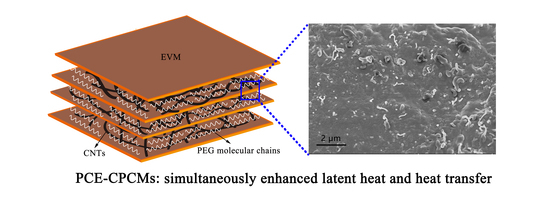
Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PCE-CPCMs
2.3. Characterization
3. Results and Discussion
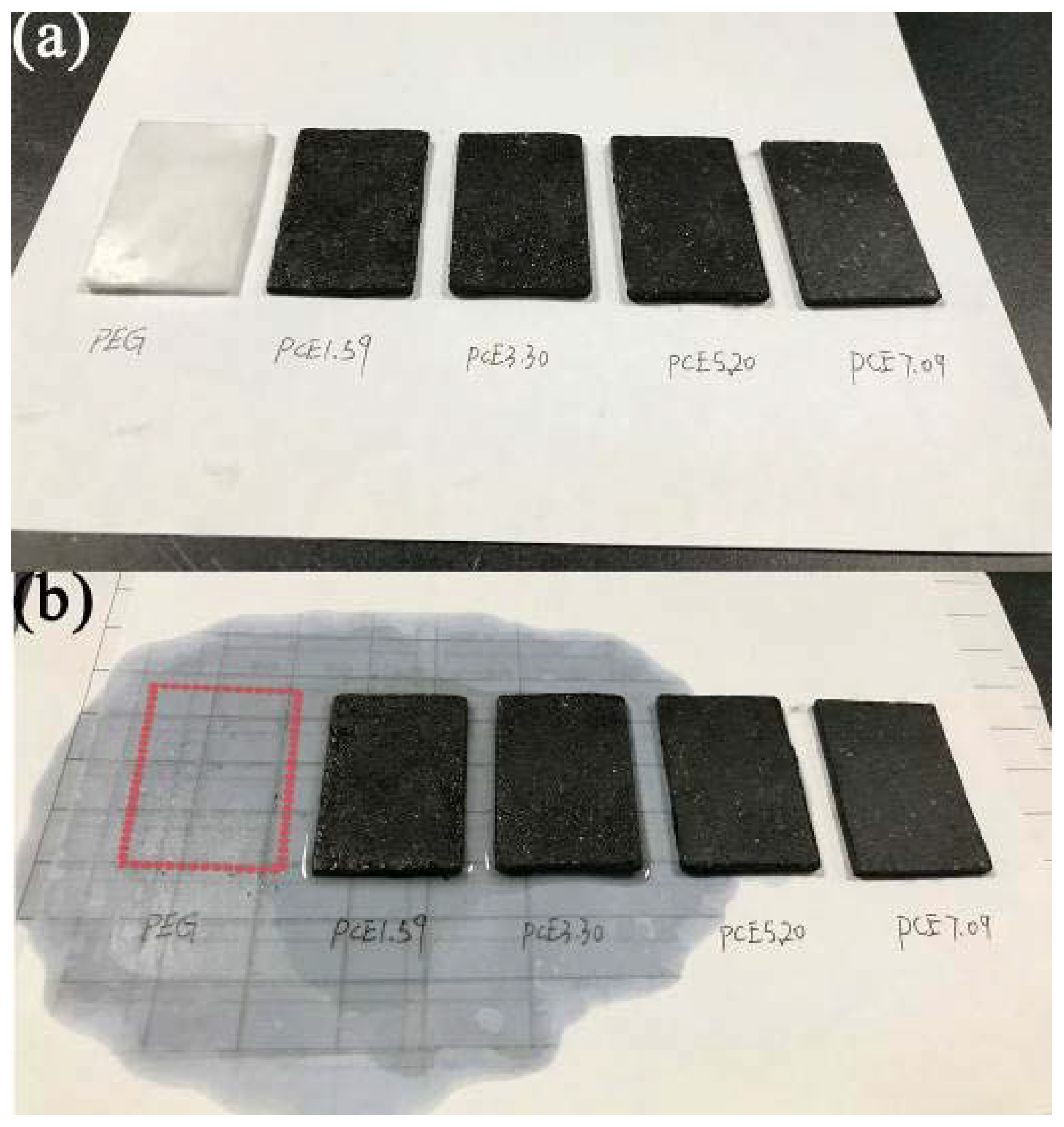
3.1. Form-Stability Analysis
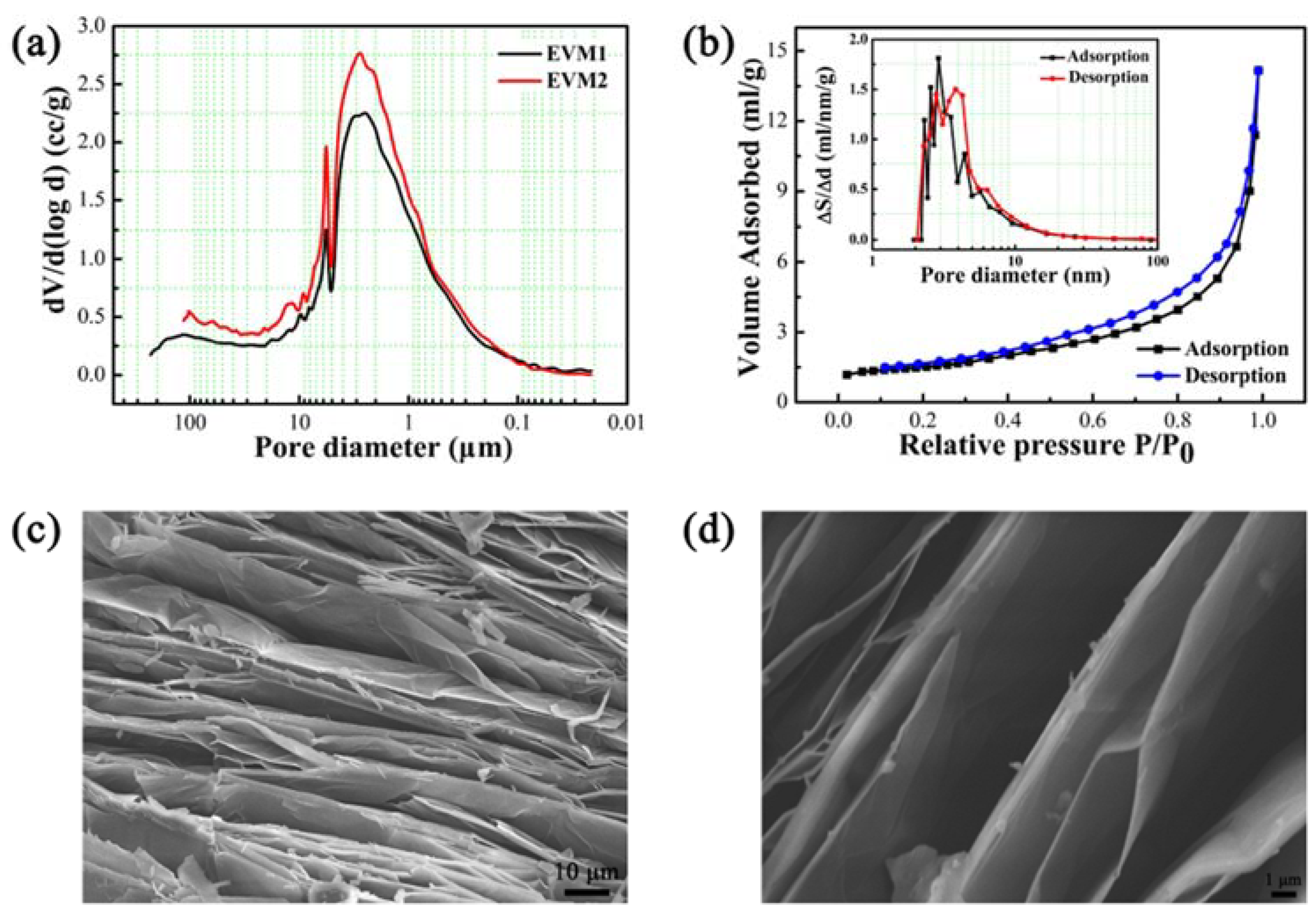
3.2. Characterization of EVM Pore Structures
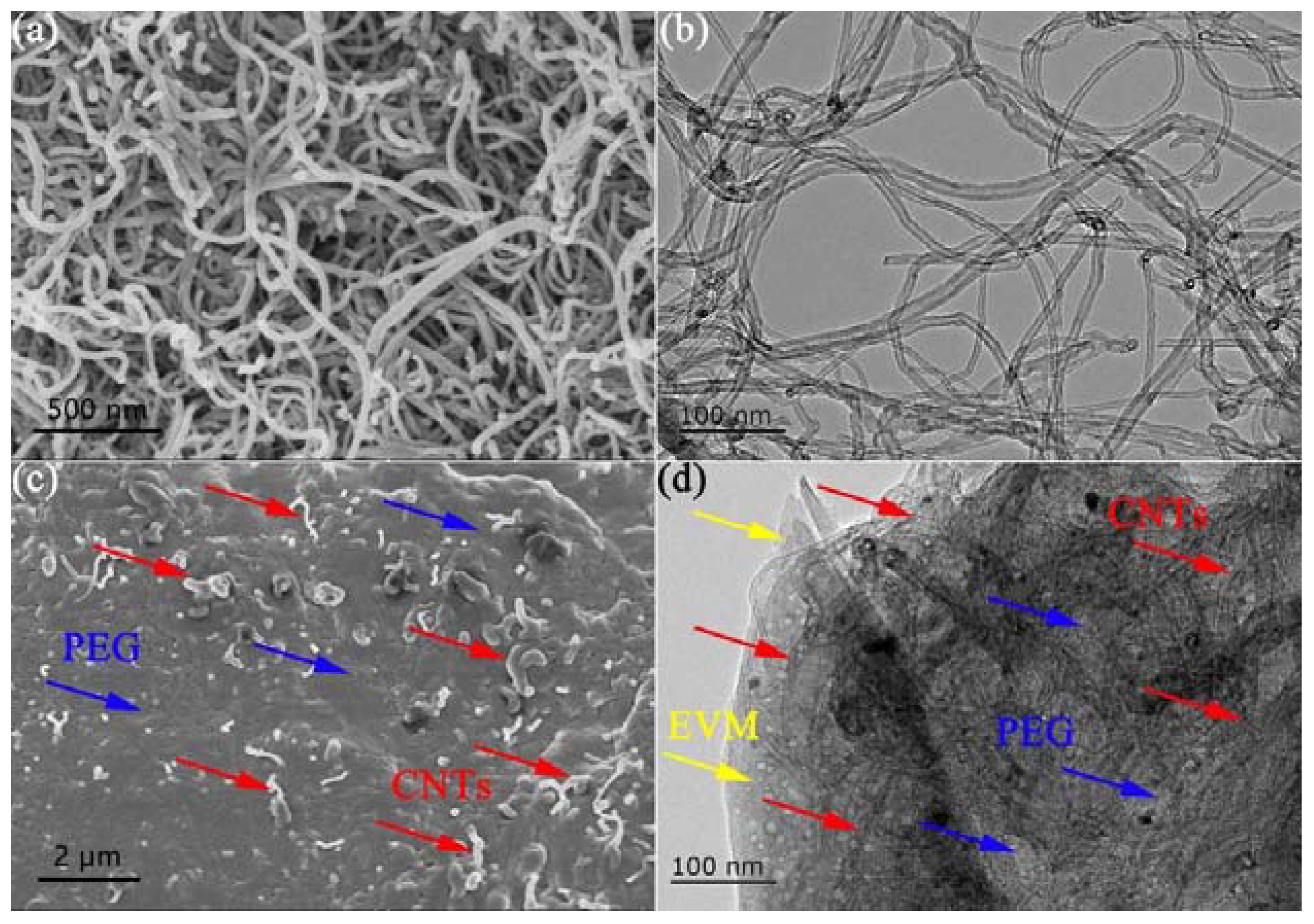
3.3. Morphology and Microstructure of CNTs and PCE-CPCMs
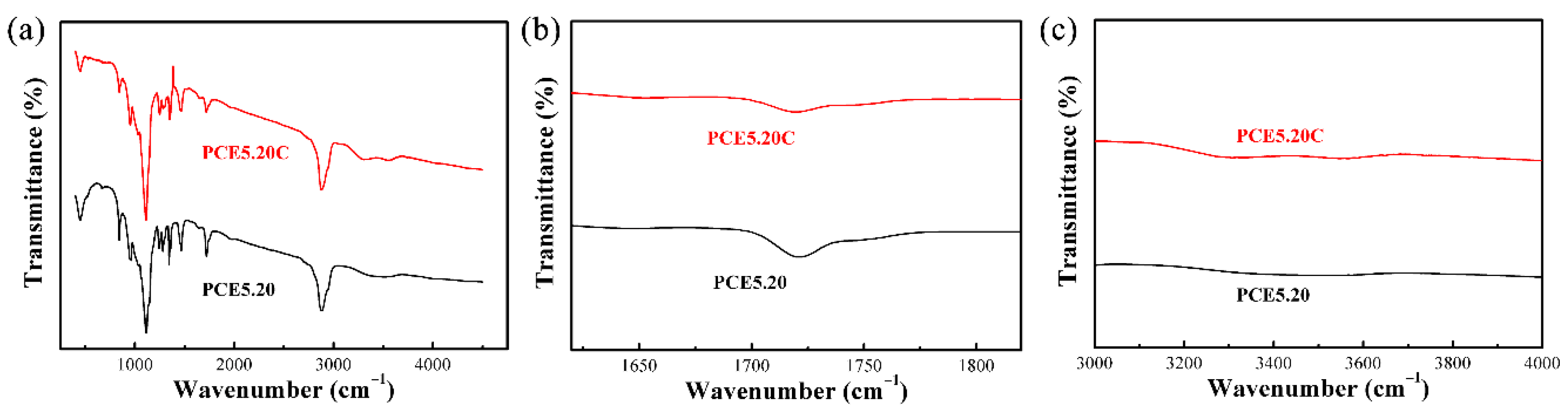
3.4. Chemical Compatibility of PCE-CPCMs
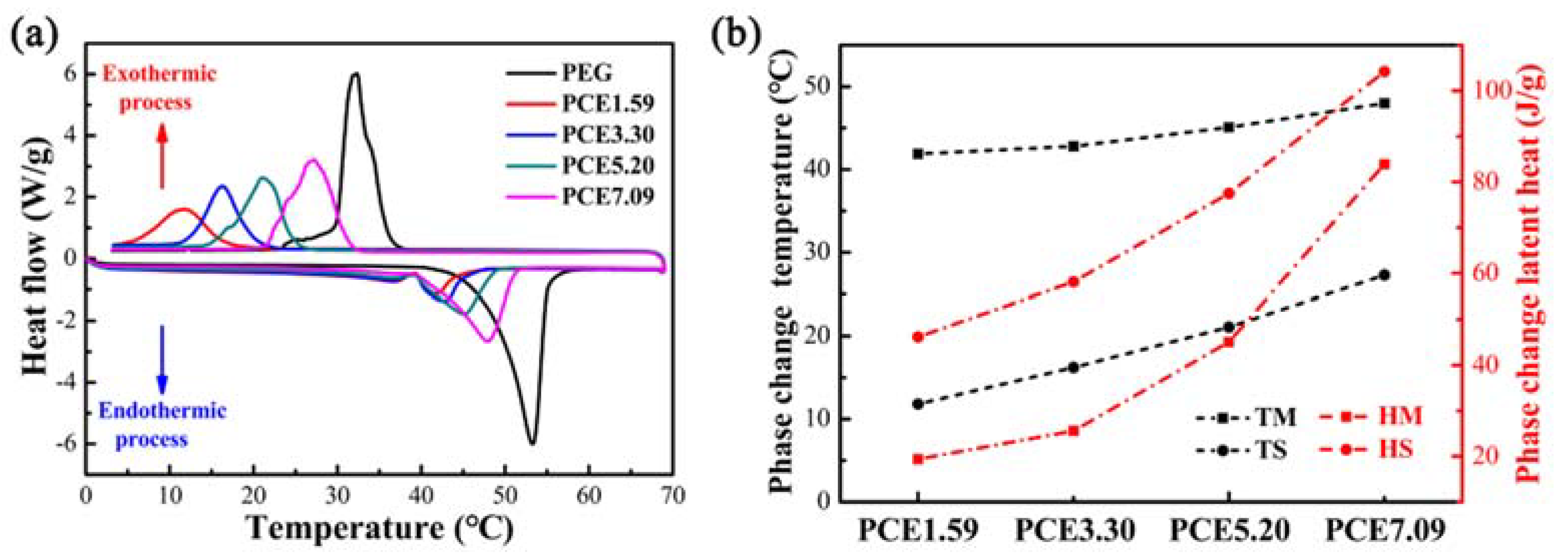
3.5. PCE-CPCMs Heat Storage Behavior
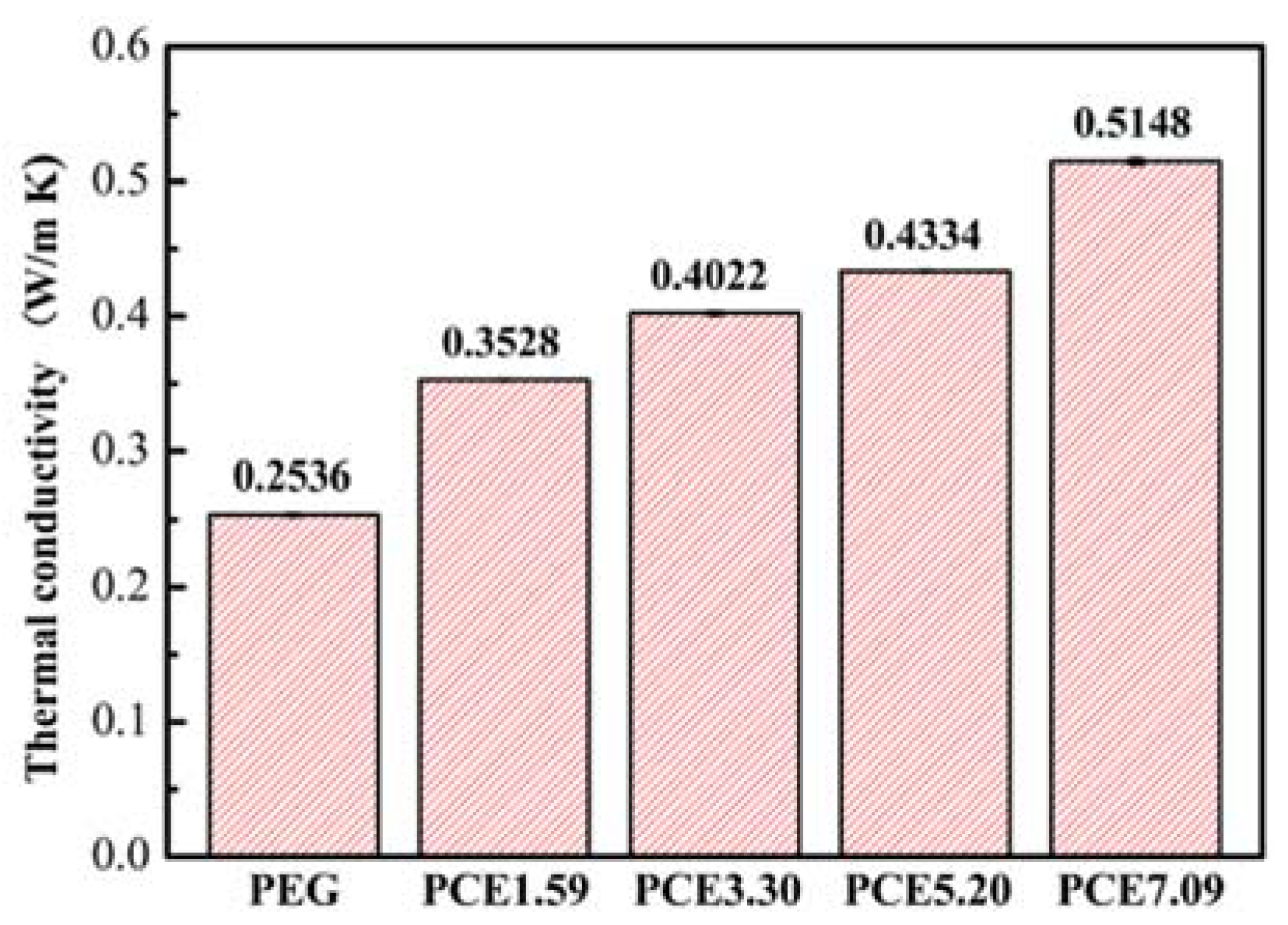
3.6. PCE-CPCMs Thermal Conductivity
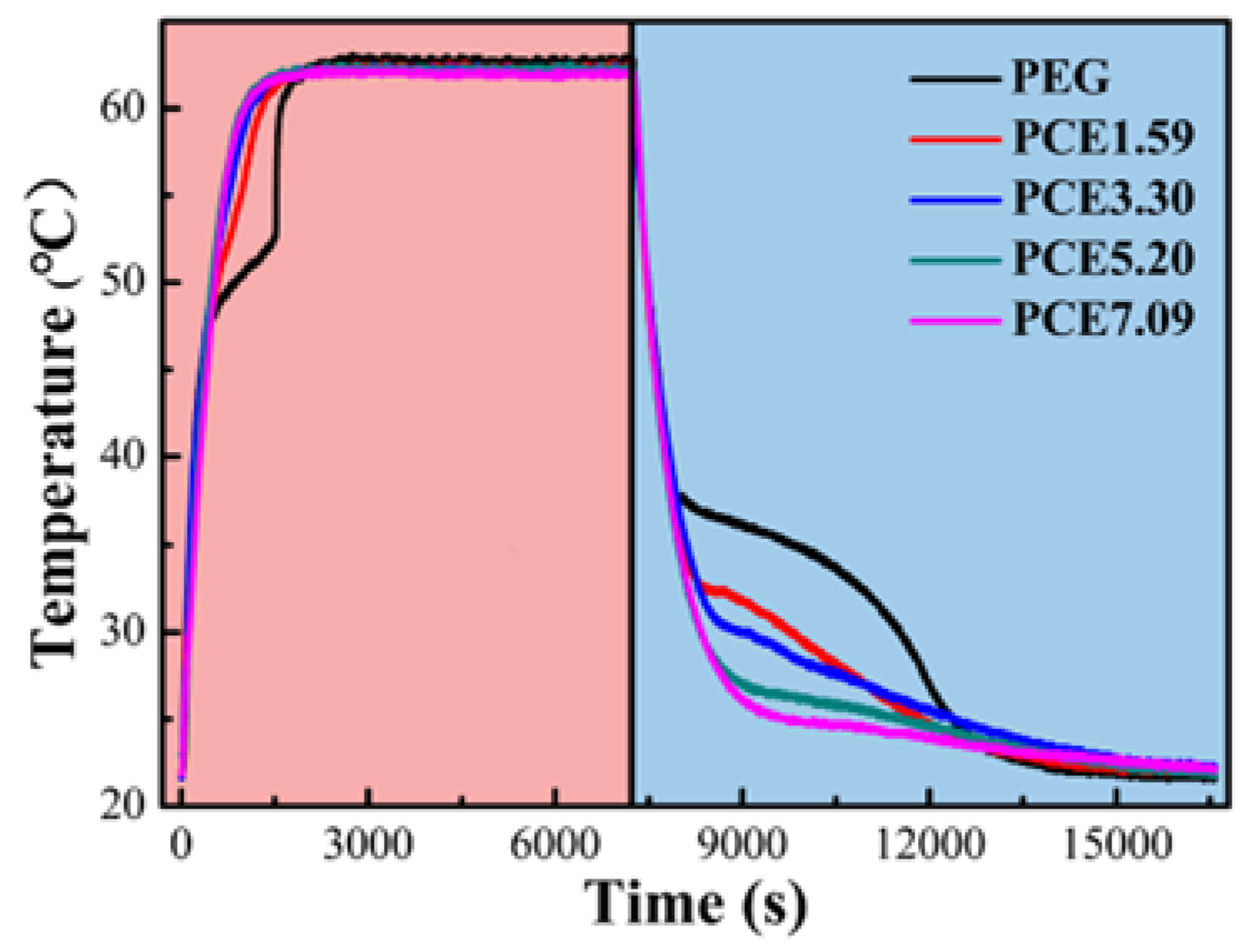
3.7. PCE-CPCMs Heat Storage and Release Properties
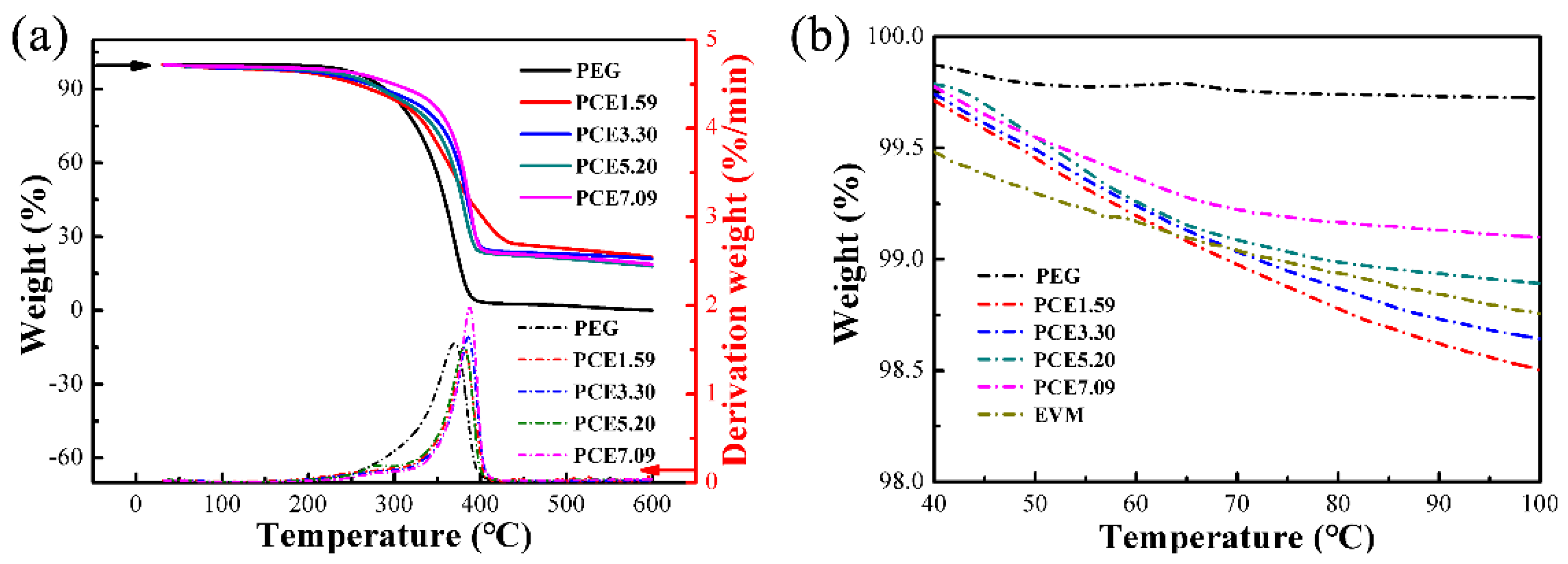
3.8. PCE-CPCMs Thermal Stability
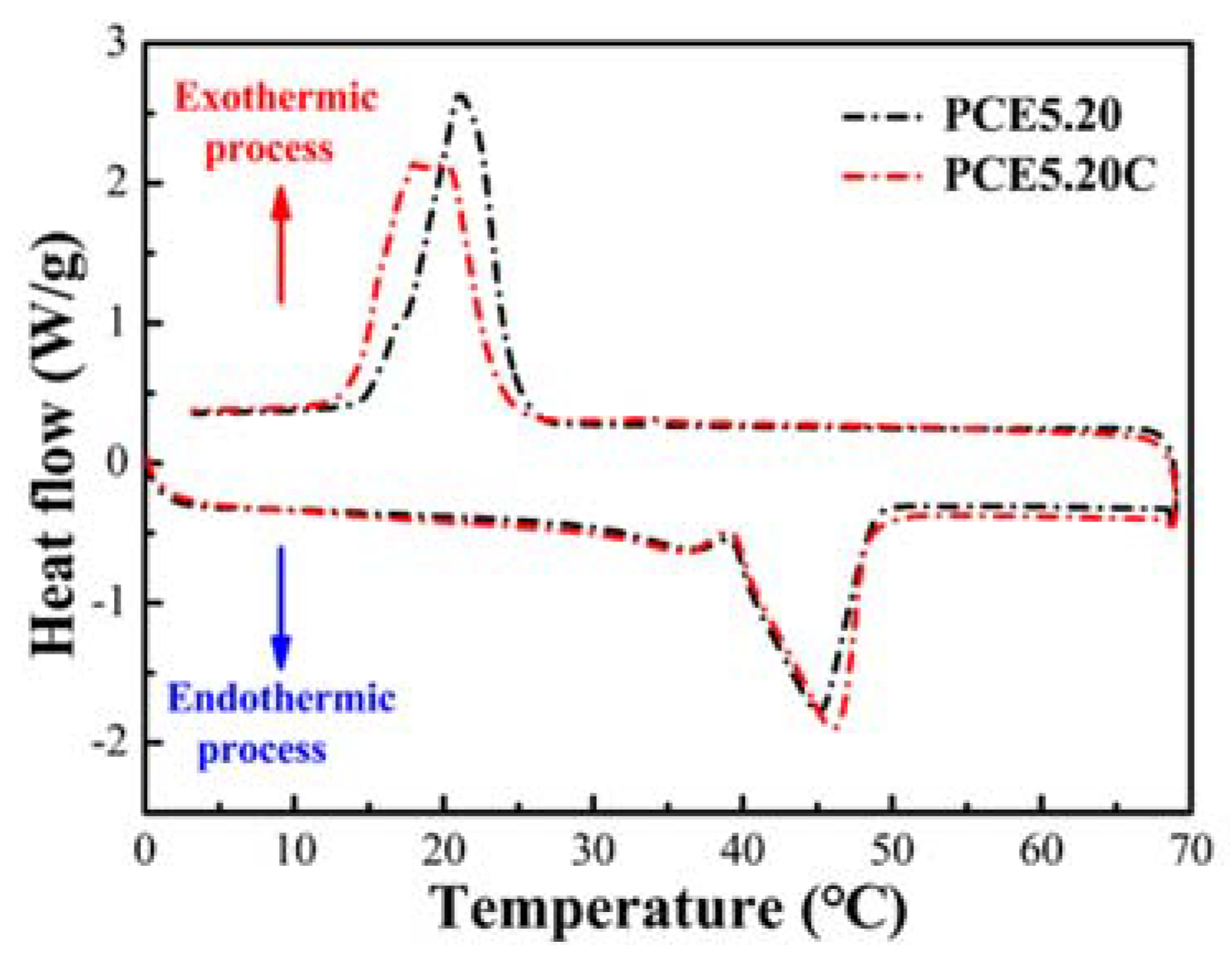
3.9. PCE-CPCMs Thermal Reliability
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Lin, Y.X.; Jia, Y.T.; Alva, G.; Fang, G.Y. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Al-Sulaiman, F.A.; Rahman, S.; Yilbas, B.S.; Sahin, A.Z. Heat transfer enhancement of phase change materials for thermal energy storage applications: A critical review. Renew. Sustain. Energy Rev. 2017, 74, 26–50. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid-liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Lv, P.Z.; Liu, C.Z.; Rao, Z.H. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Wang, W.L.; Yang, X.X.; Fang, Y.T.; Ding, J.; Yan, J.Y. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using beta-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.Q.; Zhou, W.B.; Wang, J.; Wang, Y. Thermal and electrical conductivity enhancement of graphite nanoplatelets on form-stable polyethylene glycol/polymethyl methacrylate composite phase change materials. Energy 2012, 39, 294–302. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wei, H.P.; Tang, B.T.; Xu, S.M.; Zhang, S.F. Novel light-driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Tang, B.T.; Wang, Y.M.; Qiu, M.G.; Zhang, S.F. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol. Energy Mater. Sol. Cells 2014, 123, 7–12. [Google Scholar] [CrossRef]
- Qian, T.T.; Li, J.H.; Min, X.; Guan, W.M.; Deng, Y.; Ning, L. Enhanced thermal conductivity of PEG/diatomite shape-stabilized phase change materials with Ag nanoparticles for thermal energy storage. J. Mater. Chem. A 2015, 3, 8526–8536. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.S.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Hybrid network structure of boron nitride and graphene oxide in shape-stabilized composite phase change materials with enhanced thermal conductivity and light-to-electric energy conversion capability. Sol. Energy Mater. Sol. Cells 2018, 174, 56–64. [Google Scholar] [CrossRef]
