The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Film and Sample
Refrigerated Storage
2.3. Weight Loss (LS)
2.4. Firmness Measurement
2.5. Surface Color
2.6. Soluble Solid Concentration (SSC) and Titratable Acidity (TA)
2.7. Determination of Vitamin C
2.8. Total Phenolics Content
2.9. 1-Diphenyl-2-Picrylhydrazyl (DPPH) Determination
2.10. Pyrogallol Peroxidase Assay
2.11. Sensory Evaluation of Strawberries
2.12. Microbiological Analysis
2.13. Statistical Analysis
3. Results and Discussion
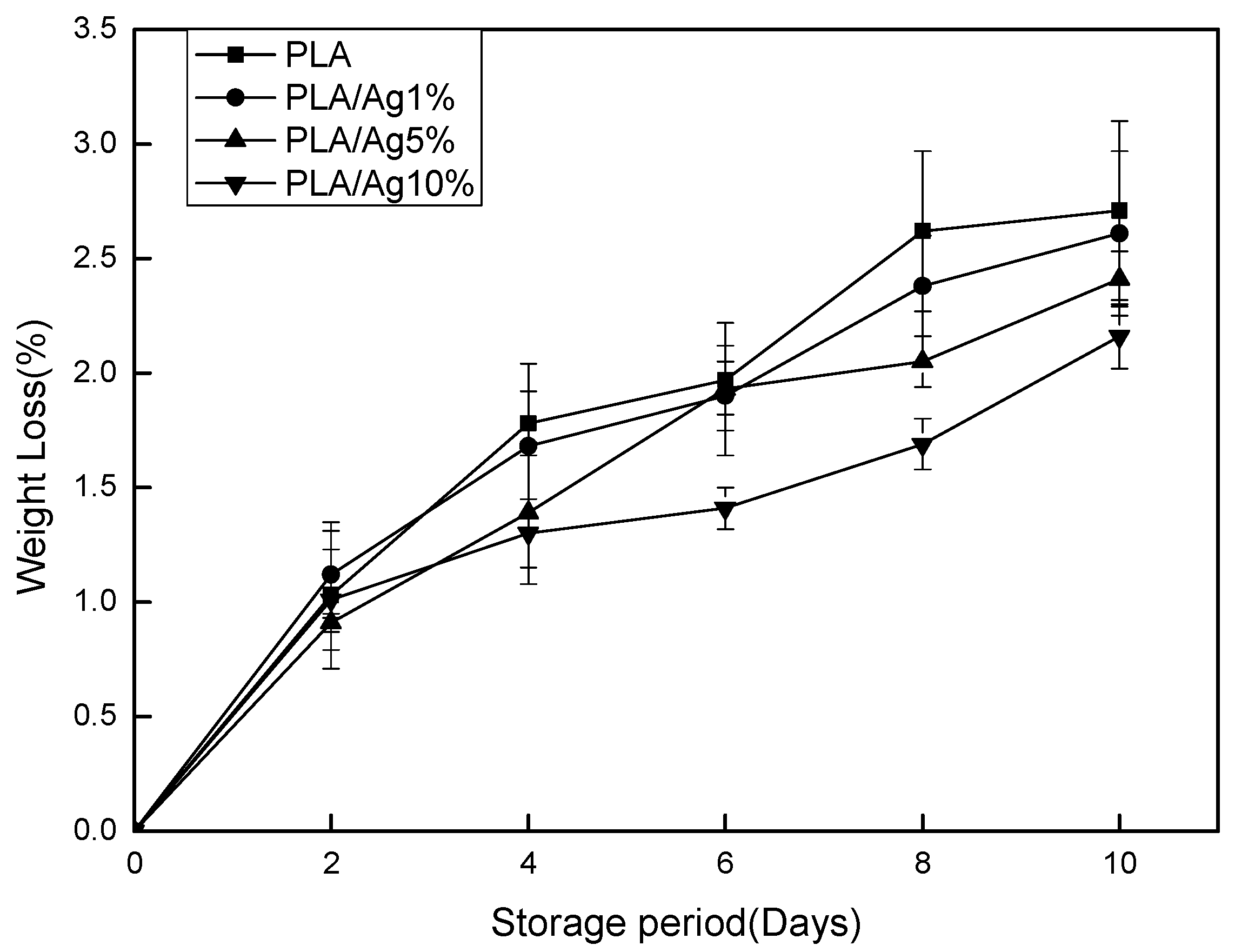
3.1. Weight Loss
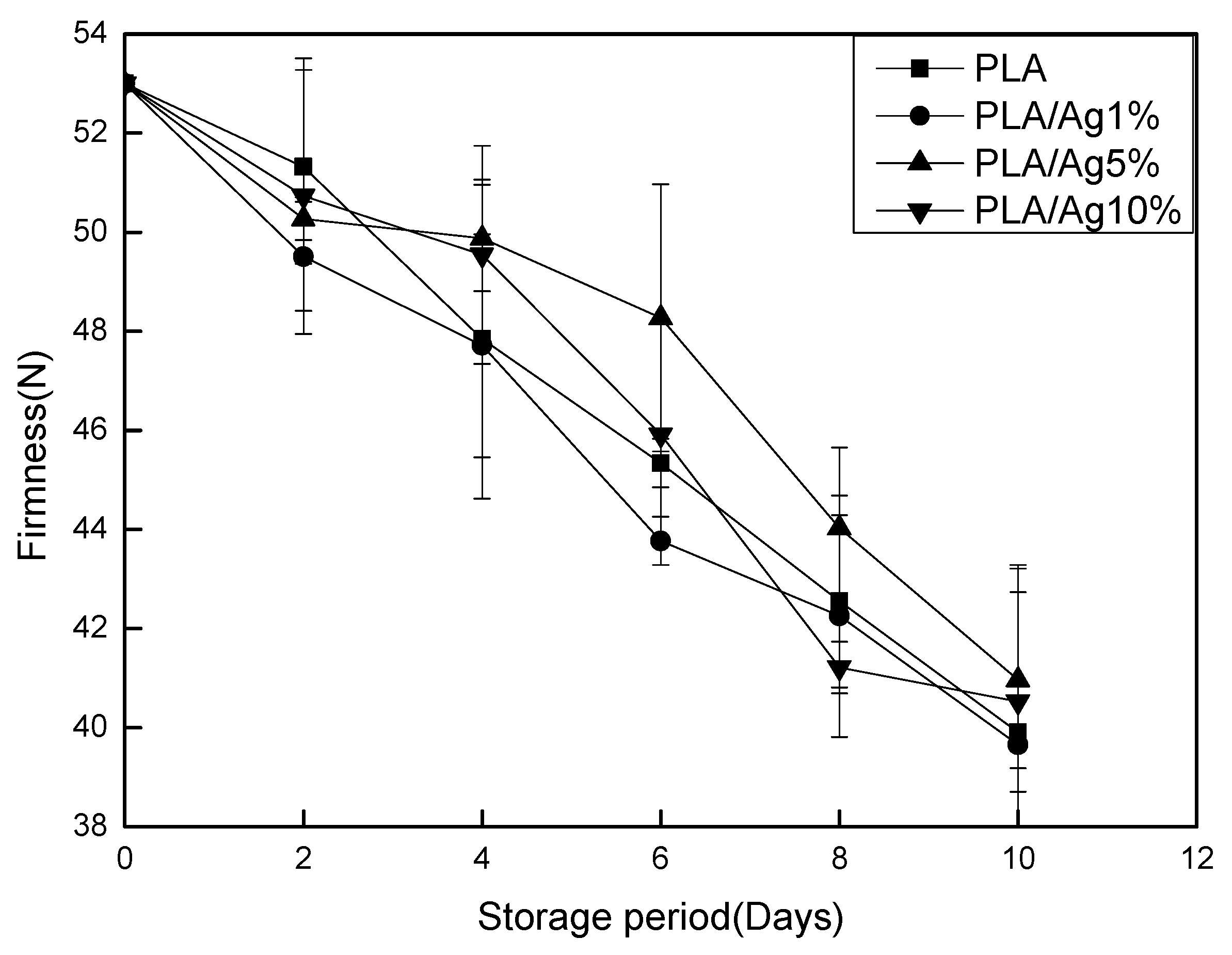
3.2. Firmness
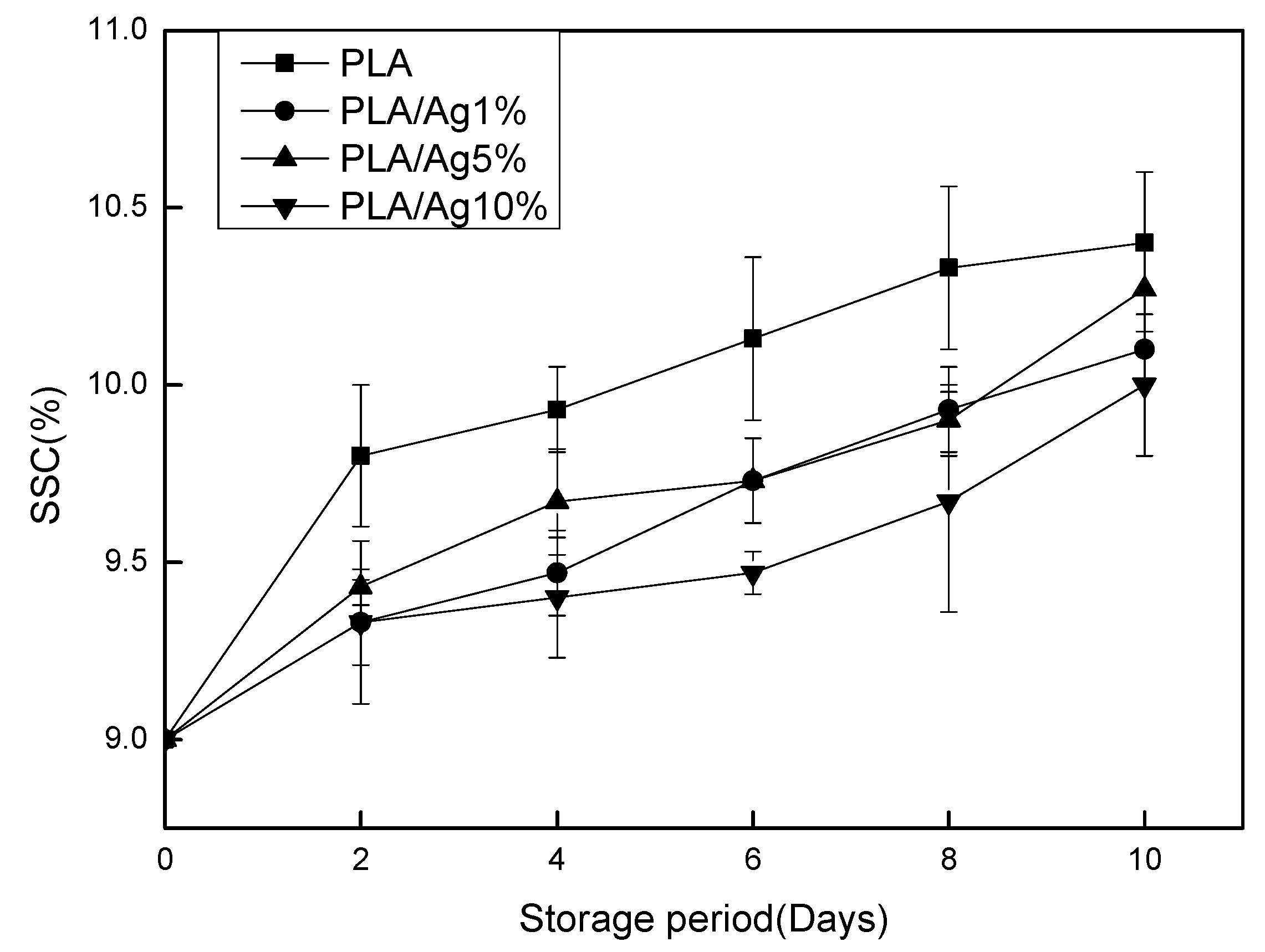
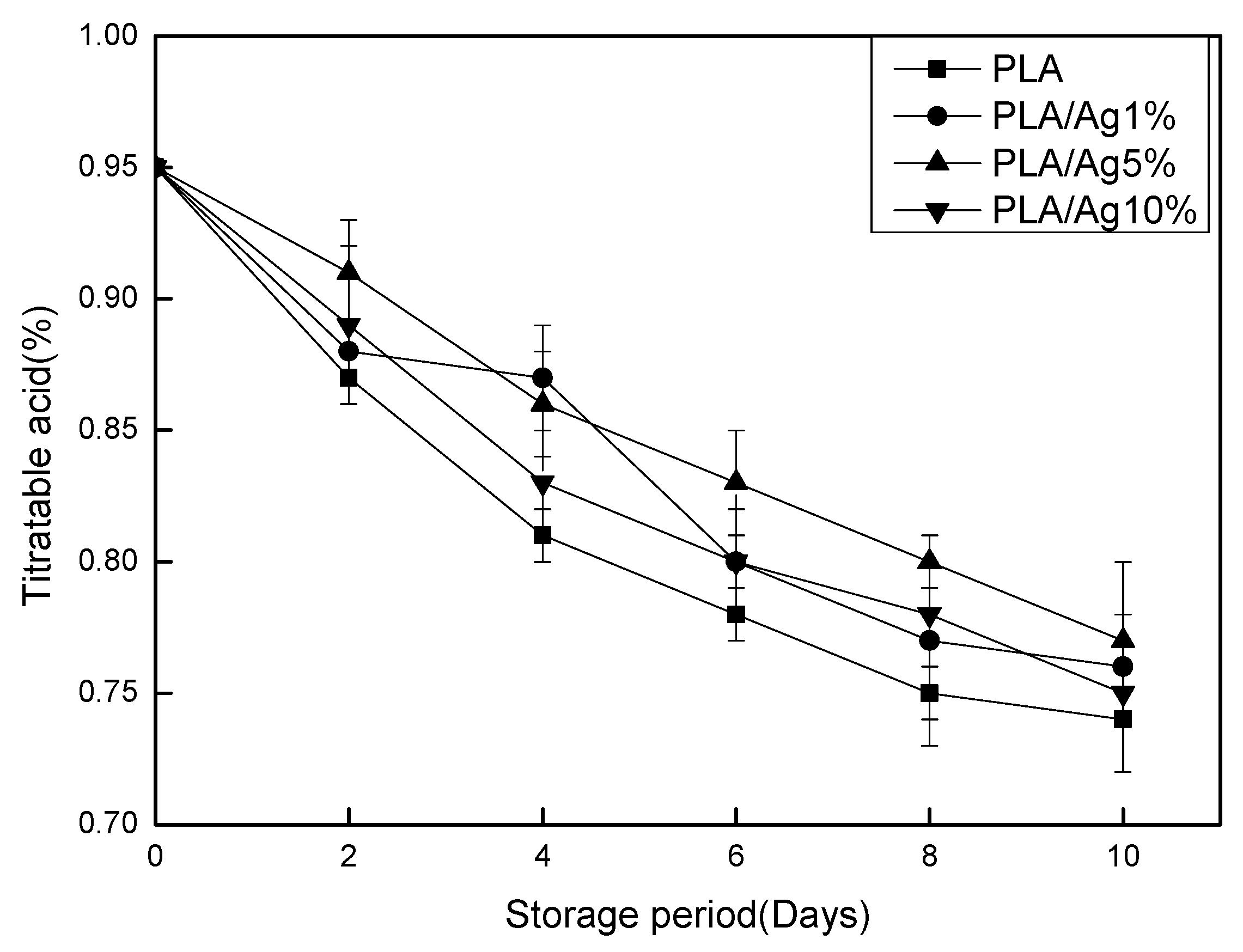
3.3. SSC and TA
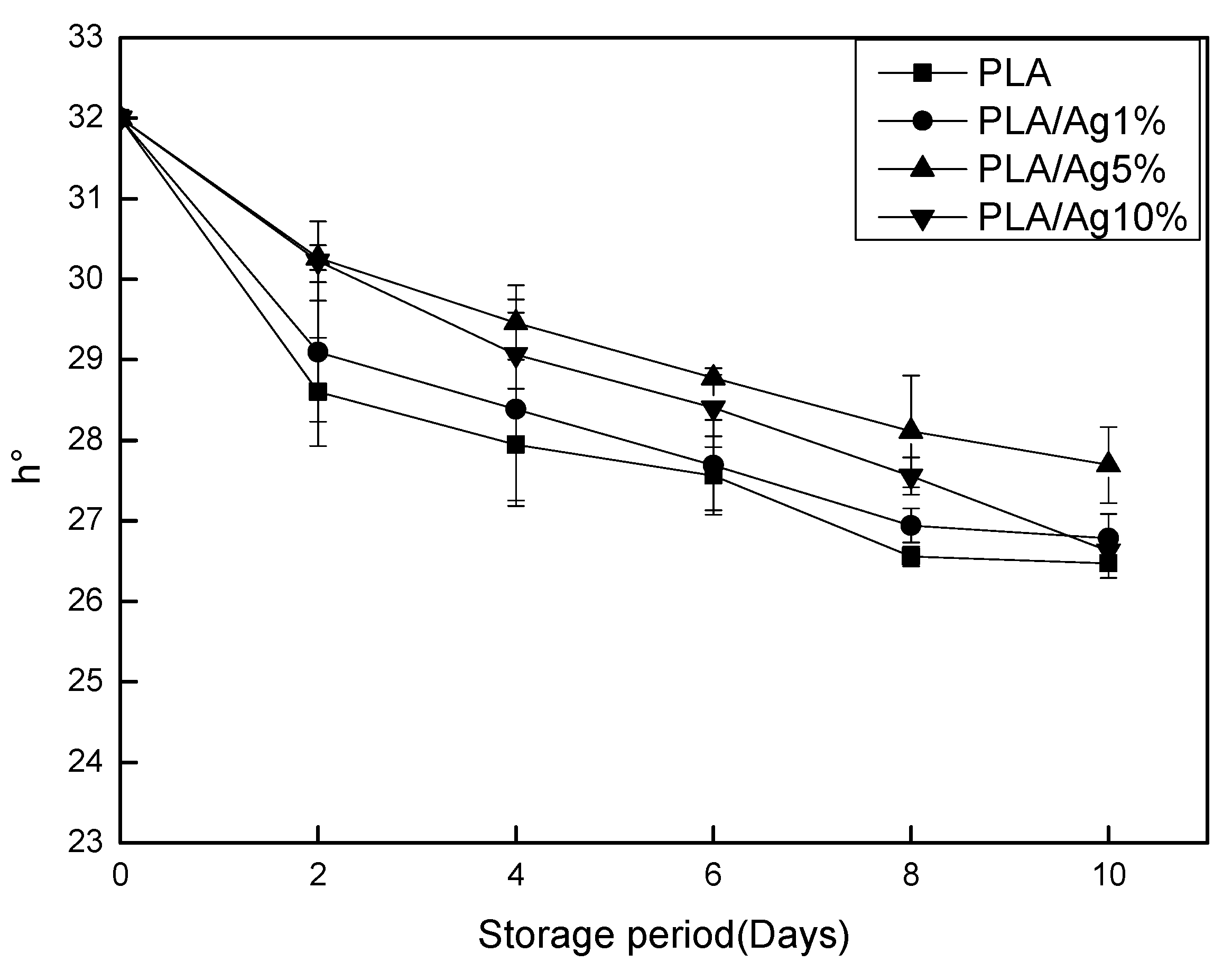
3.4. Surface Color
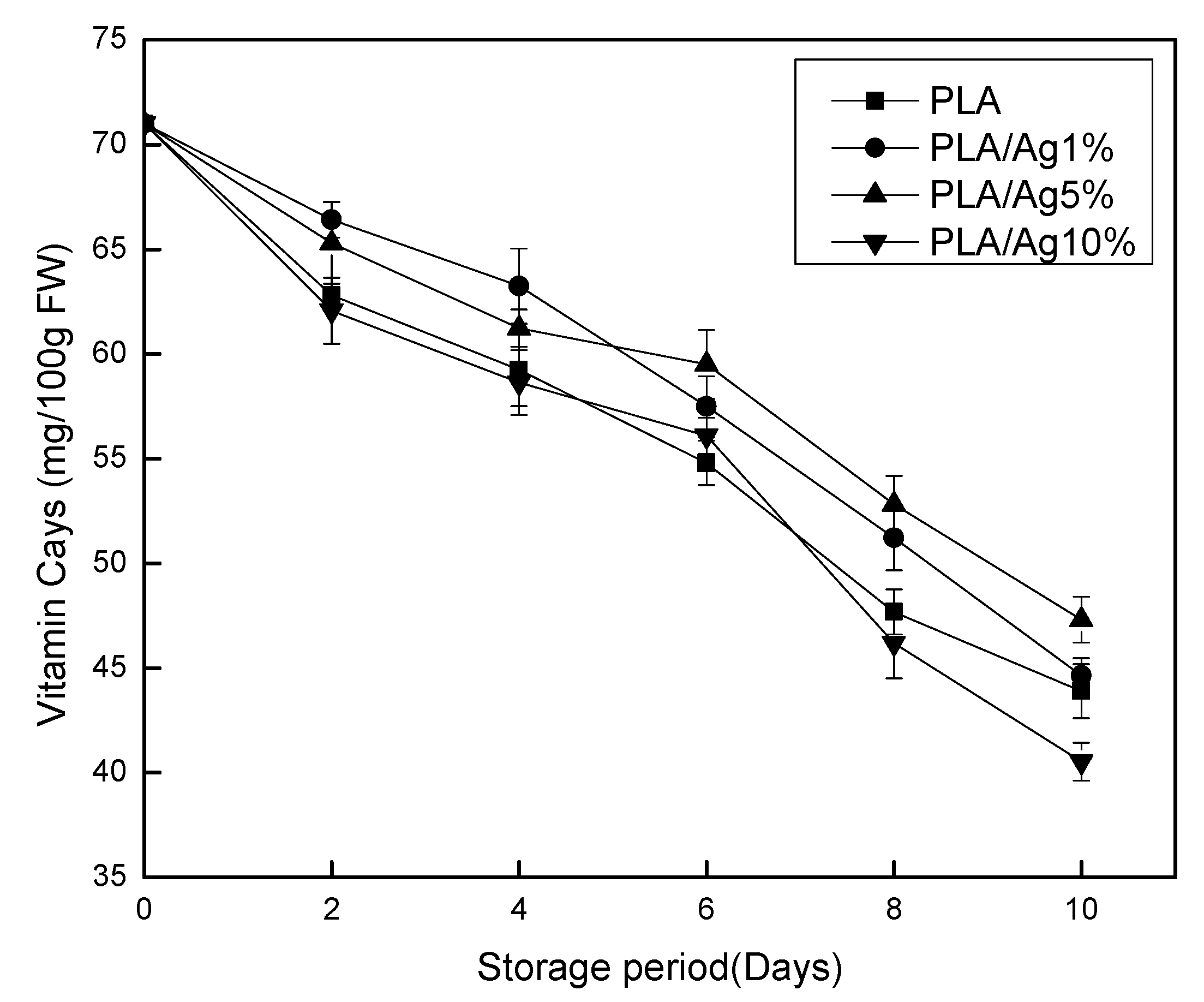
3.5. Determination of Vitamin C
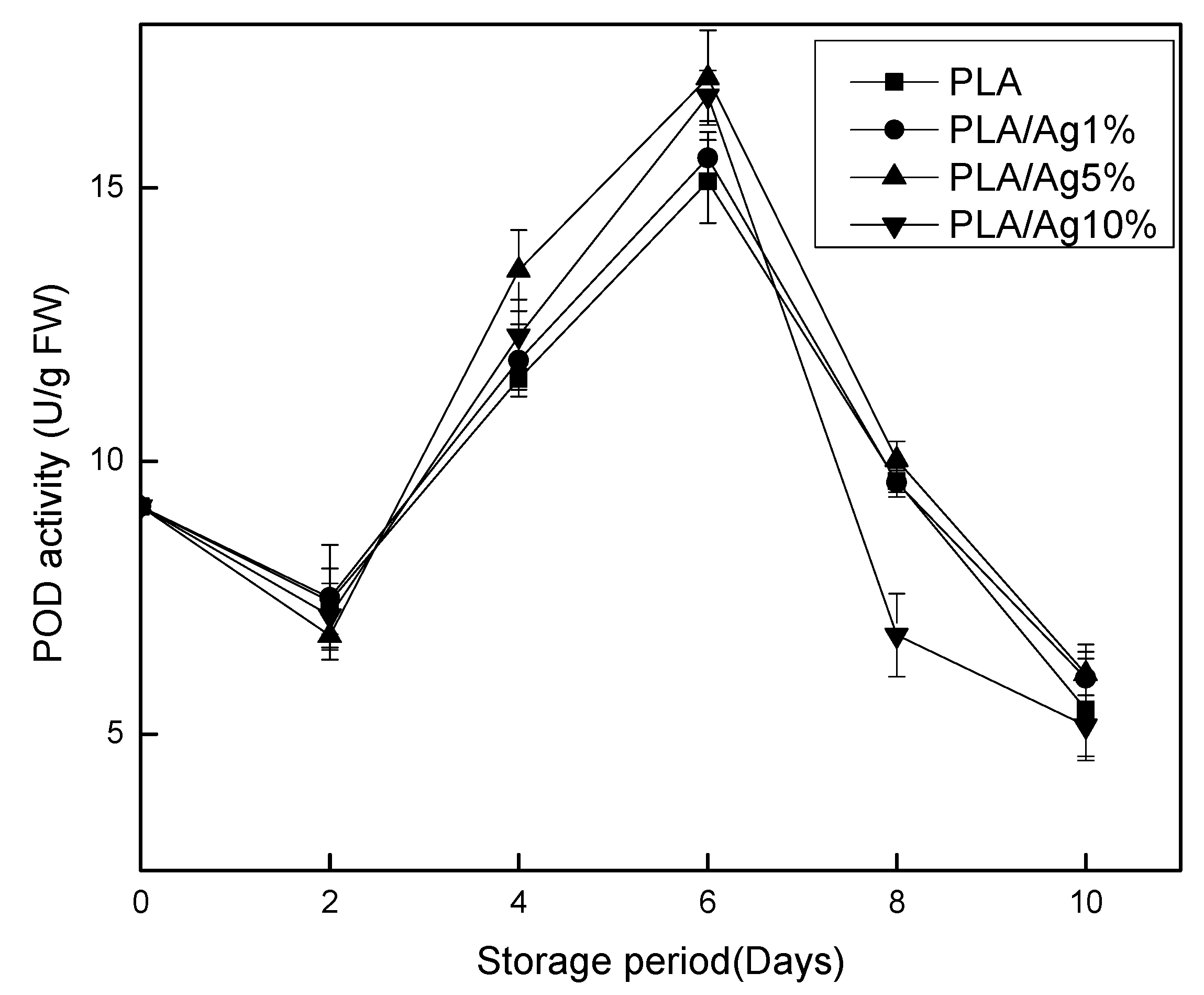
3.6. Pyrogallol Peroxidase (POD)
3.7. Total Phenolics Content
3.8. 1-Diphenyl-2-Picrylhydrazyl (DPPH)
3.9. Microbiological Analysis
3.10. Sensory Evaluation of Strawberries
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pombo, M.A.; Dotto, M.C.; Martinez, G.A.; Civello, P.M. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol. Technol. 2009, 51, 141–148. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Almenar, E.; Hernandez-Munoz, P.; Lagaron, J.M.; Catala, R.; Gavara, R. Controlled atmosphere storage of wild strawberry fruit (Fragaria vesca L.). J. Agric. Food Chem. 2006, 54, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.K.; Kim, H.J.; Lim, G.O.; Jang, S.A.; Song, K.B. The effects of aqueous chlorine dioxide or fumaric acid treatment combined with UV-C on postharvest quality of ‘Maehyang’ strawberries. Postharvest Biol. Technol. 2010, 56, 254–256. [Google Scholar]
- Chen, F.; Liu, H.; Yang, H.; Lai, S.; Cheng, X.; Xin, Y.; Yang, B.; Hou, H.; Yao, Y.; Zhang, S. Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment. Food Chem. 2011, 126, 450–459. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Singh, I.; Madaan, J. Tribological behavior of natural fiber reinforced PLA composites. Wear 2013, 297, 829–840. [Google Scholar] [CrossRef]
- Eili, M.; Shameli, K.; Ibrahim, N.A.; Yunus, W.M. Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers. Int. J. Mol. Sci. 2012, 13, 7938–7951. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Palza, H.; Delgado, K.; Rabagliati, F.M. Novel antimicrobial polyethylene composites prepared by metallocenic in situ polymerization with TiO2-based nanoparticles. J. Polym. Sci. Part A 2012, 50, 4055–4062. [Google Scholar] [CrossRef]
- Hua, Y.Q.; Zhang, Y.Q.; Wu, L.B.; Huang, Y.Q.; Wang, G.Q. Mechanical and Optical Properties of Polyethylene Filled with Nano-SiO2. J. Macromol. Sci. Part B 2005, 44, 149–159. [Google Scholar] [CrossRef]
- Li, X.H.; Li, W.L.; Xing, Y.G.; Jiang, Y.H.; Ding, Y.L.; Zhang, P.P. Effects of Nano-ZnO Power-Coated PVC Film on the Physiological Properties and Microbiological Changes of Fresh-Cut “Fuji” Apple. Adv. Mater. Res. 2011, 152–153, 450–453. [Google Scholar] [CrossRef]
