Novel Dihydroxy-Containing Ammonium Phosphate Based Poly(Lactic Acid): Synthesis, Characterization and Flame Retardancy
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
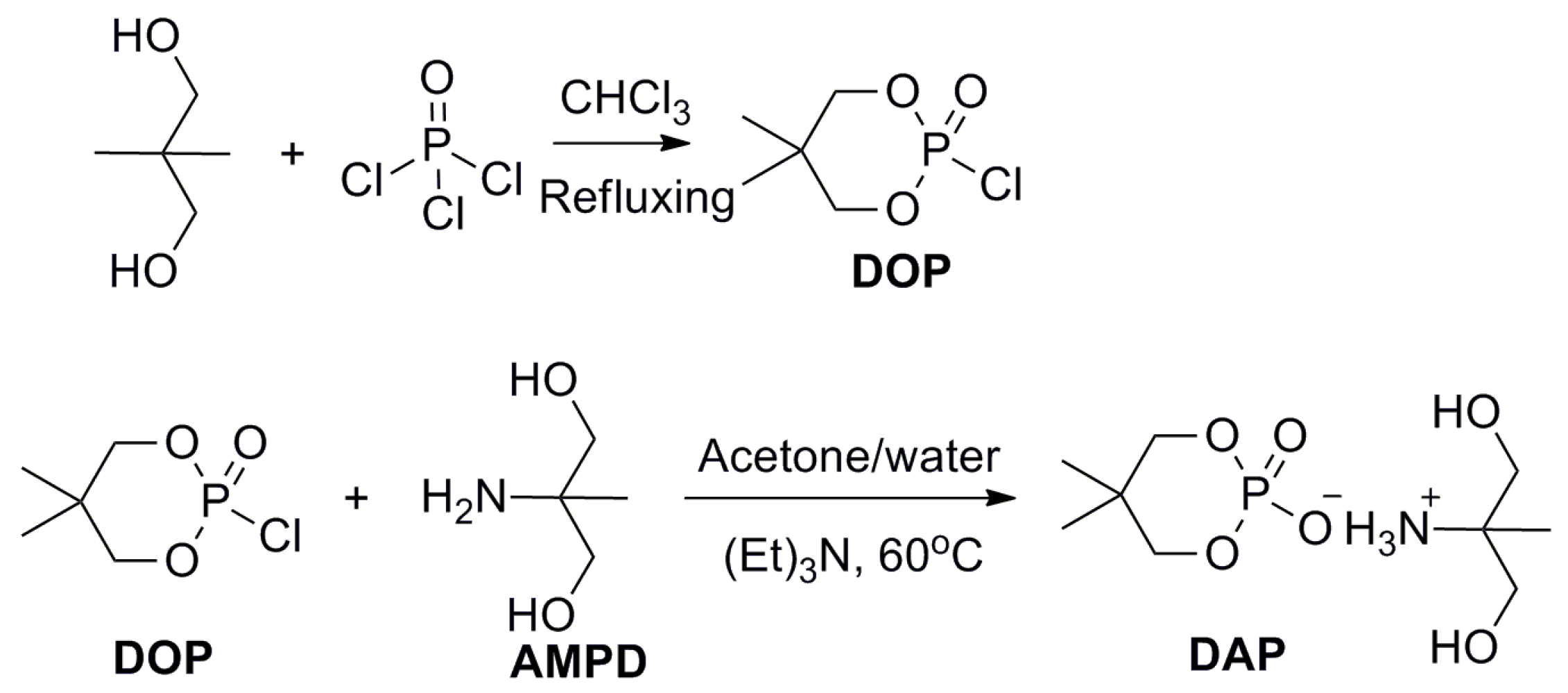
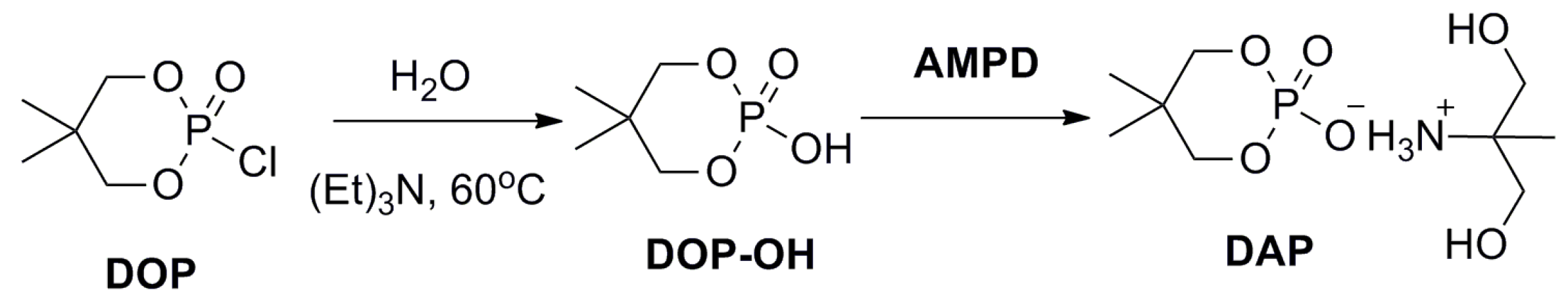
2.2. Synthesis of DAP
2.3. Preparation of PLA/DAP
2.4. Methods
3. Results and Discussion
3.1. Synthesis and Characterization of DAP
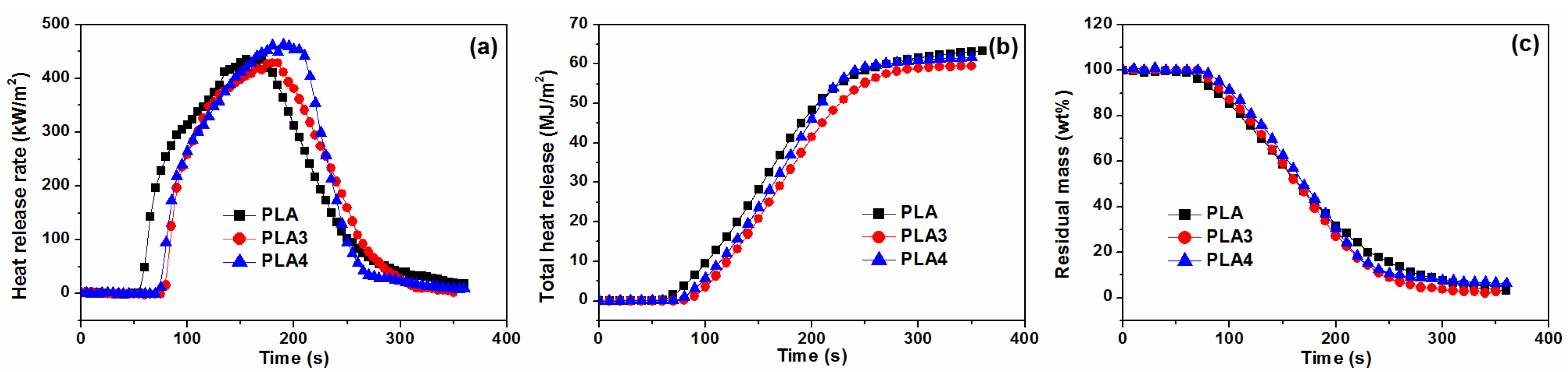
3.2. Burning Behaviors
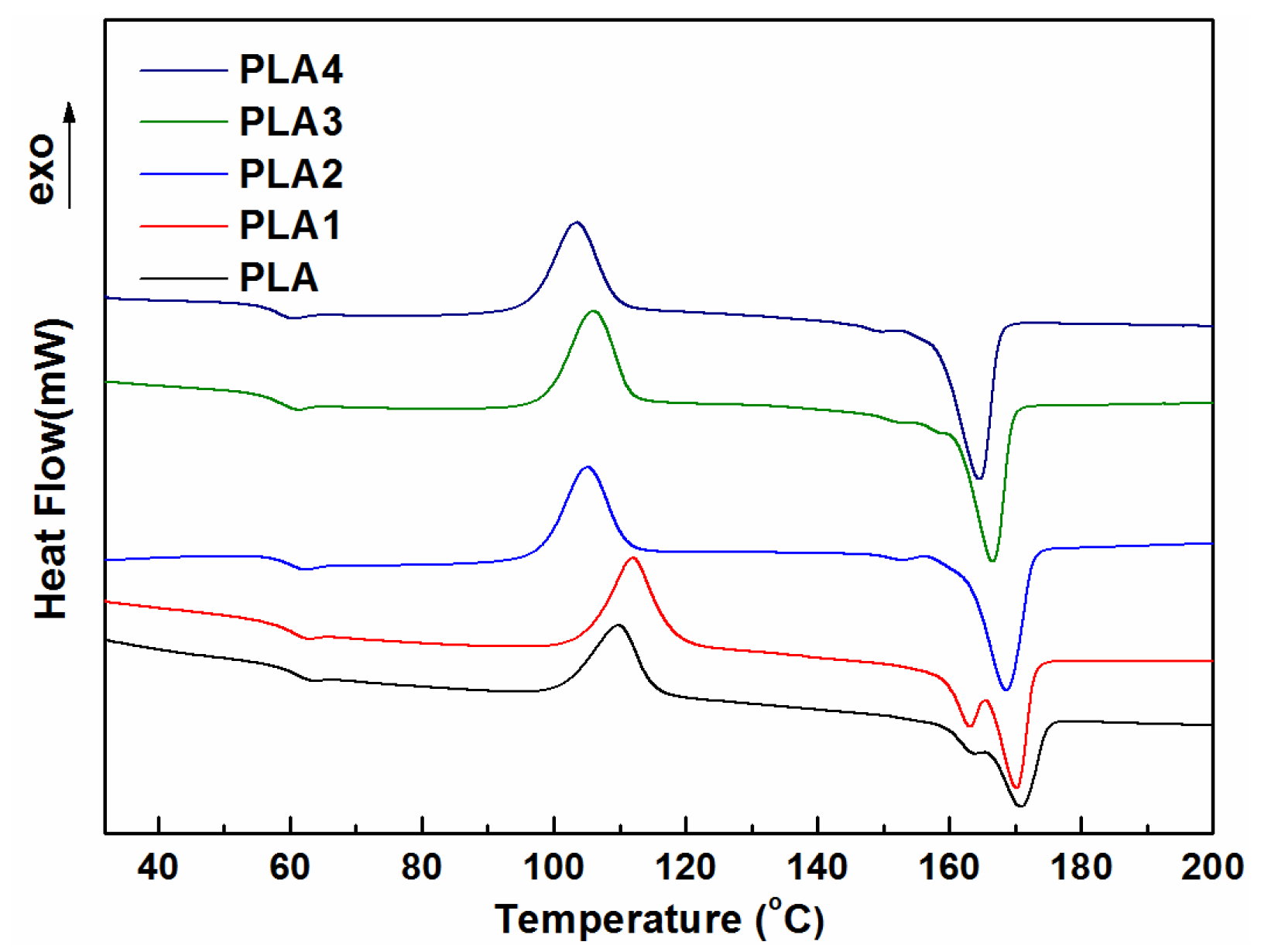
3.3. Thermal Analysis
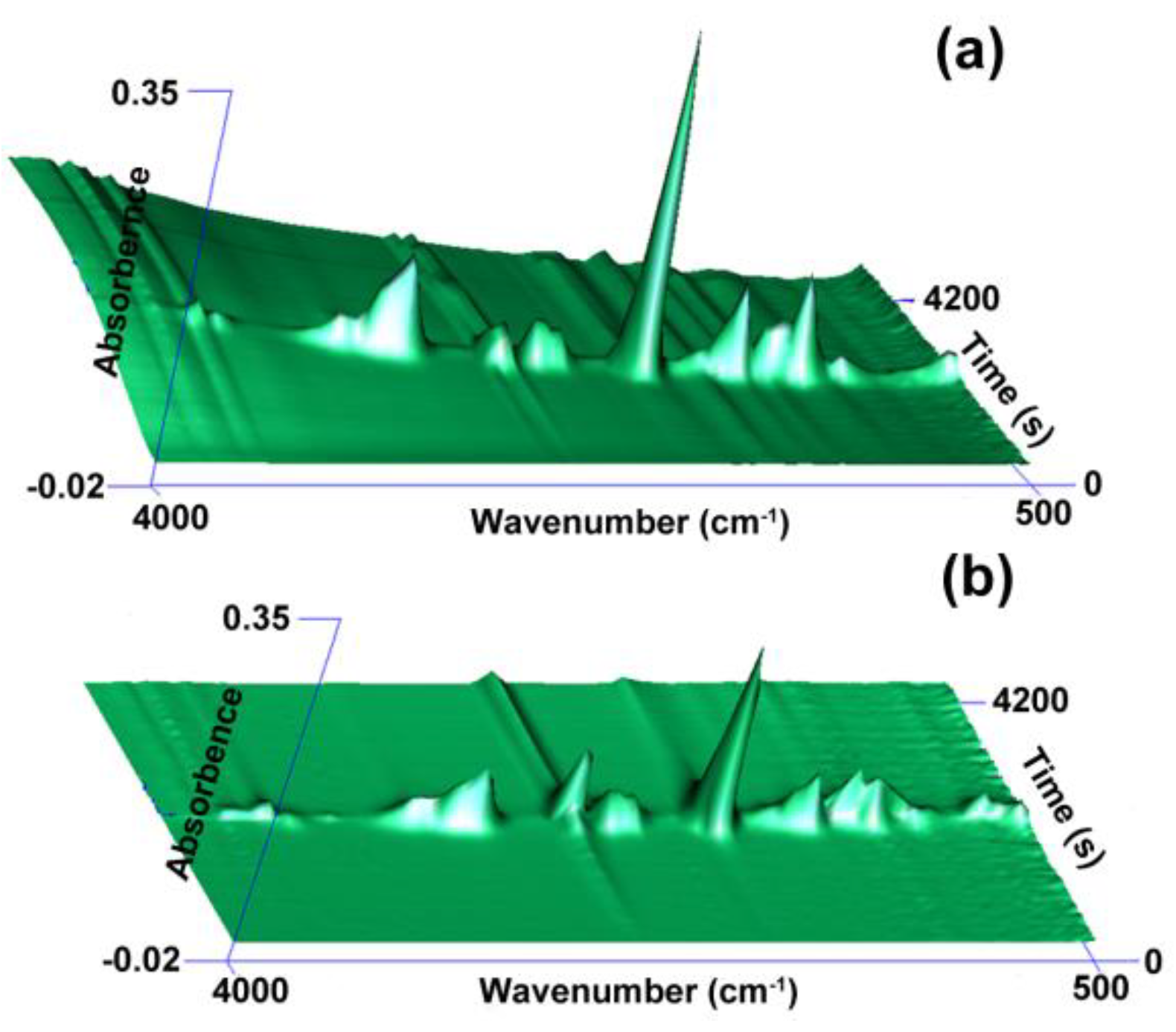
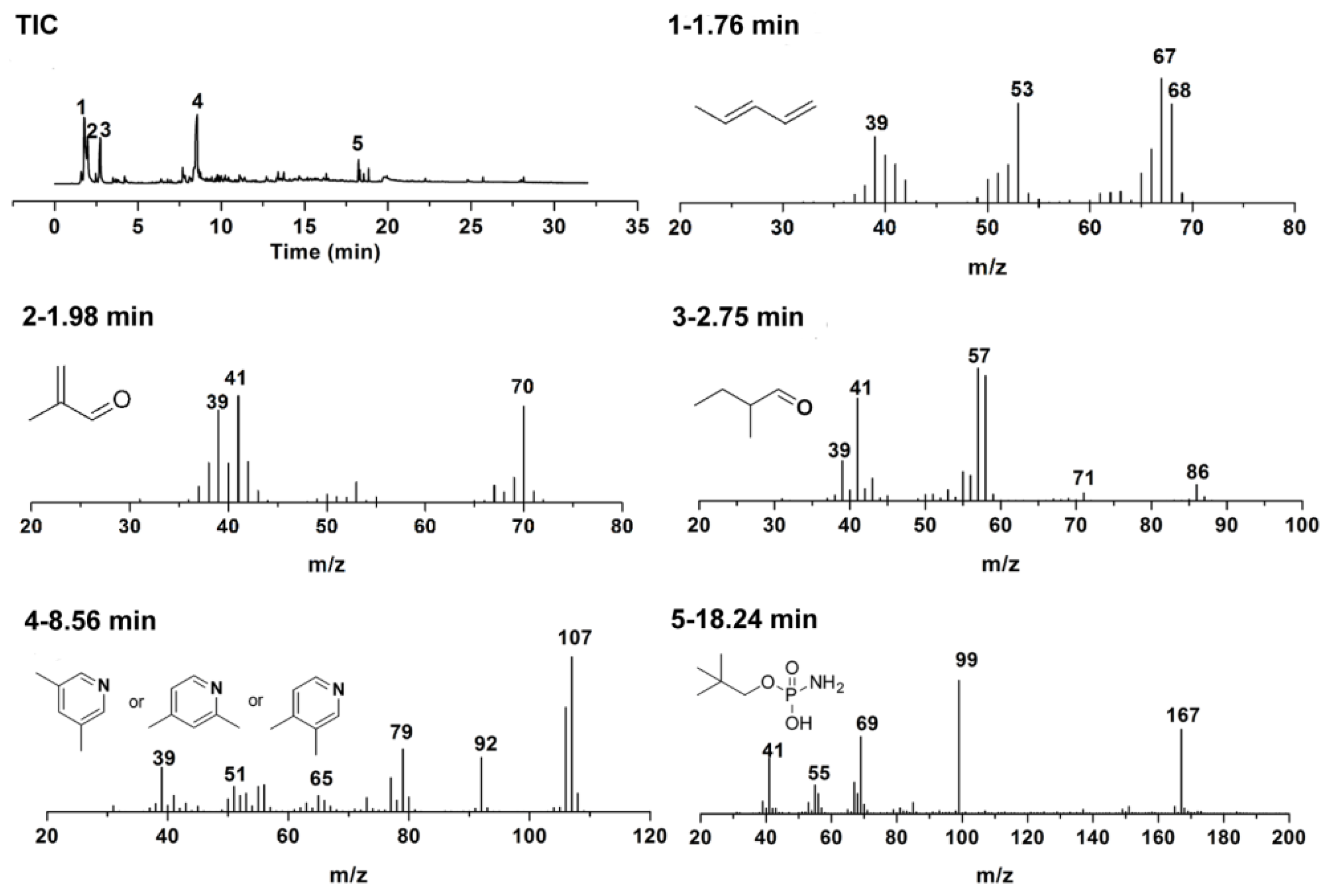
3.4. Flame-Retardant Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Zhao, H.B.; Chen, M.J.; Chen, H.B. Thermally Insulating and Flame-Retardant Polyaniline/Pectin Aerogels. ACS Sustain. Chem. Eng. 2017, 5, 7012–7019. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and characterization of PLA-based green composites: A review. J. Thermoplas. Compos. 2014, 27, 52–81. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Samper, M.D.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind. Crop. Prod. 2017, 104, 278–286. [Google Scholar] [CrossRef]