- Chen, L.J.; Zou, R.Q.; Xia, W.; Liu, Z.P.; Shang, Y.Y.; Zhu, J.L.; Wang, Y.X.; Lin, J.H.; Xia, D.G.; Cao, A.Y. Electro- and Photodriven Phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.T.; Li, J.H.; Feng, W.W.; Nian, H.E. Single-walled carbon nanotube for shape stabilization and enhanced phase change heat transfer of polyethylene glycol phase change material. Energy Convers. Manag. 2017, 143, 96–108. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Chen, Z.; Liu, X. Preparation and characterization of stearic acid/expanded graphite composites as thermal energy storage materials. Energy 2010, 35, 4622–4626. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Cao, J. An energy-efficient composite by using expanded graphite stabilized paraffin as phase change material. Compos. Part A Appl. Sci. Manuf. 2018, 107, 83–93. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.S.; Bai, L.; Bao, R.Y.; Liu, Z.; Xie, B.H.; Yang, M.B.; Yang, W. Photodriven shape-stabilized phase change materials with optimized thermal conductivity by tailoring the microstructure of hierarchically ordered hybrid porous scaffolds. ACS Sustain. Chem. Eng. 2018, 6, 6761–6770. [Google Scholar] [CrossRef]
- Qian, Z.C.; Shen, H.; Fang, X.; Fan, L.W.; Zhao, N.; Xu, J. Phase change materials of paraffin in h-BN porous scaffolds with enhanced thermal conductivity and form stability. Energy Build. 2018, 158, 1184–1188. [Google Scholar] [CrossRef]
- Zhou, D.; Shire, G.S.F.; Tian, Y. Parametric analysis of influencing factors in phase change material wallboard (PCMW). Appl. Energy 2014, 119, 33–42. [Google Scholar] [CrossRef]
- Yao, C.; Kong, X.; Li, Y.; Du, Y.; Qi, C. Numerical and experimental research of cold storage for a novel expanded perlite-based shape-stabilized phase change material wallboard used in building. Energy Convers. Manag. 2018, 155, 20–31. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Jeong, S.G.; Chang, S.J.; Wi, S.; Lee, J.; Kim, S. Energy performance evaluation of heat-storage gypsum board with hybrid SSPCM composite. J. Ind. Eng. Chem. 2017, 51, 237–243. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.H.; Qian, T.T.; Guan, W.M.; Li, Y.L.; Yin, X.P. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Nian, H. Polyethylene glycol-enwrapped silicon carbide nanowires network/expanded vermiculite composite phase change materials: Form-stabilization, thermal energy storage behavior and thermal conductivity enhancement. Sol. Energy Mater. Sol. Cells 2018, 174, 283–291. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z. Thermodynamics behavior of phase change latent heat materials in micro-/nanoconfined spaces for thermal storage and applications. Renew. Sustain. Energy Rev. 2018, 82, 2319–2331. [Google Scholar] [CrossRef]
