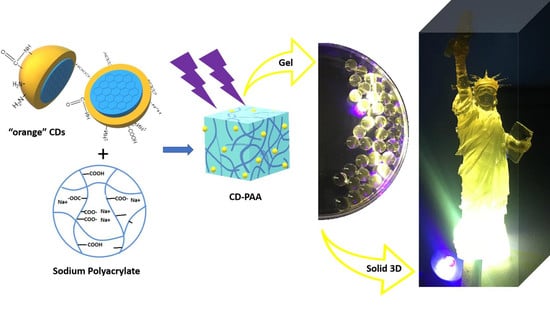
Embedding Carbon Dots in Superabsorbent Polymers for Additive Manufacturing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. O-CDs Synthesis
2.4. Embedment of O-CDs into SPA Powder as Feedstock for 3D Printing
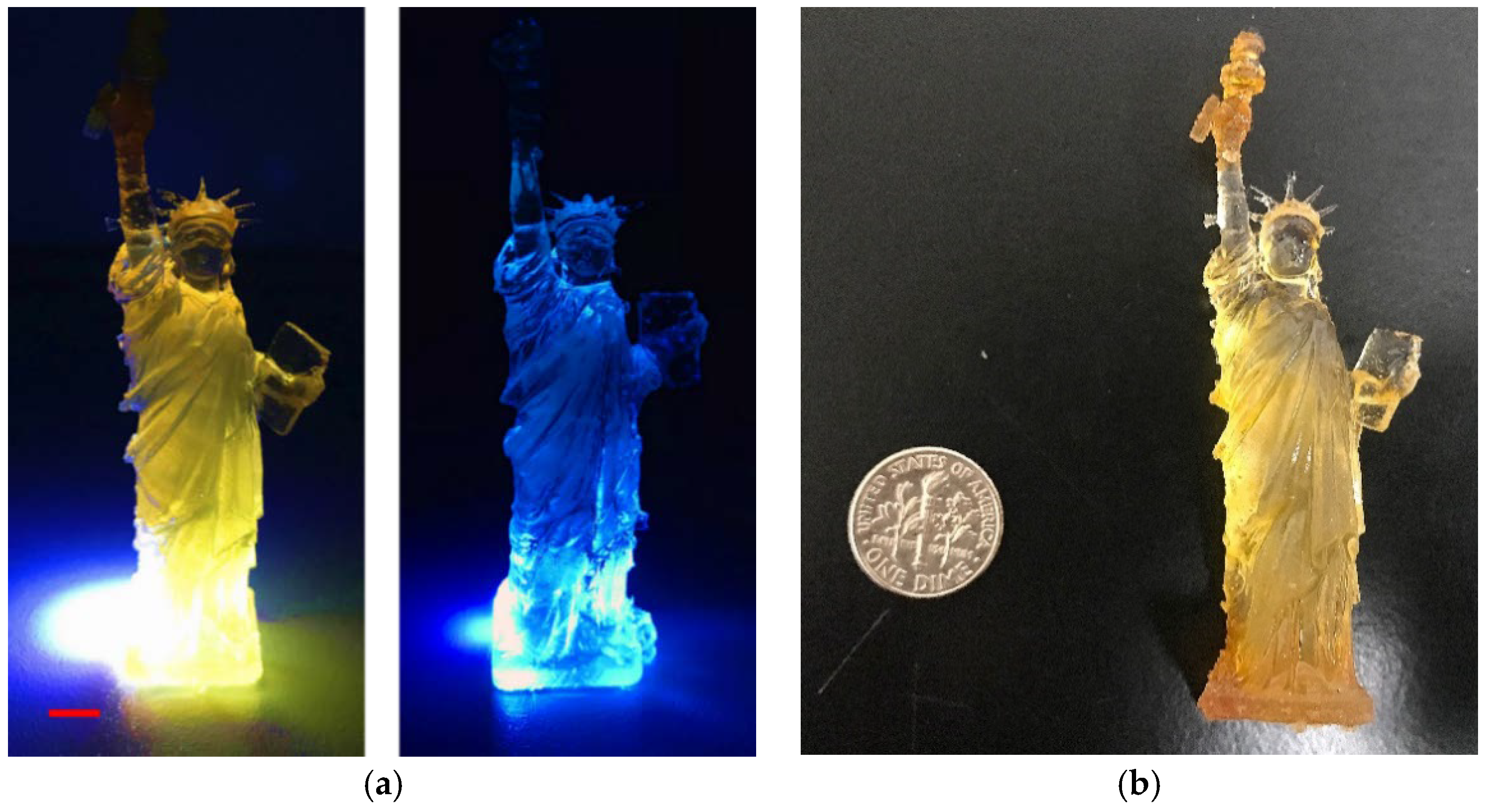
2.5. 3D Printing of the CDs-SPA Composite
3. Results and Discussion
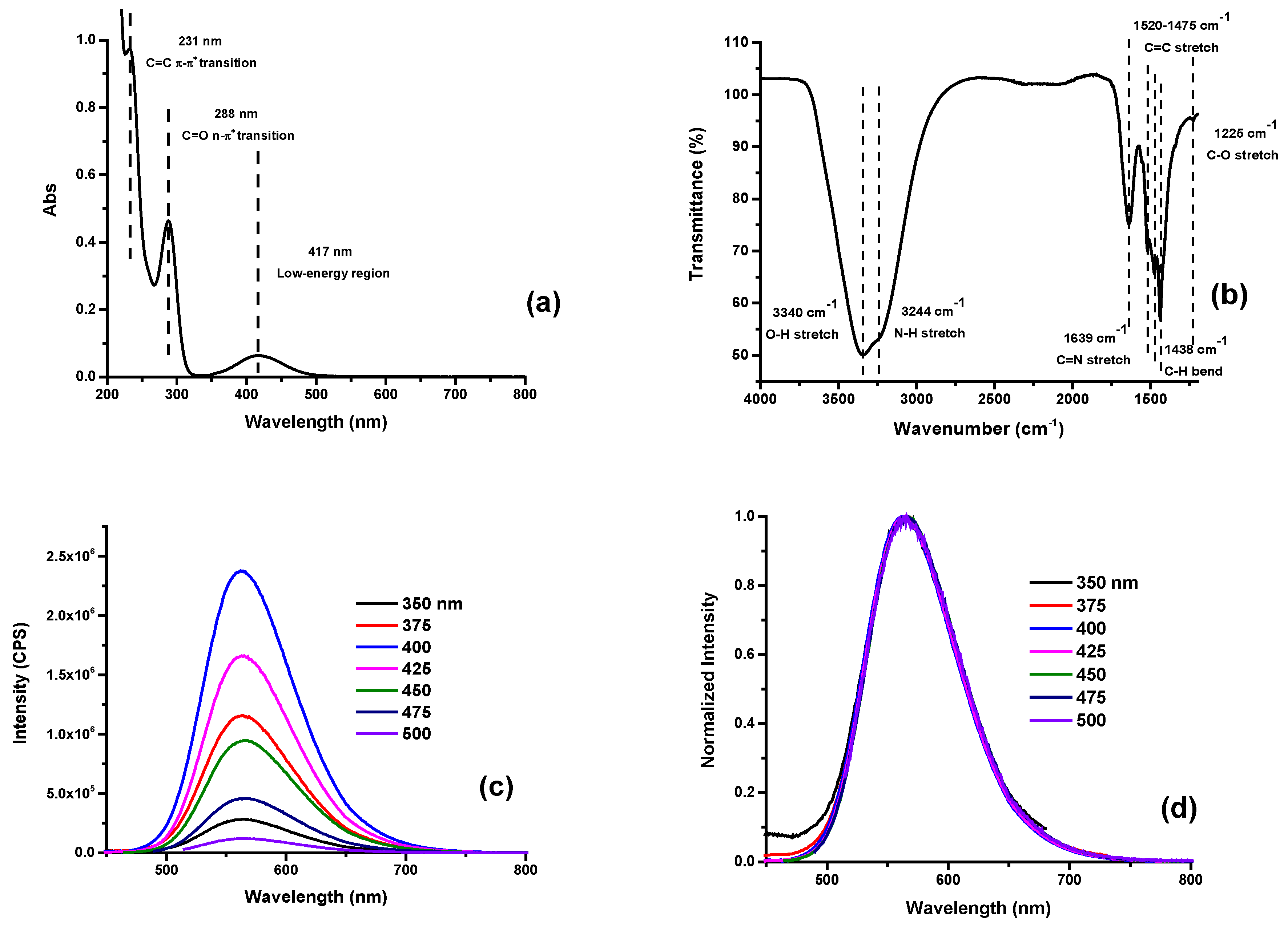
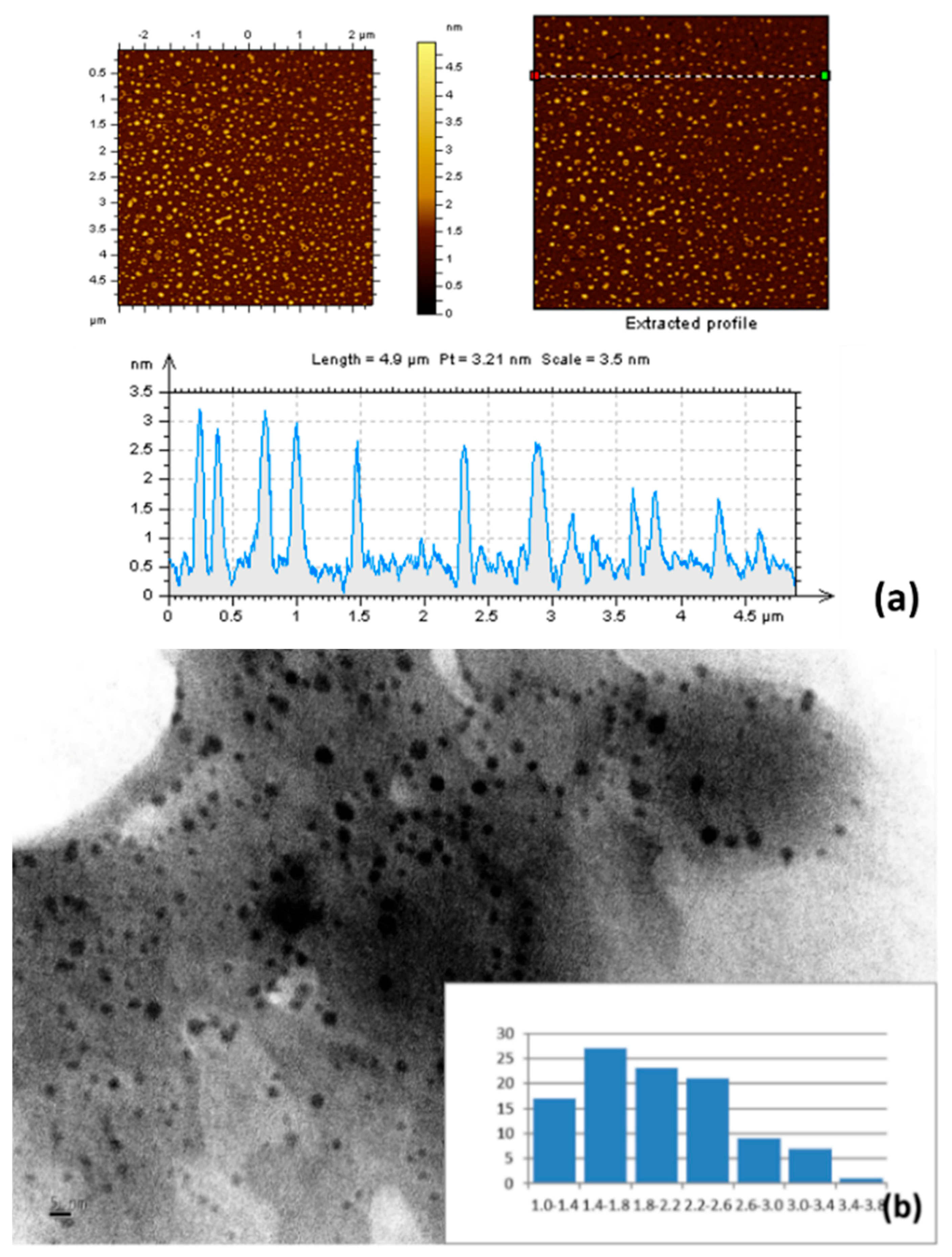
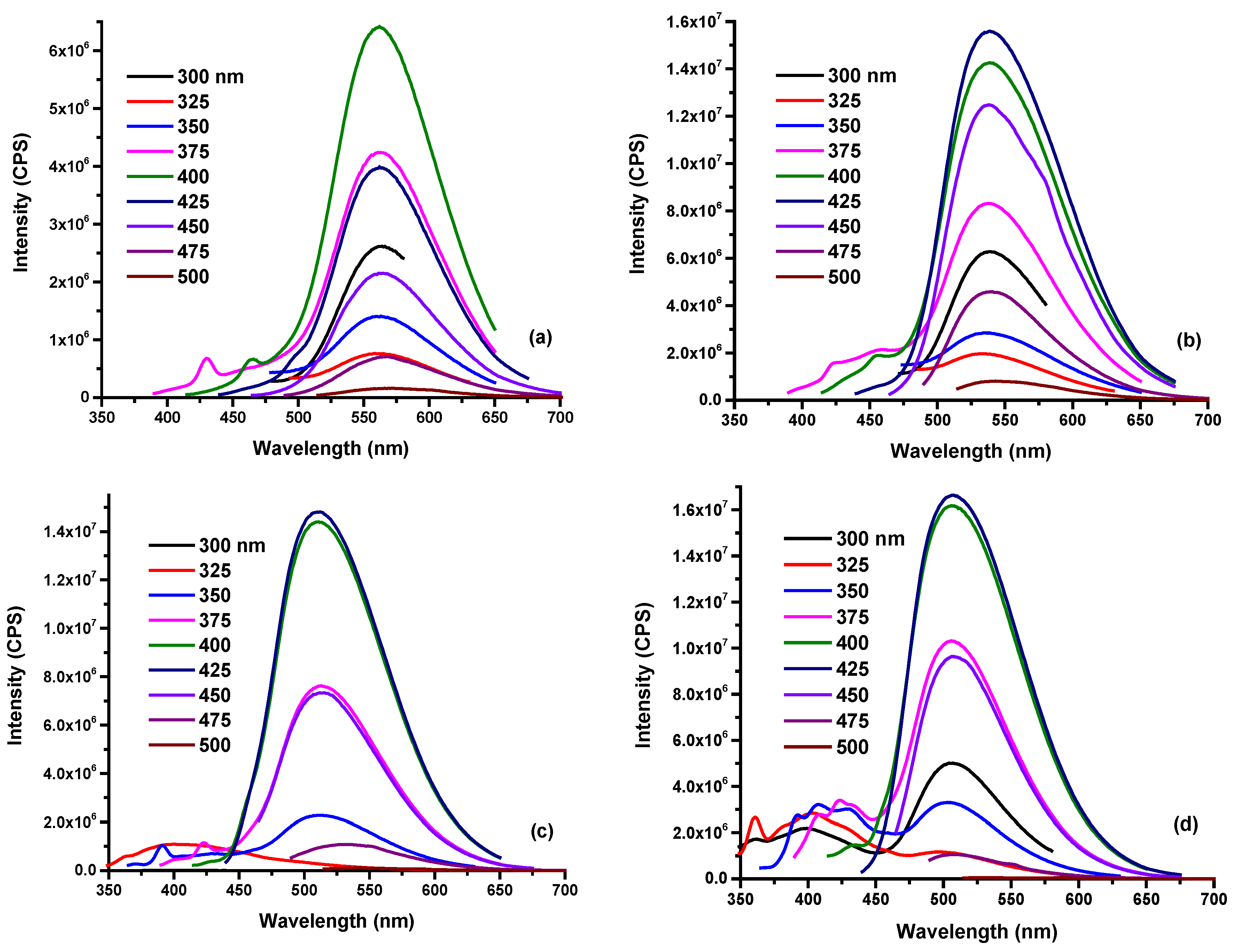
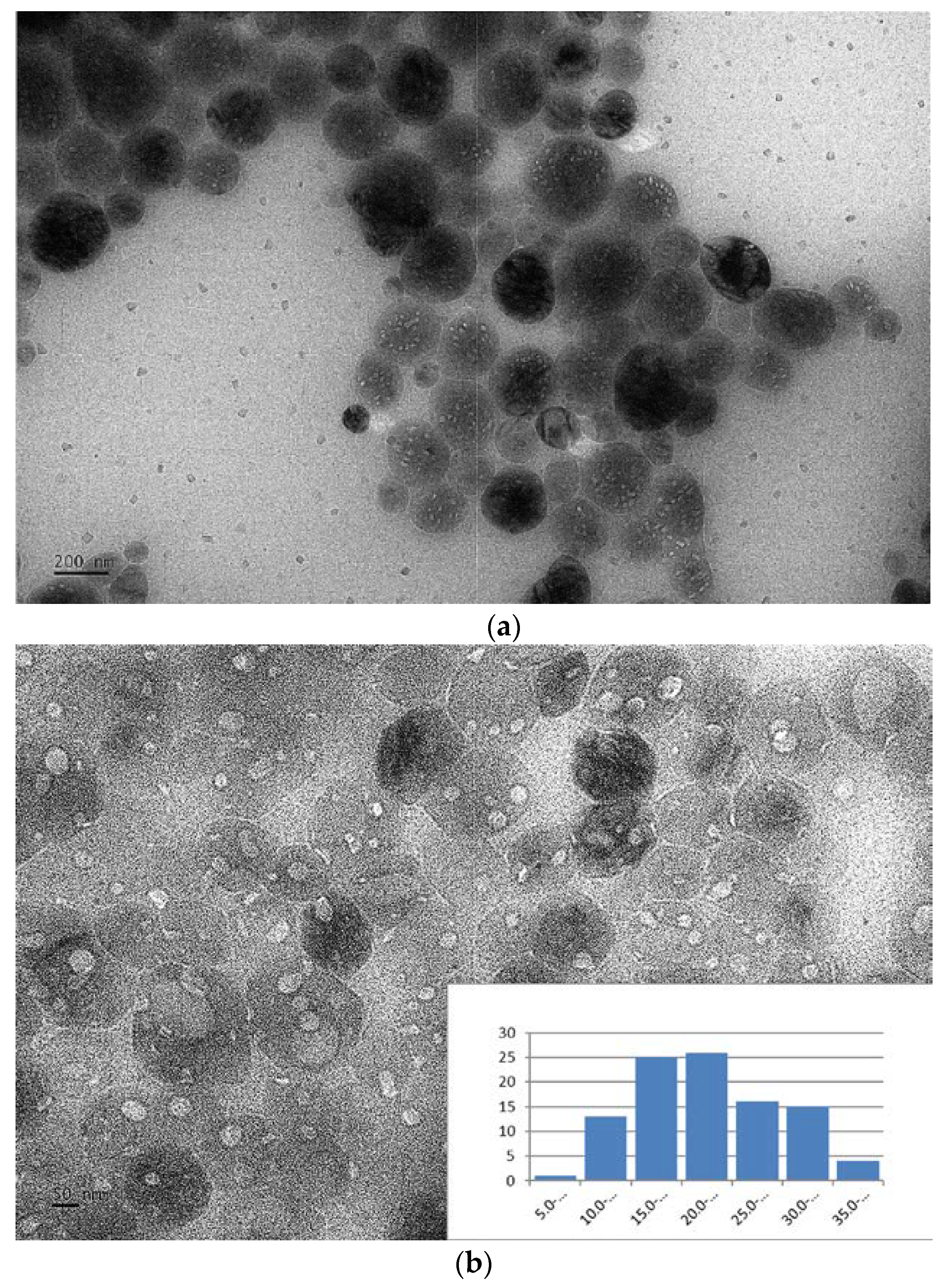
3.1. Characterization of O-CDs
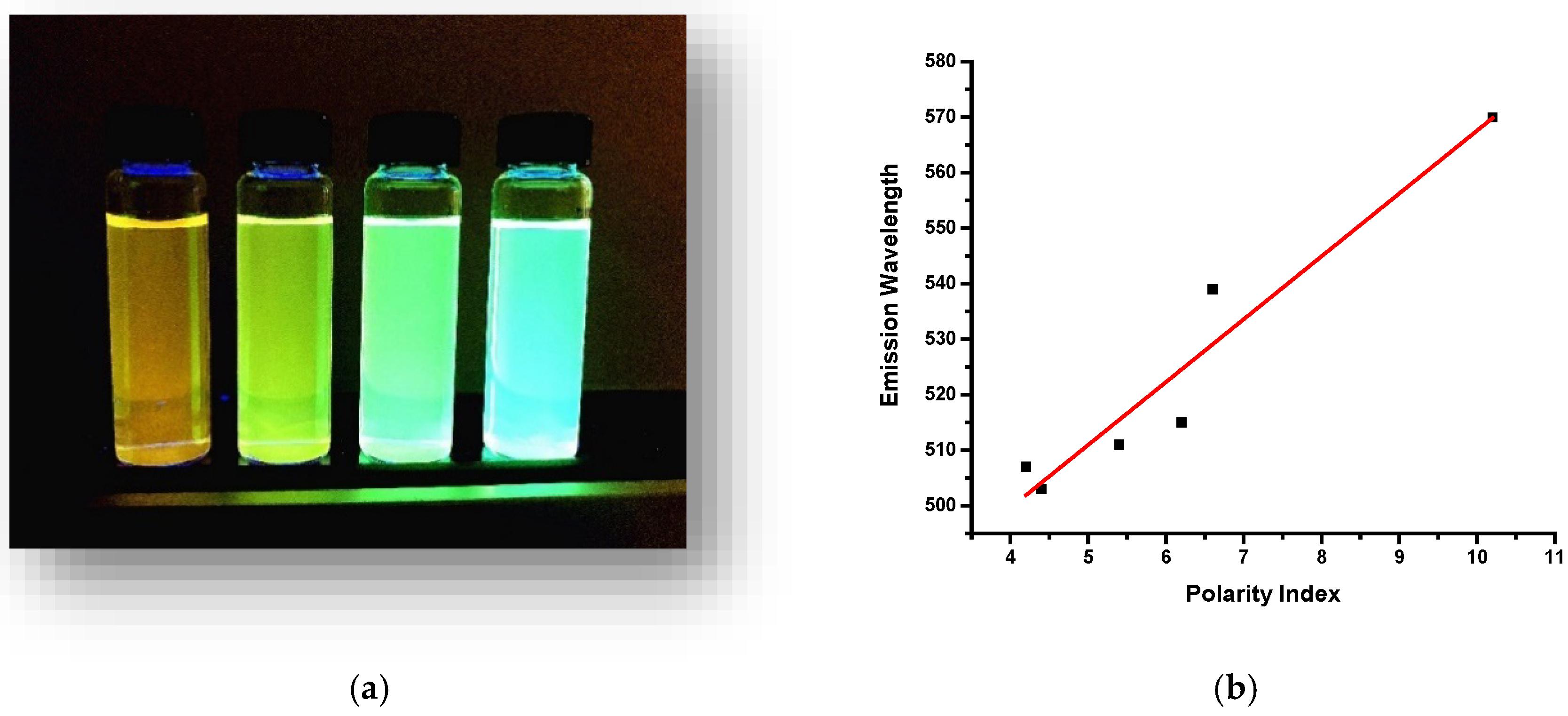
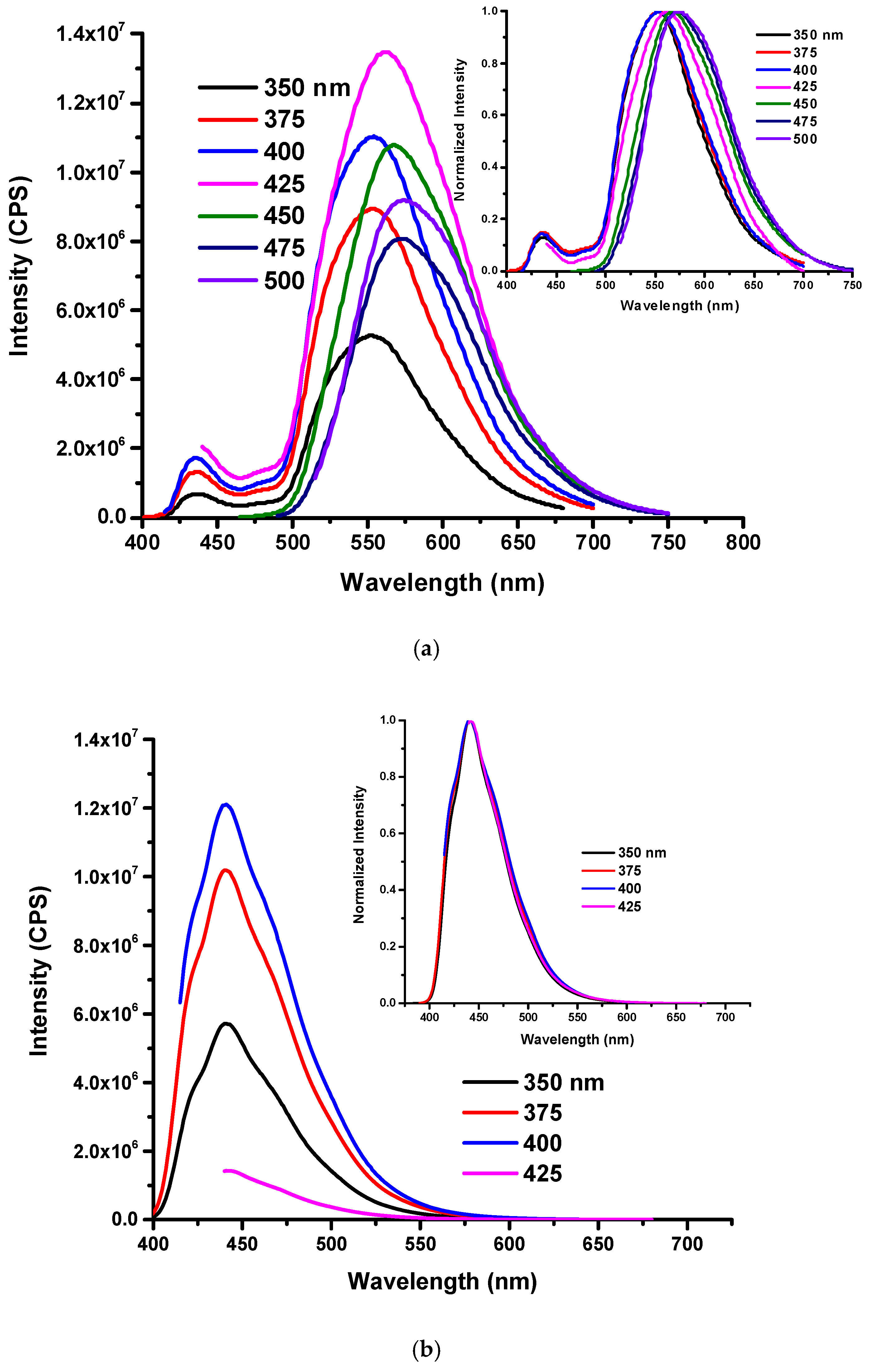
3.2. Solvent Effect of O-CDs
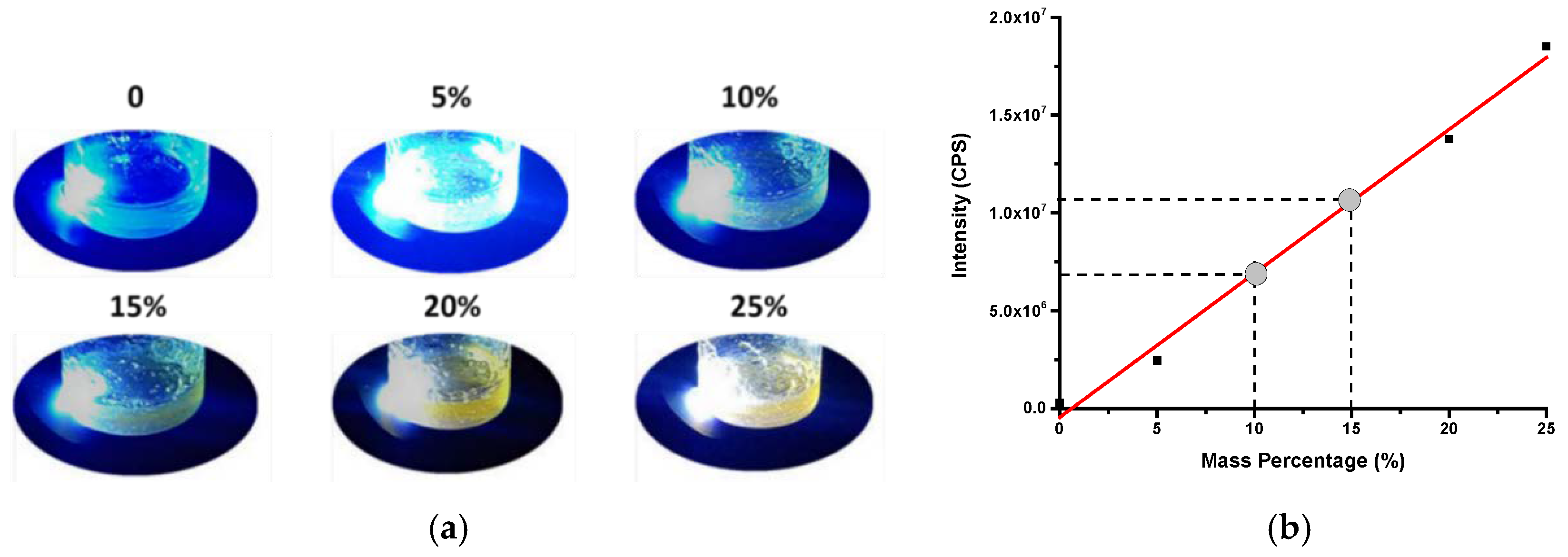
3.3. Embedment of O-CDs into SPA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lam, C.X.F.; Mo, X.; Teoh, S.-H.; Hutmacher, D. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar] [CrossRef]