- Orue, A.; Eceiza, A.; Arbelaiz, A. Preparation and characterization of poly(lactic acid) plasticized with vegetable oils and reinforced with sisal fibers. Ind. Crop. Prod. 2018, 112, 170–180. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.C.; Huang, S.W.; Mei, C.T.; Wu, Q.L. Enhancing mechanical properties of poly(lactic acid) through its in-situ crosslinking with maleic anhydride-modified cellulose nanocrystals from cottonseed hulls. Ind. Crop. Prod. 2018, 112, 449–459. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohyd. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Leuteritz, A.; Wang, Y.Z.; Wagenknecht, U.; Heinrich, G. Preparation and burning behaviors of flame retarding biodegradable poly (lactic acid) nanocomposite based on zinc aluminum layered double hydroxide. Polym. Degrad. Stabil. 2010, 95, 2474–2480. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413–1422. [Google Scholar] [CrossRef]
- Xia, L.; Jian, R.K.; Ai, Y.F.; Zheng, X.L.; Zhao, B. An effective multi-hydroxy-containing ammonium phosphate towards flame-retarding poly(lactic acid): Flame retardance, thermal and pyrolysis behaviors. J. Anal. Appl. Pyrol. 2018. [Google Scholar] [CrossRef]
- Li, D.F.; Zhao, X.; Jia, Y.W.; Wang, X.L.; Wang, Y.Z. Tough and flame-retardant poly(lactic acid) composites prepared via reactive blending with biobased ammonium phytate and in situ formed crosslinked polyurethane. Compos. Commun. 2018, 8, 52–57. [Google Scholar] [CrossRef]