- Xu, B.W.; Ma, H.Y.; Lu, Z.Y.; Li, Z.J. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Capric-myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Gubbins, K.E.; Watanabe, A.; Kaneko, K. Freezing of simple fluids in microporous activated carbon fibers: Comparison of simulation and experiment. J. Chem. Phys. 1999, 111, 9058–9067. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, S.L.; Xiao, D.Y. Experimental study on the phase change behavior of phase change material confined in pores. Sol. Energy 2007, 81, 653–660. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.Y.; Sheng, N.; Tabuchi, K.; Sagara, A.; Akiyama, T. Shape-stabilized phase change composite by impregnation of octadecane into mesoporous SiO2. Sol. Energy Mater. Sol. Cells 2015, 143, 424–429. [Google Scholar] [CrossRef]
- Burba, C.M.; Janzen, J. Confinement effects on the phase transition temperature of aqueous NaCl solutions: The extended Gibbs-Thomson equation. Thermochim. Acta 2015, 615, 81–87. [Google Scholar] [CrossRef]
- Alamo, R.G.; Viers, B.D.; Mandelkern, L. A reexamination of the relation between the melting temperature and the crystallization temperature—Linear polyethylene. Macromolecules 1995, 28, 3205–3213. [Google Scholar] [CrossRef]