- Brody, A.L. Noncomposite technology in food packaging. Food Technol. 2007, 61, 80–83. [Google Scholar]
- Silver, S.; Le, T.P. Bacterial Heavy Metal Resistance: New Surprises. Annu. Rev. Microbiol. 1996, 50, 753–789. [Google Scholar] [CrossRef] [PubMed]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Ghoshal, T.; Morris, M.A.; Cummins, E.; Kerry, J.P. Application of silver nanodots for potential use in antimicrobial packaging applications. Innov. Food Sci. Emerg. Technol. 2015, 27, 136–143. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Li, W.H.; Liu, D.; Yuan, M.L.; Li, L. Development of active packaging film made from poly(lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Liu, D.; Wu, Y.; Yuan, M.L.; Li, L.; Yang, J.Y. Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Gong, Y.; Mattheis, J.P. Effect of ethylene and 1-methylcyclopropene on chlorophyll catabolism of broccoli florets. Plant Growth Regul. 2003, 40, 33–38. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhuang, Y.; Wu, Y.; Li, L. Quality evaluation of hot peppers stored in biodegradable poly(lactic acid)-based active packaging. Sci. Hortic. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Rahman, M.M.; Islam, M.S.; Begum, S.A. A simple UV-spectrophotometric method for the determination of vitamin C content in various fruits and vegetables at Sylhet area in Bangladesh. J. Biol. Sci. 2006, 6, 2238. [Google Scholar] [CrossRef]
