourth Generation of Polymeric Materials (Nobel Lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Kingsborough, R.P.; Swager, T.M. Electroactivity Enhancement by Redox Matching in Cobalt Salen-Based Conducting Polymers. Adv. Mater. 1998, 10, 1100–1104. [Google Scholar] [CrossRef]
- Chen, W.-C.; Jenekhe, S.A. Small-Bandgap Conducting Polymers Based on Conjugated Poly(heteroarylene methines). 2. Synthesis, Structure, and Properties. Macromolecules 1995, 28, 465–480. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Liu, K.; Zhu, D.; Yang, M.; Ye, H.; Liu, X. Synthesis and characterization of novel low band gap polymers: Poly(heteroarylene methines). Synth. Met. 2006, 156, 804–808. [Google Scholar] [CrossRef]
- Yi, W.; Feng, W.; Cao, M.; Wu, H. Synthesis of third-order non-linear optical polymers based on conjugated poly(heteroarylene methines). Polym. Adv. Technol. 2004, 15, 431–438. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Yousefi, A.; Elmas, S.; Lindén, J.B.; Nann, T.; Nydén, M. Electroactive Polyhydroquinone Coatings for Marine Fouling Prevention—A Rejected Dynamic pH Hypothesis and a Deceiving Artifact in Electrochemical Antifouling Testing. ACS Omega 2017, 2, 4751–4759. [Google Scholar] [CrossRef]
- Vonlanthen, D.; Lazarev, P.; See, K.A.; Wudl, F.; Heeger, A.J. A Stable Polyaniline-Benzoquinone-Hydroquinone Supercapacitor. Adv. Mater. 2014, 26, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Elmas, S.; Butsch, K. Oxido Pincer Ligands–Exploring the Coordination Chemistry of Bis(hydroxymethyl)pyridine Ligands for the Late Transition Metals. Eur. J. Inorg. Chem. 2009, 2009, 2271–2281. [Google Scholar] [CrossRef]
- Klein, A.; Butsch, K.; Elmas, S.; Biewer, C.; Heift, D.; Nitsche, S.; Schlipf, I.; Bertagnolli, H. Oxido-pincer complexes of copper(II)–An EXAFS and EPR study of mono- and binuclear [(pydotH2)CuCl2]n (n = 1 or 2). Polyhedron 2012, 31, 649–656. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, S.; Li, B.; Fan, D. A series of [Cu(N–N)(P–P)]BF4 complexes: Luminescence quenching caused by electron-configuration transformation in excited state. Inorg. Chim. Acta 2012, 384, 225–232. [Google Scholar] [CrossRef]
- Megiatto, J.D.; Schuster, D.I. Alternative Demetalation Method for Cu(I)-Phenanthroline-Based Catenanes and Rotaxanes. Org. Lett. 2011, 13, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Butsch, K.; Neudörfl, J. Electron transfer studies on Cu(II) complexes bearing phenoxy-pincer ligands. Inorg. Chim. Acta 2010, 363, 3282–3290. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, F.; Li, M.; Peng, W.; Qu, J. Reactivity and Kinetics of Vinyl Sulfone-Functionalized Self-Assembled Monolayers for Bioactive Ligand Immobilization. Langmuir 2015, 31, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Tzorbatzoglou, F.; Brouzgou, A.; Tsiakaras, P. Electrocatalytic activity of Vulcan-XC-72 supported Pd, Rh and PdxRhy toward HOR and ORR. Appl. Catal. B Environ. 2015, 174–175, 203–211. [Google Scholar] [CrossRef]
- Jiang, L.; Hsu, A.; Chu, D.; Chen, R. Oxygen reduction on carbon supported Pt and PtRu catalysts in alkaline solutions. J. Electroanal. Chem. 2009, 629, 87–93. [Google Scholar] [CrossRef]
- Ikeuchi, H.; Hayafuji, M.; Aketagawa, Y.; Taki, J.; Sato, G.P. Diffusion coefficients of dioxygen in aqueous electrolyte solutions. J. Electroanal. Chem. 1995, 396, 553–556. [Google Scholar] [CrossRef]