- Gao, C.F.; Wang, L.P.; Li, Q.F.; Wang, C.; Nan, Z.D.; Lan, X.Z. Tuning thermal properties of latent heat storage material through confinement in porous media: The case of (1-CnH2n+1NH3)2ZnCl4 (n = 10 and 12). Sol. Energy Mater. Sol. Cells 2014, 128, 221–230. [Google Scholar] [CrossRef]
- Yang, H.Z.; Feng, L.L.; Wang, C.Y.; Zhao, W.; Li, X.G. Confinement effect of SiO2 framework on phase change of PEG in shape-stabilized PEG/SiO2 composites. Eur. Polym. J. 2012, 48, 803–810. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wen, R.L.; Tang, C.; Wu, B.G.; Huang, Z.H.; Min, X.; Huang, Y.T.; Liu, Y.G.; Fang, M.H.; Wu, X.W. Thermal conductivity enhancement of polyethylene glycol/expanded perlite with carbon layer for heat storage application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wen, R.L.; Huang, Z.H.; Tang, C.; Huang, Y.T.; Liu, Y.G.; Fang, M.H.; Wu, X.W.; Min, X.; Xu, Y.G. Enhancement of thermal conductivity by the introduction of carbon nanotubes as a filler in paraffin/expanded perlite form-stable phase-change materials. Energy Build. 2017, 149, 463–470. [Google Scholar] [CrossRef]
- Wen, R.L.; Zhang, X.G.; Huang, Z.H.; Fang, M.H.; Liu, Y.G.; Wu, X.W.; Min, X.; Gao, W.; Huang, S.F. Preparation and thermal properties of fatty acid/diatomite form-stable composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 273–279. [Google Scholar] [CrossRef]