- Xu, G.H.; Liu, D.H.; Chen, J.C.; Ye, X.Q.; Ma, Y.Q.; Shi, J. Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem. 2008, 106, 545–551. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Sanchez-Moreno, C.; Saura-Calixto, F. Effect of temperature on the free radical scavenging capacity of extracts from red and white grape pomace peels. J. Agric. Food Chem. 1998, 46, 2694–2697. [Google Scholar] [CrossRef]
- Chen, X.N.; Ren, L.P.; Li, M.L.; Qian, J.; Fan, J.F.; Du, B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldao-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Ahmadi-Afzadi, M.; Tahir, I.; Nybom, H. Impact of harvesting time and fruit firmness on the tolerance to fungal storage diseases in an apple germplasm collection. Postharvest Biol. Technol. 2013, 82, 51–58. [Google Scholar] [CrossRef]
- Jiang, M.; Xiao-Jun, H.U.; Jiao, M.S.; Huang, S.J.; Qiu-Ping, L.U.; Qiao-Lin, F.U. Preservative effect of citric acid and nano-ZnO treatment on storage of mango at room temperature. North. Hortic. 2012, 11, 172–176. [Google Scholar]
- Li, H.; Li, F.; Wang, L.; Sheng, J.; Xin, Z.; Zhao, L.; Xiao, H.; Zheng, Y.; Hu, Q. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd). Food Chem. 2009, 114, 547–552. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y.G.; Chen, P. Elucidation of the mechanism of enzymatic browning inhibition by sodium chlorite. Food Chem. 2008, 110, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A.; Hadley, P. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 2012, 92, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Mcerlain, L.; Marson, H.; Ainsworth, P.; Burnett, S.A. Ascorbic acid loss in vegetables: Adequacy of a hospital cook-chill system. Int. J. Food Sci. Nutr. 2001, 52, 205–211. [Google Scholar] [PubMed]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int. J. Food Sci. Technol. 2012, 47, 2318–2324. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Changes in color, antioxidant, and free radical scavenging enzyme activity of mushrooms under high oxygen modified atmospheres. Postharvest Biol. Technol. 2012, 69, 1–6. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007, 55, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Junliang, L.; Chen, K.; Zhang, S. Study on the change in ethylene production, the activity of superoxide dismutase and peroxidase in Chinese gooseberry fruits during ripening. J. Zhejiang Agric. Univ. 1993, 19, 135–138. [Google Scholar]
- Ku, H.S.; Yang, S.F.; Pratt, H.K. Ethylene production and peroxidase activity during tomato fruit ripening. Plant Cell Physiol. 1970, 11, 241–246. [Google Scholar] [CrossRef]
- Mazza, G.; Brouillard, R. The mechanism of co-pigmentation of anthocyanins in aqueous solutions. Phytochemistry 1990, 29, 1097–1102. [Google Scholar] [CrossRef]
- Bhat, R.; Stamminger, R. Impact of ultraviolet radiation treatments on the physicochemical properties, antioxidants, enzyme activity and microbial load in freshly prepared hand pressed strawberry juice. Food Sci. Technol. Int. 2015, 21, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.M.; Li, H.M.; Li, F.; Xin, Z.H.; Zhao, L.Y.; Zheng, Y.H.; Hu, Q.H. Effect of Nano-Packing on Preservation Quality of Fresh Strawberry (Fragaria ananassa Duch. cv Fengxiang) during Storage at 4 degrees C. J. Food Sci. 2010, 75, C236–C240. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Ryu, J.A.; Liu, R.H.; Nock, J.F.; Watkins, C.B. Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol. 2008, 49, 201–209. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; Gonzalez-Aguilar, G.A. High oxygen treatment increases antioxidant capacity and postharvest life of strawberry fruit. Food Technol. Biotechnol. 2007, 45, 166–173. [Google Scholar]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Li, D.; Ye, Q.; Lei, J.; Luo, Z. Effects of nano-TiO2-LDPE packaging on postharvest quality and antioxidant capacity of strawberry (Fragaria ananassa Duch.) stored at refrigeration temperature. J. Sci. Food Agric. 2016, 97, 1116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Hu, J.L.; Kim, S.H.; Park, Y.K.; Yong, H.P.; Hwang, C.Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Bensaid, S.; Geobaldo, F.; Saracco, G.; Russo, N. Photocatalytic Degradation of Ethylene Emitted by Fruits with TiO2 Nanoparticles. Ind. Eng. Chem. Res. 2010, 50, 2536–2543. [Google Scholar] [CrossRef]