- Tang, G.; Huang, X.; Ding, H.; Wang, X.; Jiang, S.D.; Zhou, K.Q.; Wang, B.B.; Yang, W.; Hu, Y. Combustion properties and thermal degradation behaviors of biobased polylactide composites filled with calcium hypophosphite. RSC Adv. 2014, 4, 8985–8993. [Google Scholar] [CrossRef]
- Wang, D.Y.; Song, Y.P.; Lin, L.; Wang, X.L.; Wang, Y.Z. A novel phosphorus-containing poly(lactic acid) toward its flame retardation. Polymer 2011, 52, 233–238. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Wang, D.Y.; Chen, L.; Wang, X.L.; Wang, Y.Z. Inherent flame retardation of bio-based poly(lactic acid) by incorporating phosphorus linked pendent group into the backbone. Polym. Degrad. Stabil. 2011, 96, 1669–1675. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, B.W.; Wang, X.L.; Chen, L.; Wang, X.L.; Wang, Y.Z. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]
- Wei, L.L.; Wang, D.Y.; Chen, H.B.; Chen, L.; Wang, X.L.; Wang, Y.Z. Effect of a phosphorus-containing flame retardant on the thermal properties and ease of ignition of poly(lactic acid). Polym. Degrad. Stabil. 2011, 96, 1557–1561. [Google Scholar] [CrossRef]
- Zhao, X.M.; Guerrero, F.R.; Llorca, J.; Wang, D.Y. New Superefficiently Flame-Retardant Bioplastic Poly(lactic acid): Flammability, Thermal Decomposition Behavior, and Tensile Properties. ACS Sustain. Chem. Eng. 2016, 4, 202–209. [Google Scholar] [CrossRef]
