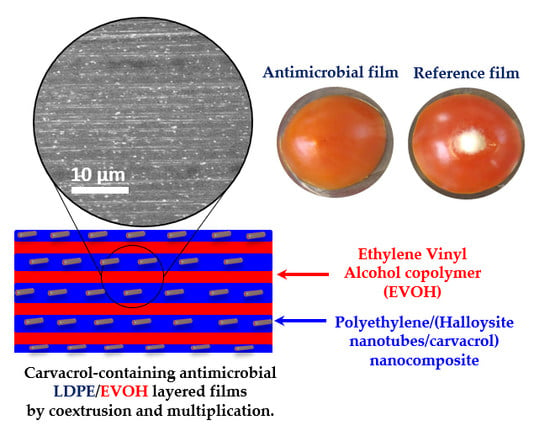
Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HNTs Loaded with Carvacrol
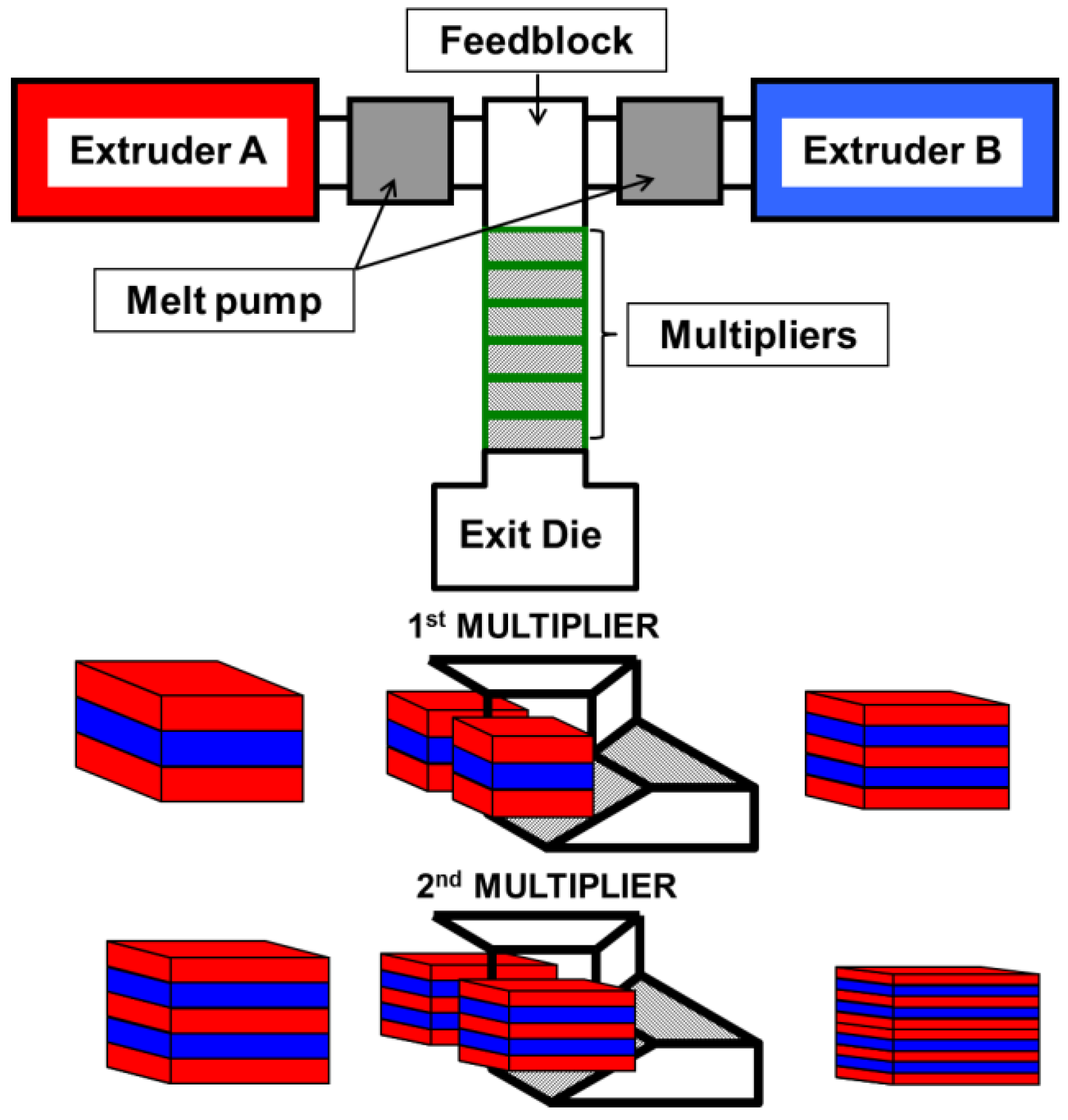
2.3. Preparation of Films
2.4. Characterization of Films
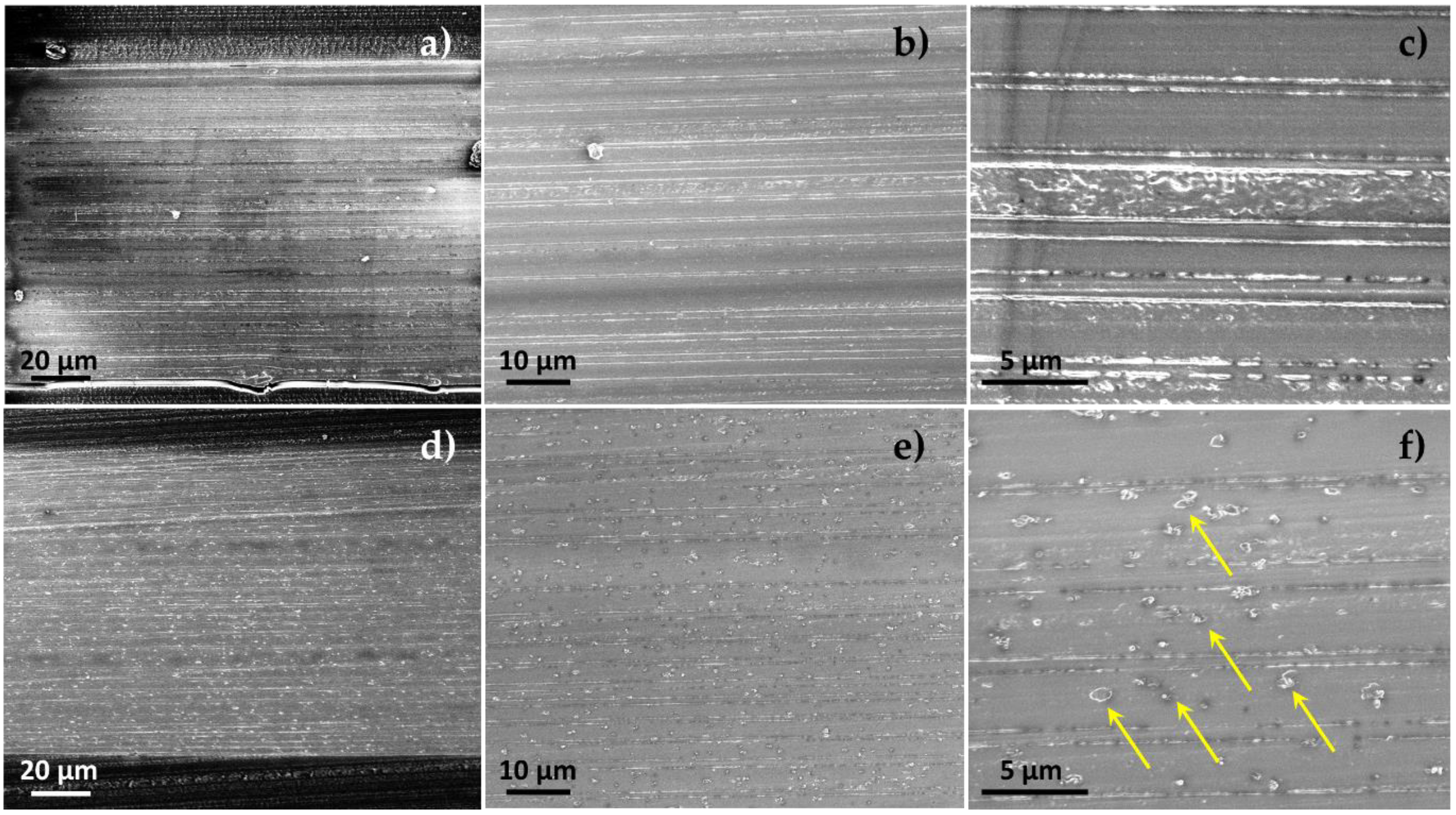
2.4.1. High-Resolution Scanning Electron Microscopy
2.4.2. Thermal Gravimetric Analysis (TGA)
2.4.3. Carvacrol Release Studies
2.4.4. Oxygen Transmission Rate
2.4.5. Antimicrobial In Vitro Assays
3. Results
3.1. Films Preparation and Morphology
3.2. Thermal Gravimetric Analysis (TGA)
3.3. Carvacrol Release Studies
3.4. Oxygen Transmission Rate
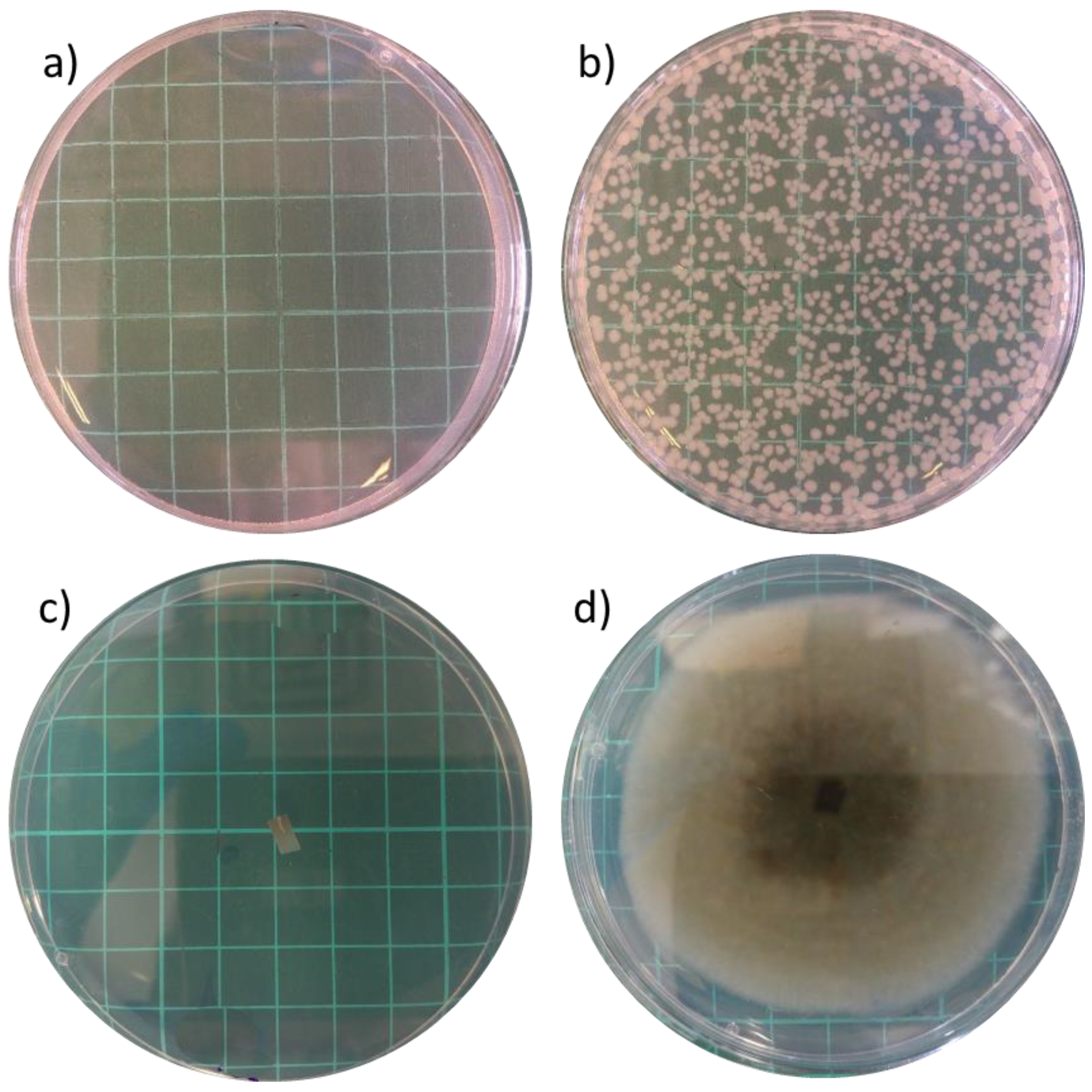
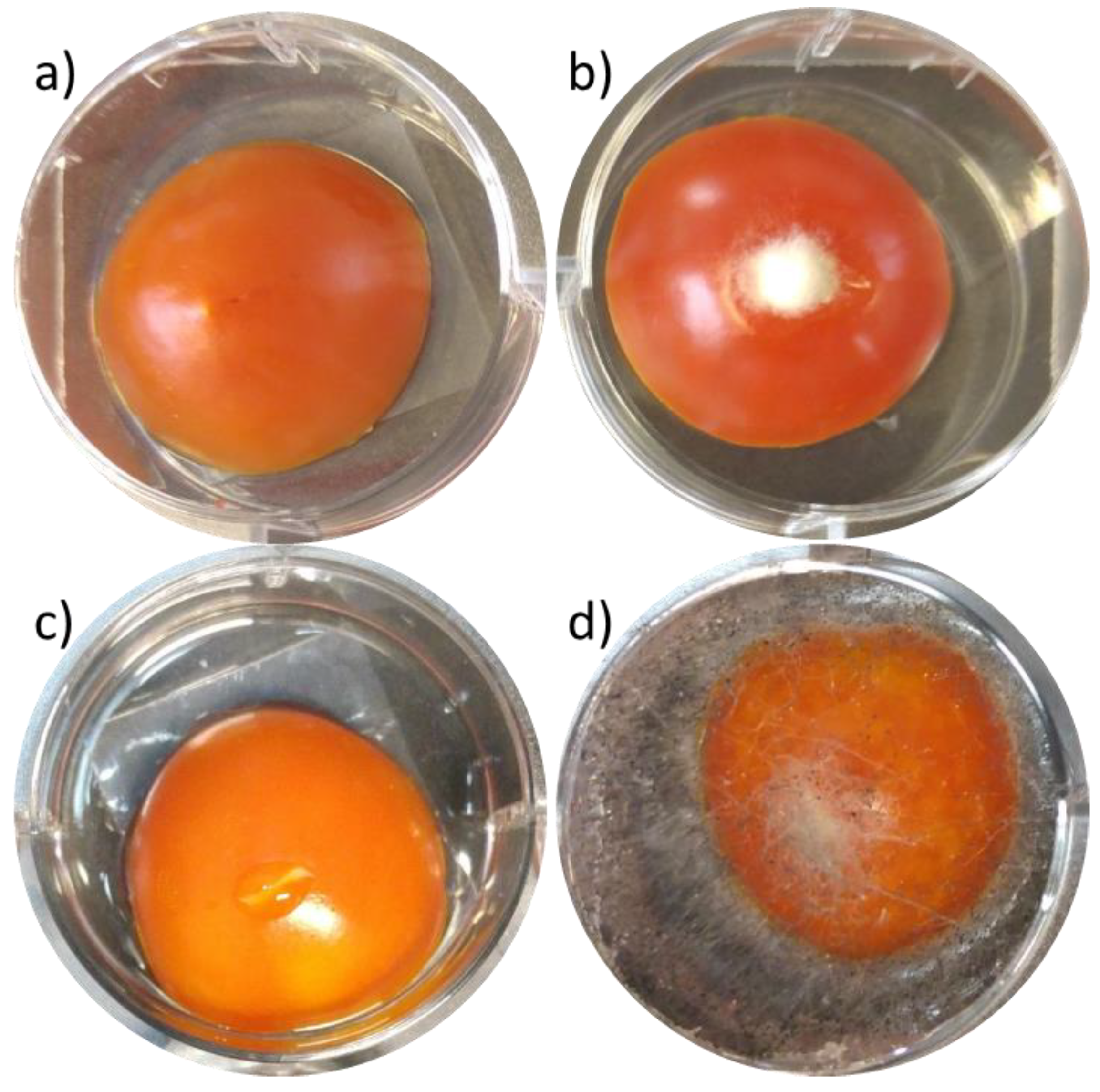
3.5. Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muñoz-Bonilla, A.; Fernández-García, M.; Mu, A. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent developments in antimicrobial polymers: A review. Materials. 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, J.T.; Coorey, R.; Dykes, G.A. Integration of Emerging Biomedical Technologies in Meat Processing to Improve Meat Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 615–632. [Google Scholar] [CrossRef] [Green Version]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Carvacrol-Based Films: Usage and Potential in In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 329–338. [Google Scholar]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sarita; Nehra, M.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog. Polym. Sci. 2018, 80, 1–38. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Characterization of antimicrobial films containing basil extracts. Packag. Technol. Sci. 2006, 19, 259–268. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.A.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef] [Green Version]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries−In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018, 258, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/halloysite nanotube films with sustained carvacrol release for broad-spectrum antimicrobial activity. Rsc Adv 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Nitzan, N.; Vaxman, A.; Segal, E. Active packaging containing encapsulated carvacrol for control of postharvest decay. Postharvest Biol. Technol. 2016, 118, 175–182. [Google Scholar] [CrossRef]
- Krepker, M.; Prinz-Setter, O.; Shemesh, R.; Vaxman, A.; Alperstein, D.; Segal, E. Antimicrobial carvacrol-containing polypropylene films: Composition, structure and function. Polymers 2018, 10, 79. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Hendessi, S.; Sevinis, E.B.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Antibacterial sustained-release coatings from halloysite nanotubes/waterborne polyurethanes. Prog. Org. Coat. 2016, 101, 253–261. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/clay hybrids loaded into in situ gelling films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Ponting, M.; Hiltner, A.; Baer, E. Polymer Nanostructures by Forced Assembly: Process, Structure, and Properties. Macromol. Symp. 2010, 294, 19–32. [Google Scholar] [CrossRef]
- Ponting, M.; Burt, T.M.; Korley, L.T.J.; Andrews, J.; Hiltner, A.; Baer, E. Gradient multilayer films by forced assembly coextrusion. Ind. Eng. Chem. Res. 2010, 49, 12111–12118. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Yam, K.L. The Wiley Encyclopedia of Packaging Technology; Yam, K.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Catalá, R.; Muriel-Galet, V.; Cerisuelo, J.P.; Domínguez, I.; Carballo, G.L.; Hernández-Muñoz, P.; Gavara, R. Antimicrobial Active Packaging Systems Based on EVOH Copolymers. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 297–303. [Google Scholar]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Natural antimicrobial-Containing EVOH coatings on PP and PET films: Functional and active property characterization. Packag. Technol. Sci. 2014, 27, 901–920. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Cerisuelo, J.P.; Bermúdez, J.M.; Aucejo, S.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Describing and modeling the release of an antimicrobial agent from an active PP/EVOH/PP package for salmon. J. Food Eng. 2013, 116, 352–361. [Google Scholar] [CrossRef]
- Koivisto, A.J.; Bluhme, A.B.; Kling, K.I.; Fonseca, A.S.; Redant, E.; Andrade, F.; Hougaard, K.S.; Krepker, M.; Prinz, O.S.; Segal, E.; et al. Occupational exposure during handling and loading of halloysite nanotubes–A case study of counting nanofibers. NanoImpact 2018, 10, 153–160. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Cran, M.J.; Rupika, L.A.S.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Release of Naturally Derived Antimicrobial Agents from LDPE Films. J. Food Sci. 2010, 75, E126–E133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suppakul, P.; Sonneveld, K.; Bigger, S.W.; Miltz, J. Loss of AM additives from antimicrobial films during storage. J. Food Eng. 2011, 105, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.-T.; Tung, M.A. Vapor Pressure of Allyl Isothiocyanate and Its Transport in PVDC/PVC Copolymer Packaging Film. J. Food Sci. 1997, 62, 1061–1062. [Google Scholar] [CrossRef]