- Fan, J.; Chu, Z.; Li, L.; Zhao, T.; Yin, M.; Qin, Y. Physicochemical Properties and Microbial Quality of Tremella aurantialba Packed in Antimicrobial Composite Films. Molecules 2017, 22, 500. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, L.; Yun, C.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA Film Incorporated with ZnO Nanoparticle on the Quality Attributes of Fresh-Cut Apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Odor | Color | Texture | Overall Acceptability |
|---|---|---|---|---|
| Day 0 | 9 | 9 | 9 | 9 |
| Day 2 | ||||
| PLA | 8.18 ± 0.03 ab | 7.87 ± 0.32 a | 8.07 ± 0.17 a | 7.94 ± 0.02 a |
| PLA/Ag1% | 8.05 ± 0.18 a | 8.06 ± 0.32 a | 8.22 ± 0.04 ab | 8.05 ± 0.59 a |
| PLA/Ag5% | 8.29 ± 0.07 b | 8.08 ± 0.05 a | 8.28 ± 0.07 b | 8.13 ± 0.11 a |
| PLA/Ag10% | 8.22 ± 0.04 ab | 8.07 ± 0.15 a | 8.19 ± 0.04 ab | 8.04 ± 0.02 a |
| Day 4 | ||||
| PLA | 7.19 ± 0.04 a | 7.05 ± 0.39 a | 6.98 ± 0.14 a | 6.89 ± 0.27 a |
| PLA/Ag1% | 7.37 ± 0.18 a | 6.85 ± 0.22 a | 7.05 ± 0.18 a | 7.07 ± 0.01 ab |
| PLA/Ag5% | 7.37 ± 0.13 a | 7.51 ± 0.44 a | 7.18 ± 0.01 a | 7.2 ± 0.08 b |
| PLA/Ag10% | 7.24 ± 0.02 a | 7.19 ± 0.21 a | 7.12 ± 0.08 a | 7.12 ± 0.06 ab |
| Day 6 | ||||
| PLA | 6.68 ± 0.06 ab | 6.27 ± 0.21 a | 6.45 ± 0.05 a | 6.03 ± 0.4 a |
| PLA/Ag1% | 6.67 ± 0.02 ab | 6.31 ± 0.17 a | 6.49 ± 0.01 ab | 6.52 ± 0.02 b |
| PLA/Ag5% | 6.76 ± 0.09 b | 6.63 ± 0.02 b | 6.55 ± 0.04 b | 6.71 ± 0.05 b |