- Zhao, X.M.; Juan, S.D.; Guerrero, F.R.; Li, Z.; Llorca, J.; Wang, D.Y. Effect of N,N′-diallyl-phenylphosphoricdiamide on ease of ignition, thermal decomposition behavior and mechanical properties of poly (lactic acid). Polym. Degrad. Stabil. 2016, 127, 2–10. [Google Scholar] [CrossRef]
- Li, Z.; Expósito, D.F.; González, A.J.; Wang, D.Y. Natural Halloysite Nanotube Based Functionalized Nanohybrid Assembled via Phosphorus-containing Slow Release Method: A Highly Efficient Way to Impart Flame Retardancy to Polylactide. Eur. Polym. J. 2017, 93, 458–470. [Google Scholar] [CrossRef]
- Shi, X.W.; Dai, X.; Cao, Y.; Li, J.W.; Huo, C.G.; Wang, X.L. Degradable Poly(lactic acid)/Metal–Organic Framework Nanocomposites Exhibiting Good Mechanical, Flame Retardant, and Dielectric Properties for the Fabrication of Disposable Electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Lin, H.; Han, L.; Dong, L. Thermal degradation behavior and gas phase flame-retardant mechanism of polylactide/PCPP blends. J. Appl. Polym. Sci. 2014, 131, 378. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Zammarano, M.; Gilman, J.W.; Shields, J.R.; Boday, D.J. Synthesis and characterization of isosorbide-based polyphosphonates as biobased flame-retardants. Polym. Chem. 2014, 5, 5139–5146. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Chen, C.; Gu, X.Y.; Jin, X.D.; Sun, J.; Zhang, S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohyd. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zhang, Y.; Tang, X.; Zhou, Y.; Li, X.N.; Kandola, B.K.; Fang, Z.P. Layer by layer deposition of polyethylenimine and bio-based polyphosphate on ammonium polyphosphate: A novel hybrid for simultaneously improving the flame retardancy and toughness of polylactic acid. Polymer 2017, 108, 361–371. [Google Scholar] [CrossRef]
- Liu, T.; Jing, J.; Zhang, Y.; Fang, Z.P. Synthesis of a novel polyphosphate and its application with APP in flame retardant PLA. RSC Adv. 2018, 8, 4483–4493. [Google Scholar] [CrossRef]
- Liu, G.S.; Gao, S. Synergistic effect between aluminum hypophosphite and a new intumescent flame retardant system in poly(lactic acid). J Appl. Polym. Sci. 2018, 135, 46359. [Google Scholar] [CrossRef]
- Long, L.J.; Yin, J.B.; He, W.T.; Qin, S.H.; Yu, J. Influence of a phenethyl-bridged DOPO derivative on the flame retardancy, thermal and mechanical properties of poly(lactic acid). Ind. Eng. Chem. Res. 2016, 55, 10803–10812. [Google Scholar] [CrossRef]
- Hai, V.; Nguyen, C.; Lee, K.; Kim, J. Thermal stability and flame retardancy of novel phloroglucinol based organo phosphorus compound. Polym. Degrad. Stabil. 2010, 95, 1092–1098. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2015, 31, 327–354. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, Y.Z. Design and synthesis of PET-based copolyesters with flame-retardant and antidripping performance. Macromol. Rapid Comm. 2017, 38, 1700451. [Google Scholar] [CrossRef] [PubMed]
- Jian, R.K.; Wang, P.; Xia, L.; Zheng, X.L. Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J. Anal. Appl. Pyrol. 2017, 127, 360–368. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, Y.; Feng, J.; Zhang, X.; Zhang, C.; Tang, Z.; Zhu, J. Preparation and characterization of poly(lactic acid)/starch composites toughened with epoxidized soybean oil. Carbohydr. Polym. 2013, 92, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyesters—A review of the recent literature. Polym. Int. 2005, 54, 11–35. [Google Scholar] [CrossRef]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Aanl. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Jian, R.K.; Wang, P.; Duan, W.S.; Wang, J.S.; Zheng, X.L.; Weng, J.B. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance and pyrolysis behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Jian, R.K.; Wang, P.; Xia, L.; Yu, X.Q.; Zheng, X.L.; Shao, Z.B. Low-flammability epoxy resins with improved mechanical properties using a Lewis base based on phosphaphenanthrene and 2-aminothiazole. J. Mater. Sci. 2017, 52, 1–15. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.K.; Zheng, X.L.; Chen, G.L.; Wang, J.S. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stabil. 2018, 149, 69–77. [Google Scholar] [CrossRef]