- Karaipekli, A.; Bicer, A.; Sari, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]

| Constituent | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | H2O |
|---|---|---|---|---|---|---|---|
| Ratio (wt %) | 43.2 | 12.68 | 4.56 | 24.2 | 0.96 | 5.95 | 7.6 |
| PCE-CPCMs | PEG (g) | CNT (g) | EVM (g) | PEG Weight Fractions (wt %) | CNT Weight Fractions (wt %) | EVM Weight Fractions (wt %) |
|---|---|---|---|---|---|---|
| PCE1.59 | 7.5360 | 0.1541 | 2.0025 | 77.75 | 1.59 | 20.66 |
| PCE3.30 | 9.1336 | 0.3800 | 2.0027 | 79.31 | 3.30 | 17.39 |
| PCE5.20 | 12.3063 | 0.7849 | 2.0030 | 81.53 | 5.20 | 13.27 |
| PCE7.09 | 14.3588 | 1.2485 | 2.0022 | 81.54 | 7.09 | 11.37 |
| Sample | Melting Process | Solidification Process | ||
|---|---|---|---|---|
| TM (°C) | HM (J/g) | TS (°C) | HS (J/g) | |
| PEG | 53.27 | 176.7 | 32.28 | 160.8 |
| PCE1.59 | 41.88 | 19.4 | 11.81 | 46.2 |
| PCE3.30 | 42.80 | 25.6 | 16.23 | 58.1 |
| PCE5.20 | 45.09 | 45.1 | 21.09 | 77.5 |
| PCE7.09 | 47.98 | 83.9 | 27.26 | 104.2 |
| Sample (Mass Ratio) | Melting Process | Solidification Process | ||||
|---|---|---|---|---|---|---|
| TM (°C) | HM (J/g) | HC (J/g) | TS (°C) | HS (J/g) | HC (J/g) | |
