1. Introduction
Methods such as ring opening metathesis [
1] and polyhomologation [
2] can lead to macrocycles, yet perhaps the most common technique involves the chain-end closure of telechelic linear polymer precursors. This much-used sequence will often follow the same general plan: synthesize α,ω-telechelic polymers with reactive chain ends, and coerce an intramolecular coupling reaction by appropriate choice of chain ends and dilution (
Scheme 1). This approach is conceptually simple, with the overall process involving end-group transformations of one or both of the chain ends to prepare the linear precursors capable of cyclization. Examples of reactive chain ends for cyclization include, but are not limited to: (i) alkynes and azides for “click” reactions [
3,
4,
5]; (ii) dienes and dienophiles for Diels–Alder chemistry [
6]; (iii) alkenes and thiols for thiol-ene reactions [
7]; (iv) anthracenes for photodimerization reactions [
8]; (v) carbanions for reactions with external electrophiles (by consecutive S
N2 reactions between a polymer anion and bifunctional electrophile) [
9,
10]; and (vi) C–Br bonds capable of activation in a radical transfer coupling sequence [
11,
12].
A common solvent for both polymerizations, leading to linear precursors and post-polymerization transformation reactions, is tetrahydrofuran (THF). This solvent is compatible with all the cyclization reactions listed above, while also being a compatible polymerization medium for anionic techniques [
13,
14] and reversible activation/deactivation radical polymerization (RDRP) methods such as atom transfer radical polymerization (ATRP) [
15,
16] and nitroxide-mediated polymerization (NMP) [
17]. The success of cyclization reactions is almost always evaluated, at least in part, using gel permeation chromatography (GPC), with the shift to longer elution times indicative of the topological transformation from linear to cyclic [
18,
19]. Further analysis, such as via MALDI-TOF [
20,
21,
22], is often used to verify the cyclic structure and validate the proposed mechanistic pathway leading to its creation.
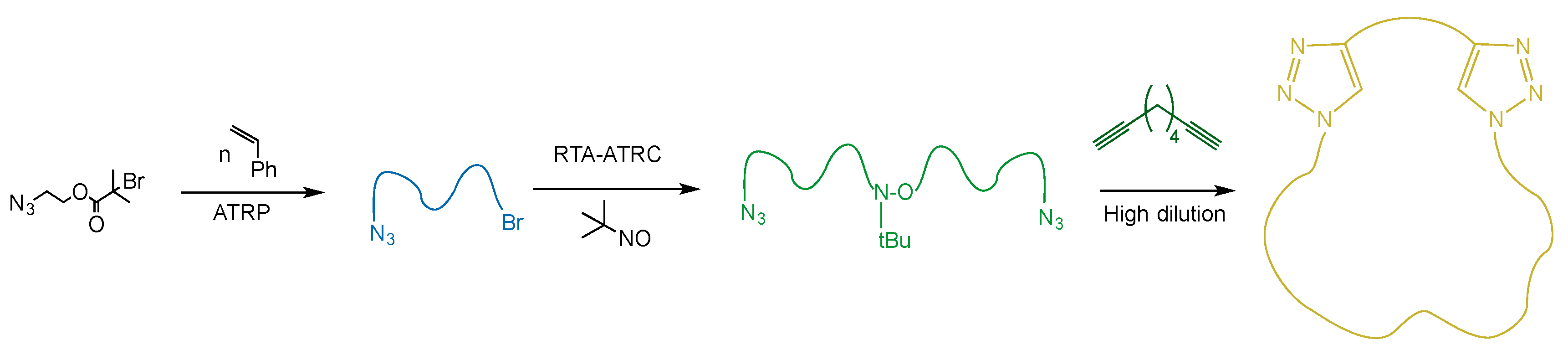
In this submission, the outcomes of intramolecular, chain-end azide–alkyne “click” reactions and atom transfer radical coupling (ATRC) reactions, both leading to the formation of macrocyclic polystyrene (PS), were analyzed. All cyclization reactions were performed under highly dilute conditions in THF, and the raw cyclization reaction mixtures, concentrated by rotary evaporation, were analyzed by GPC. In addition to the expected signals attributed to cyclic material and higher-molecular-weight species formed by competing intermolecular chain-end coupling, signals at lower molecular weights (~1100 Da) were consistently observed. This had previously been seen by our group in trials using ATRC-type ring closing reactions, but was never investigated. By systematically altering and removing reaction mixture contents, the identity of the low-molecular-weight species was determined and is reported here.
2. Materials and Methods
2.1. Materials
Copper(I) bromide (CuBr, 98%, Aldrich, St. Louis, MO, USA) and copper metal (Cu0, fine powder, Baker & Adamson, Easton, PA, USA) were used as received. Tetrahydrofuran (THF, inhibitor free ≥99.9%, Aldrich, St. Louis, MO, USA) was obtained from the Aldrich Pure-PacTM System. Styrene (S, ≥99%, Aldrich) was purified through prepacked column inhibitor removers (Aldrich). 2-Azidoethyl-2-bromoisobutyrate (Aldrich) and 1,6-heptadiyne (Aldrich) were used as received. The following were used as received and stored in the refrigerator: 1-bromoethylbenzene (BEB, 97%, Aldrich), α,α-dibromotoluene (97%, Aldrich) and N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA, 99%, Aldrich). The N-tert-butyl-α-phenylnitrone (tBuPN, Aldrich) and 2-methyl-2-nitrosopropane (MNP, Aldrich) were used as received and stored in the freezer.