- Miltz, J. Migration of low molecular weight species from packaging materials: Theoretical and practical considerations. In Food Product-Package Compatibility; Technomic Publishing Company: Lancaster, PA, USA, 1987; pp. 30–43. [Google Scholar]
- Cardiet, G.; Fuzeau, B.; Barreau, C.; Fleurat-Lessard, F. Contact and fumigant toxicity of some essential oil constituents against a grain insect pest Sitophilus oryzae and two fungi, Aspergillus westerdijkiae and Fusarium graminearum. J. Pest Sci. 2012, 85, 351–358. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Cai, X.; Jia, Z.; Liu, M.; Jia, D. Morphology and properties of halloysite nanotubes reinforced polypropylene nanocomposites. e-Polymers 2008, 8, 130. [Google Scholar] [CrossRef]
- Cabedo, L.; Giménez, E.; Lagaron, J.M.; Gavara, R.; Saura, J.J. Development of EVOH-kaolinite nanocomposites. Polymer 2004, 45, 5233–5238. [Google Scholar] [CrossRef]
- Han, J.H. Antimicrobial packaging systems. In Innovations in Food Packaging; Elsevier: New York, NY, USA, 2005; pp. 80–107. [Google Scholar]
- Ouali, L.; Léon, G.; Normand, V.; Johnsen, H.; Dyrli, A.; Schmid, R.; Benczedi, D. Mechanism of Romascone® release from hydrolyzed vinyl acetate nanoparticles: Thermogravimetric method. Polym. Adv. Technol. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Alonso, J.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Modifications induced by the addition of a nanoclay in the functional and active properties of an EVOH film containing carvacrol for food packaging. J. Membr. Sci. 2012, 423–424, 247–256. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Muriel-Galet, V.; Bermúdez, J.M.; Aucejo, S.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Mathematical model to describe the release of an antimicrobial agent from an active package constituted by carvacrol in a hydrophilic EVOH coating on a PP film. J. Food Eng. 2012, 110, 26–37. [Google Scholar] [CrossRef]
- Persico, P.; Ambrogi, V.; Carfagna, C.; Cerruti, P.; Ferrocino, I.; Mauriello, G. Nanocomposite polymer films containing carvacrol for antimicrobial active packaging. Polym. Eng. Sci. 2009, 49, 1447–1455. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and food spoilage. In Fungi and Food Spoilage; Springer: Berlin, Germany, 2009; pp. 1–519. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in wound—A review. Microbiol. Int. J. Food 2004, 94, 223–254. [Google Scholar] [CrossRef] [PubMed]
| System | LDPE/EVOH Ratio | No. of Layers | Carvacrol Content * (w/w %) | HNTs Content (w/w %) |
|---|---|---|---|---|
| LDPE/EVOH | 80/20 | 9, 17, 33, 65 | 0 | 0 |
| (LDPE/carvacrol)/EVOH | 80/20 | 9, 17, 33, 65 | 6 | 0 |
| (LDPE/HNTs)/EVOH | 80/20 | 9, 17, 33, 65 | 0 | 3 |
| (LDPE/[HNTs/carvacrol])/EVOH | 80/20 | 9, 17, 33, 65 | 6 | 3 |
| Neat LDPE | N/A | 1 | 0 | 0 |
| LDPE/carvacrol | N/A | 1 | 4 | 0 |
| LDPE/(HNTs/carvacrol) | N/A | 1 | 4 | 2 |
| Polymer Films | No. of Layers | Pre-Processing Content of Carvacrol (% w/w) | Post-Processing Content of Carvacrol by TGA (% w/w) |
|---|---|---|---|
| (LDPE/carvacrol)/EVOH | 9 | 4.8 | 3.2 (0.1) |
| (LDPE/carvacrol)/EVOH | 17 | 4.8 | 3.1 (0.1) |
| (LDPE/carvacrol)/EVOH | 33 | 4.8 | 3.4 (0.1) |