- Lipson, H.; Kurman, M. Fabricated: The New World of 3D Printing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Haring, A.P.; Khan, A.U.; Liu, G.; Johnson, B.N. 3D printed functionally graded plasmonic constructs. Adv. Opt. Mater. 2017, 5, 1700367–1700375. [Google Scholar] [CrossRef]
- Brubaker, C.D.; Davies, M.A.; McBride, J.R.; Rosenthal, S.J.; Jennings, G.K.; Adams, D.E. Nondestructive evaluation and detection of defects in 3D printed materials using the optical properties of gold nanoparticles. ACS Appl. Nano Mater. 2018, 1, 1377–1384. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots-emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Chusuei, C.C.; Suarez, V.M.; Blackwelder, P.L.; Micic, M.; Orbulescu, J.; Leblanc, R.M. Nontoxic carbon dots potently inhibit human insulin fibrillation. Chem. Mater. 2015, 27, 1764–1771. [Google Scholar] [CrossRef]
- Chae, A.; Choi, Y.; Jo, S.; Nur’aeni; Paoprasert, P.; Park, S.Y.; In, I. Microwave-assisted synthesis of fluorescent carbon quantum dots from an A2/B3 monomer set. RSC Adv. 2017, 7, 12663–12669. [Google Scholar] [CrossRef]
- Zheng, C.; An, X.; Gong, J. Novel pH sensitive n-doped carbon dots with both long fluorescence lifetime and high quantum yield. RSC Adv. 2015, 5, 32319–32322. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Desserre, A.; Sharma, S.K.; Li, S.; Marksberry, M.H.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Gel-like carbon dots: Characterization and their potential applications. ChemPhysChem 2017, 18, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Shi, L.; Xian, M.; Dong, C.; Shuang, S. Folic acid-conjugated carbon dots as green fluorescent probes based on cellular targeting imaging for recognizing cancer cells. RSC Adv. 2017, 7, 42159–42167. [Google Scholar] [CrossRef]