| PLA/Ag10% | 6.59 ± 0.1 a | 6.52 ± 0.15 ab | 6.46 ± 0.04 a | 6.4 ± 0.08 ab |
| Day 8 | ||||
| PLA | 6.22 ± 0.02 ab | 5.17 ± 0.14 a | 5.99 ± 0.27 a | 4.78 ± 0.06 a |
| PLA/Ag1% | 6.13 ± 0.16 a | 5.38 ± 0.21 a | 6.1 ± 0.07 a | 5.9 ± 0.04 ab |
| PLA/Ag5% | 6.29 ± 0.05 b | 5.43 ± 0.21 a | 6.12 ± 0.35 a | 6.3 ± 0.56 b |
| PLA/Ag10% | 6.16 ± 0.03 ab | 5.34 ± 0.06 a | 6.08 ± 0.05 a | 4.82 ± 1.02 a |
| Day 10 | ||||
| PLA | 5.71 ± 0.23 b | 5.01 ± 0.55 a | 5.45 ± 0.12 ab | 4.23 ± 0.57 a |
| PLA/Ag1% | 5.05 ± 0.08 a | 5.03 ± 0.09 a | 5.36 ± 0.12 a | 5.36 ± 0.24 b |
| PLA/Ag5% | 5.66 ± 0.32 ab | 5.14 ± 0.25 a | 5.6 ± 0.03 b | 5.73 ± 0.04 b |
| PLA/Ag10% | 5.13 ± 0.49 ab | 4.99 ± 0.02 a | 5.46 ± 0.06 ab | 5.35 ± 0.15 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers 2018, 10, 894. https://doi.org/10.3390/polym10080894
Zhang C, Li W, Zhu B, Chen H, Chi H, Li L, Qin Y, Xue J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers. 2018; 10(8):894. https://doi.org/10.3390/polym10080894
Chicago/Turabian StyleZhang, Cheng, Wenhui Li, Bifen Zhu, Haiyan Chen, Hai Chi, Lin Li, Yuyue Qin, and Jing Xue. 2018. "The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature" Polymers 10, no. 8: 894. https://doi.org/10.3390/polym10080894
APA StyleZhang, C., Li, W., Zhu, B., Chen, H., Chi, H., Li, L., Qin, Y., & Xue, J. (2018). The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers, 10(8), 894. https://doi.org/10.3390/polym10080894