| (LDPE/carvacrol)/EVOH | 65 | 4.8 | 2.9 (0.2) |
| (LDPE/[HNTs/carvacrol])/EVOH | 9 | 4.8 | 3.2 (0.1) |
| (LDPE/[HNTs/carvacrol])/EVOH | 17 | 4.8 | 3.4 (0.2) |
| (LDPE/[HNTs/carvacrol])/EVOH | 33 | 4.8 | 3.2 (0.1) |
| (LDPE/[HNTs/carvacrol])/EVOH | 65 | 4.8 | 3.3 (0.1) |
| Polymer Film | No of Layers | Carvacrol Effective Diffusivity ×1014, m2 s−1 |
|---|---|---|
| (LDPE/carvacrol)/EVOH | 9 | 1.11 a (0.19) |
| (LDPE/carvacrol)/EVOH | 17 | 1.57 b (0.23) |
| (LDPE/carvacrol)/EVOH | 33 | 2.22 c (0.37) |
| (LDPE/carvacrol)/EVOH | 65 | 1.35 a,b (0.22) |
| (LDPE/[HNTs/carvacrol])/EVOH | 9 | 2.45 e (0.08) |
| (LDPE/[HNTs/carvacrol])/EVOH | 17 | 2.39 e,d (0.33) |
| (LDPE/[HNTs/carvacrol])/EVOH | 33 | 2.54 e,d (0.39) |
| (LDPE/[HNTs/carvacrol])/EVOH | 65 | 2.76 d (0.02) |
| LDPE/carvacrol | 1 | 418 f (72) |
| LDPE/(HNTs/carvacrol) | 1 | 285 g (24) |
| Systems | No. of Layers | |||
|---|---|---|---|---|
| 9 | 17 | 33 | 65 | |
| LDPE/EVOH | 0.26 (0.03) | 0.29 (0.02) | 0.35 (0.02) | 0.41 (0.02) |
| (LDPE/carvacrol)/EVOH | 1.49 (0.04) | 1.67 (0.04) | 1.94 (0.04) | 2.14 (0.06) |
| (LDPE/HNTs)/EVOH | 0.26 (0.02) | 0.28 (0.01) | 0.30 (0.02) | 0.33 (0.02) |
| (LDPE/[HNTs/carvacrol])/EVOH | 1.36 (0.19) | 2.31 (0.06) | 2.52 (0.09) | 3.24 (0.11) |
| LDPE/carvacrol | 237.0 (6.5) | |||
| LDPE/(HNTs/carvacrol) | 254.2 (7.0) | |||
| Neat LDPE | 181.1 (5.1) | |||
| Polymer Composition | No. of Layers | A. alternata In Vitro Growth Reduction (%) x | A. alternata Growth on Cherry Tomato y | Rhisopus spp. Growth on Cherry Tomato y |
|---|---|---|---|---|
| LDPE control 100% | 1 | 0 ± 0 | 3/3 | 3/3 |
| LDPE/EVOH control | 9 | 1.1 ± 2.3 | 3/3 | 3/3 |
| LDPE/EVOH control | 17 | 2.9 ± 6.8 | 3/3 | 3/3 |