- Bergau, N.; Navarette Santos, A.; Henning, A.; Balcke, G.U.; Tissier, A. Autofluorescence as a signal to sort developing glandular trichomes by flow cytometry. Front. Plant Sci. 2016, 7, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, M.M.; William, M.C.; May, P.S.; QuocAnh, L.; Grant, A.C.; Jon, J.K. Security printing of covert quick response codes using upconverting nanoparticle inks. Nanotechnology 2012, 23, 395201–395210. [Google Scholar]
- Tyler, B.; Jeevan, M.; May, P.S.; Jon, K.; William, C.; Krishnamraju, A.; Swathi, V.; QuocAnh, N.L. Patterned direct-write and screen-printing of NIR-to-visible upconverting inks for security applications. Nanotechnology 2012, 23, 185305–185313. [Google Scholar]
- Kong, Y.L.; Tamargo, I.A.; Kim, H.; Johnson, B.N.; Gupta, M.K.; Koh, T.-W.; Chin, H.-A.; Steingart, D.A.; Rand, B.P.; McAlpine, M.C. 3D printed quantum dot light-emitting diodes. Nano Lett. 2014, 14, 7017–7023. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, T. Preparation and swelling properties of crosslinked sodium polyacrylate. J. Appl. Polym. Sci. 2001, 82, 1515–1520. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. ChemComm 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta 2015, 866, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vandaele, A.C.; Hermans, C.; Simon, P.C.; Van Roozendael, M.; Guilmot, J.M.; Carleer, M.; Colin, R. Fourier transform measurement of NO2 absorption cross-section in the visible range at room temperature. J. Atmos. Chem. 1996, 25, 289–305. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Rider, A.E.; Furman, S.A.; Ostrikov, K. Fast, energy-efficient synthesis of luminescent carbon quantum dots. Green Chem. 2014, 16, 2566–2570. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C. Chemically induced fluorescence switching of carbon-dots and its multiple logic gate implementation. Sci. Rep. 2015, 5, 10012–10021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.H.; Liu, Z.X.; Li, R.S.; Zou, H.Y.; Lin, M.; Liu, H.; Huang, C.Z. Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale 2016, 8, 6770–6776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liyanage, P.Y.; Geleroff, D.L.; Peng, Z.; Mintz, K.J.; Hettiarachchi, S.D.; Pandey, R.R.; Chusuei, C.C.; Blackwelder, P.L.; Leblanc, R.M. Photoluminescent carbon dots: A mixture of heterogeneous fractions. ChemPhysChem 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhu, S.; Liu, H.; Song, Y.; Tao, S.; Yang, B. Full-color emission polymer carbon dots with quench-resistant solid-state fluorescence. Adv. Sci. 2017, 4, 1700395–1700402. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C.K. Time-resolved emission reveals ensemble of emissive states as the origin of multicolor fluorescence in carbon dots. Nano Lett. 2015, 15, 8300–8305. [Google Scholar] [CrossRef] [PubMed]