| Samples | PLA (wt %) | DAP (wt %) | LOI (%) | UL-94 (3.2 mm) | Dripping | Igniting Cotton |
|---|---|---|---|---|---|---|
| PLA | 100 | 0 | 19.5 | NR | Yes | Yes |
| PLA1 | 99.75 | 0.25 | 23.0 | V-2 | Yes | Yes |
| PLA2 | 99.5 | 0.5 | 24.6 | V-0 | Yes | No |
| PLA3 | 99 | 1 | 30.3 | V-0 | Yes | No |
| PLA4 | 98 | 2 | 38.3 | V-0 | Yes | No |
| Samples | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | TTP (s) | FIGRA (kW m−1 s−1) | TSP (m3) | av-EHC (MJ/kg) |
|---|---|---|---|---|---|---|---|
| PLA | 54 ± 1 | 436 ± 25 | 64.2 ± 0.7 | 170 ± 10 | 2.56 | 24.6 ± 1.5 | 17.5 ± 0.5 |
| PLA3 | 71 ± 2 | 439 ± 10 | 62.8 ± 1.5 | 175 ± 10 | 2.50 | 24.6 ± 2.0 | 16.7 ± 0.5 |
| PLA4 | 69 ± 1 | 463 ± 18 | 60.8 ± 1.0 | 190 ± 15 | 2.44 | 25.1 ± 2.5 | 15.6 ± 0.9 |
| Samples | T5% (°C) | Tmax (°C) | Mass Loss Rate at Tmax (wt %·min−1) | Residual at 600 °C (wt %) |
|---|---|---|---|---|
| P | 237 | 306 | 21.7 | 17.1 |
| PLA | 335 | 368 | 32.5 | 2.3 |
| PLA1 | 333 | 370 | 31.7 | 2.6 |
| PLA2 | 335 | 370 | 32.5 | 3.1 |
| PLA3 | 335 | 370 | 32.0 | 3.5 |
| PLA4 | 329 | 370 | 31.3 | 3.5 |