| LDPE/EVOH control | 33 | 7.2 ± 1.2 | 3/3 | 3/3 |
| LDPE/EVOH control | 65 | 7.9 ± 3.1 | 3/3 | 3/3 |
| LDPE/carvacrol/EVOH | 9 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/carvacrol/EVOH | 17 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/carvacrol/EVOH | 33 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/carvacrol/EVOH | 65 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/[HNT/carvacrol]/EVOH | 9 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/[HNT/carvacrol]/EVOH | 17 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/[HNT/carvacrol]/EVOH | 33 | 100 ± 0.0 | 0/3 | 0/3 |
| LDPE/[HNT/carvacrol]/EVOH | 65 | 100 ± 0.0 | 0/3 | 0/3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krepker, M.; Zhang, C.; Nitzan, N.; Prinz-Setter, O.; Massad-Ivanir, N.; Olah, A.; Baer, E.; Segal, E. Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers 2018, 10, 864. https://doi.org/10.3390/polym10080864
Krepker M, Zhang C, Nitzan N, Prinz-Setter O, Massad-Ivanir N, Olah A, Baer E, Segal E. Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers. 2018; 10(8):864. https://doi.org/10.3390/polym10080864
Chicago/Turabian StyleKrepker, Max, Cong Zhang, Nadav Nitzan, Ofer Prinz-Setter, Naama Massad-Ivanir, Andrew Olah, Eric Baer, and Ester Segal. 2018. "Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion" Polymers 10, no. 8: 864. https://doi.org/10.3390/polym10080864
APA StyleKrepker, M., Zhang, C., Nitzan, N., Prinz-Setter, O., Massad-Ivanir, N., Olah, A., Baer, E., & Segal, E. (2018). Antimicrobial LDPE/EVOH Layered Films Containing Carvacrol Fabricated by Multiplication Extrusion. Polymers, 10(8), 864. https://doi.org/10.3390/polym10080864