- Noboru, M.; Yozo, K.; Masao, K. Solvent effects upon fluorescence spectra and the dipolemoments of excited molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar]
- Lippert, E. Spektroskopische bestimmung des dipolmomentes aromatischer verbindungen im ersten angeregten singulettzustand. Z. Elektrochem. Ber. Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar]
- Junwu, C.; Jiarui, S. Swelling behaviors of polyacrylate superabsorbent in the mixtures of water and hydrophilic solvents. J. Appl. Polym. Sci. 2000, 75, 1331–1338. [Google Scholar]
- He, M.; Zhao, Y.; Wang, B.; Xi, Q.; Zhou, J.; Liang, Z. 3D printing fabrication of amorphous thermoelectric materials with ultralow thermal conductivity. Small 2015, 11, 5889–5894. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Mintz, K.J.; Oztan, C.Y.; Hettiarachchi, S.D.; Peng, Z.; Seven, E.S.; Liyanage, P.Y.; De La Torre, S.; Celik, E.; Leblanc, R.M. Embedding Carbon Dots in Superabsorbent Polymers for Additive Manufacturing. Polymers 2018, 10, 921. https://doi.org/10.3390/polym10080921
Zhou Y, Mintz KJ, Oztan CY, Hettiarachchi SD, Peng Z, Seven ES, Liyanage PY, De La Torre S, Celik E, Leblanc RM. Embedding Carbon Dots in Superabsorbent Polymers for Additive Manufacturing. Polymers. 2018; 10(8):921. https://doi.org/10.3390/polym10080921
Chicago/Turabian StyleZhou, Yiqun, Keenan J. Mintz, Cagri Y. Oztan, Sajini D. Hettiarachchi, Zhili Peng, Elif S. Seven, Piumi Y. Liyanage, Sabrina De La Torre, Emrah Celik, and Roger M. Leblanc. 2018. "Embedding Carbon Dots in Superabsorbent Polymers for Additive Manufacturing" Polymers 10, no. 8: 921. https://doi.org/10.3390/polym10080921
APA StyleZhou, Y., Mintz, K. J., Oztan, C. Y., Hettiarachchi, S. D., Peng, Z., Seven, E. S., Liyanage, P. Y., De La Torre, S., Celik, E., & Leblanc, R. M. (2018). Embedding Carbon Dots in Superabsorbent Polymers for Additive Manufacturing. Polymers, 10(8), 921. https://doi.org/10.3390/polym10080921