- Tammeveski, K.; Kontturi, K.; Nichols, R.J.; Potter, R.J.; Schiffrin, D.J. Surface redox catalysis for O2 reduction on quinone-modified glassy carbon electrodes. J. Electroanal. Chem. 2001, 515, 101–112. [Google Scholar] [CrossRef]
- Yuan, X.; Pham, A.N.; Miller, C.J.; Waite, T.D. Copper-Catalyzed Hydroquinone Oxidation and Associated Redox Cycling of Copper under Conditions Typical of Natural Saline Waters. Environ. Sci. Technol. 2013, 47, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO●, H● and HOO●) Formation and Ionomer Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2011, 158, B755. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. XPS Analysis of Polymer Membrane Degradation in PEMFCs. J. Electrochem. Soc. 2009, 156, B1218. [Google Scholar] [CrossRef]
- Shah, A.A.; Ralph, T.R.; Walsh, F.C. Modeling and Simulation of the Degradation of Perfluorinated Ion-Exchange Membranes in PEM Fuel Cells. J. Electrochem. Soc. 2009, 156, B465. [Google Scholar] [CrossRef]
- Song, A.; Cao, L.; Yang, W.; Li, Y.; Qin, X.; Shao, G. Uniform Multilayer Graphene-Coated Iron and Iron-Carbide as Oxygen Reduction Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 4890–4898. [Google Scholar] [CrossRef]
- Pan, F.; Cao, Z.; Zhao, Q.; Liang, H.; Zhang, J. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction. J. Power Sources 2014, 272, 8–15. [Google Scholar] [CrossRef]
- Chen, X.; He, F.; Shen, Y.; Yang, Y.; Mei, H.; Liu, S.; Mori, T.; Zhang, Y. Effect of Carbon Supports on Enhancing Mass Kinetic Current Density of Fe-N/C Electrocatalysts. Chem. Eur. J. 2017, 23, 14597–14603. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, X.; Wang, X.; Müllen, K. Graphene-Based Carbon Nitride Nanosheets as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reactions. Angew. Chem. Int. Ed. 2011, 50, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous Graphitic-C 3 N 4 @Carbon Metal-Free Electrocatalysts for Highly Efficient Oxygen Reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Zhou, W.; Lin, J.; Shen, Z. Nitrogen-doped Graphene-Supported Transition-metals Carbide Electrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]

| Catalyst | Electrolyte | Ered,ons (V vs. RHE) (a) | Ired,ons (mA/cm2) | N | Lit. |
|---|---|---|---|---|---|
| Fe3C/Fe@G-800 | 0.1M KOH | 0.91 | n.r. (b) | 3.71 | [71] |
| NPCN | 0.1M KOH | 0.72 | 13.57 | 3.70 | [72] |
| Pt/C | 0.1M KOH | 0.72–0.8 | 9.28–14.00 | 4.00 | [64,72,73] |
| GC-N800 | 0.1M KOH | 0.58 | 7.30 | 4.00 | [74] |
| g-CN3@C | 0.1M KOH | 0.37 | 11.30 | 3.90 | [75] |
| FeMo-NG | 0.1M KOH | 0.17 | 3.50 | 3.90 | [76] |
| PdRh/C, Rh/C | 0.5M H2SO4 | 0.70 (c) | 0.89–5.20 | 4.00 | [63] |
| Cu MP | 0.1M KCl | −0.15 | 22.62–30.78 | 3.75–3.90 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmas, S.; Beelders, W.; Pan, X.; Nann, T. Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers 2018, 10, 1002. https://doi.org/10.3390/polym10091002
Elmas S, Beelders W, Pan X, Nann T. Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers. 2018; 10(9):1002. https://doi.org/10.3390/polym10091002
Chicago/Turabian StyleElmas, Sait, Wesley Beelders, Xun Pan, and Thomas Nann. 2018. "Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density" Polymers 10, no. 9: 1002. https://doi.org/10.3390/polym10091002
APA StyleElmas, S., Beelders, W., Pan, X., & Nann, T. (2018). Conducting Copper(I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers, 10(9), 1002. https://doi.org/10.3390/polym10091002