| PEG-EVM (68.59:31.41) | 57.11 | 95.15 | 137.46 | 32.62 | 82.48 | 127.66 |
| PEG-CNTs (90:10) | 55.30 | 165.4 | 165.1 | 43.63 | 151.6 | 150.8 |
| PEG-CNTs (92:8) | 55.76 | 169.4 | 168.7 | 43.25 | 154.6 | 154.2 |
| PEG-CNTs (94:6) | 56.03 | 172.4 | 172.4 | 43.03 | 157.6 | 157.5 |
| PEG-CNTs (96:4) | 56.32 | 176.4 | 176.1 | 42.81 | 160.6 | 160.9 |
| PEG-CNTs (98:2) | 56.68 | 179.4 | 179.7 | 42.66 | 163.6 | 164.2 |
| Sample | Melting Process | Solidification Process | ||
|---|---|---|---|---|
| PCE1.59 | 14.12 | 85.88 | 36.95 | 63.05 |
| PCE3.30 | 18.27 | 81.73 | 45.56 | 54.44 |
| PCE5.20 | 31.31 | 68.69 | 59.12 | 40.88 |
| PCE7.09 | 58.23 | 41.77 | 79.47 | 20.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; He, M.; Li, J.; Yang, Z. Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer. Polymers 2018, 10, 889. https://doi.org/10.3390/polym10080889
Deng Y, He M, Li J, Yang Z. Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer. Polymers. 2018; 10(8):889. https://doi.org/10.3390/polym10080889
Chicago/Turabian StyleDeng, Yong, Mingyue He, Jinhong Li, and Zhiwei Yang. 2018. "Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer" Polymers 10, no. 8: 889. https://doi.org/10.3390/polym10080889
APA StyleDeng, Y., He, M., Li, J., & Yang, Z. (2018). Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer. Polymers, 10(8), 889. https://doi.org/10.3390/polym10080889