| Samples | Tg (°C) | Tc (°C) | ΔHc (J g−1) | Tm (°C) | ΔHm (J g−1) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 61.0 | 109.8 | 28.48 | 170.4 | 31.55 | 3.3 |
| PLA1 | 60.3 | 112.0 | 34.03 | 169.8 | 38.39 | 4.7 |
| PLA2 | 59.4 | 105.1 | 31.03 | 168.4 | 39.27 | 8.9 |
| PLA3 | 58.2 | 106.1 | 33.10 | 166.1 | 42.28 | 10.0 |
| PLA4 | 58.0 | 103.4 | 30.08 | 164.1 | 41.99 | 12.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, R.-K.; Xia, L.; Ai, Y.-F.; Wang, D.-Y. Novel Dihydroxy-Containing Ammonium Phosphate Based Poly(Lactic Acid): Synthesis, Characterization and Flame Retardancy. Polymers 2018, 10, 871. https://doi.org/10.3390/polym10080871
Jian R-K, Xia L, Ai Y-F, Wang D-Y. Novel Dihydroxy-Containing Ammonium Phosphate Based Poly(Lactic Acid): Synthesis, Characterization and Flame Retardancy. Polymers. 2018; 10(8):871. https://doi.org/10.3390/polym10080871
Chicago/Turabian StyleJian, Rong-Kun, Long Xia, Yuan-Fang Ai, and De-Yi Wang. 2018. "Novel Dihydroxy-Containing Ammonium Phosphate Based Poly(Lactic Acid): Synthesis, Characterization and Flame Retardancy" Polymers 10, no. 8: 871. https://doi.org/10.3390/polym10080871
APA StyleJian, R.-K., Xia, L., Ai, Y.-F., & Wang, D.-Y. (2018). Novel Dihydroxy-Containing Ammonium Phosphate Based Poly(Lactic Acid): Synthesis, Characterization and Flame Retardancy. Polymers, 10(8), 871. https://doi.org/10.3390/polym10080871