2.2. Procedure for the Synthesis of Monobrominated Polystyrene Using ATRP
Styrene (4.0 mL, 34.9 mmol), CuBr (100 mg, 0.698 mmol), and (1-bromoethyl)benzene (BEB, 95 μL, 0.698 mmol) were added into a custom-made Schlenk flask, capped with a rubber septum, that was attached to the Schlenk line. The contents of the flask were exposed to three freeze−pump−thawing cycles before being placed on a heat plate with custom-fit block heaters and equipped with a digital contact thermoregulator (Chemglass CG-1994-V015) set to 80 °C and left to stir for 5 min. PMDETA (146 μL, 0.698 mmol) was then added to the reaction flask via syringe to begin polymerization. Dibrominated polystyrene was synthesized using the same material as listed above except using α,α-dibromotoluene (115 μL, 0.698 mmol) as the initiator instead of BEB.
2.3. Typical Procedure for the ATRC of PSBr
A representative ATRC reaction was performed as follows: a molar ratio of [PsBr]:[CuBr]:[Cu0]:[PMDETA] = 1:5:5:10, monobrominated polystyrene (PSBr, 195 mg, 0.075 mmol, 2600 g/mol), CuBr (53.8 mg, 0.375 mmol), Cu0 (16 mg, 0.375 mmol), and THF (5 mL) were added into a custom-made Schlenk line round-bottom flask. Concentrations of PSBr were typically kept at 15 mM for intermolecular reactions. The reaction flask, sealed with a rubber septum, was attached to the Schlenk line. The flask was exposed to three freeze−pump−thawing cycles before being placed onto a heat plate set to 60 °C. After 5 min of heating and stirring, PMDETA (157 μL, 0.75 mmol) was added into the reaction chamber via syringe. The coupling reaction was stopped after 60 min by exposing the content of the flask to the atmosphere and placing the flask in an ice bath. The reaction mixture was filtered into a GPC vial, diluted with THF, and analyzed. A radical trap-assisted ATRC (RTA-ATRC) reaction was performed using the same material listed above with the addition of N-tert-butyl-α-phenylnitrone (tBuPN, 13 mg, 0.075 mmol), and then following the same procedure described.
2.4. Typical Procedure for the End-To-End Coupling Condition, Using the Highly Dilute RTA-ATRC of PSBr as an Example
A representative highly dilute RTA-ATRC reaction was performed as follows: a molar ratio of [BrPsBr]:[CuBr]:[Cu0]:[PMDETA] = 1:20:20:40, dibrominated polystyrene (BrPSBr, 13 mg, 0.005 mmol, 2700 g/mol), CuBr (214.3 mg, 0.1 mmol), Cu0 (6.32, 0.1 mmol), 2-methyl-2-nitrosopropane dimer (MNP, 157 mM MNP-dimer solution in THF, 160 μL, 0.025 mmol), and THF (50 mL) were added into a custom-made Schlenk line round-bottom flask. Concentrations of BrPSBr were consistently kept at 10−4 mM. The reaction flask, sealed with a rubber septum, was attached to the Schlenk line. The flask was exposed to three freeze−pump−thawing cycles before being placed onto a heat plate set to 60 °C. After 5 min of heating and stirring, PMDETA (42 μL, 0.2 mmol) was added into the reaction chamber via syringe. The RTA-ATRC reaction was stopped after 24 h by exposing the content of the flask to the atmosphere and placing the flask in an ice bath. The reaction mixture was filtered into a GPC vial, diluted with THF, and analyzed. Analogous end-to-end coupling reactions were conducted using azide–alkyne “click” chemistry at similar concentrations.
2.5. Procedure of Synthesis of α,ω-Diazido PS
N3–SBr was synthesized using established ATRP methods and 2-azidoethyl-2-bromoisobutyrate as the initiator. N3–PS–N3 was synthesized subsequently using established RTA-ATRC methods with N3–PSBr and MNP as the radical trap.
2.6. Characterization
Raw reaction products were placed on a rotary evaporator (Buchi Rotavapor R-114, New Castle, DE, USA) to concentrate the crude products for GPC analysis. GPC analysis was done on a system comprised of a Shimadzu CBM-20A communications module, a Shimadzu DGU-20A degassing unit, a Shimadzu SIL-20A auto sampler, a Shimadzu LC-20AD pump, and a Wyatt T-rEX RI detector (Santa Barbara, CA, USA). The instrument was interfaced with a PC and was operated using PSS WinGPC Unichrom software (Mainz, Germany). Separations were performed using a PSS SDV analytical 1000-angstrom and a 100,000-angstrom column in sequence, housed inside a Shimadzu CTO-2A column oven set (Kyoto, Japan) to 40 °C. THF was used as an eluent at an optimized flow rate of 1.00 mL/min, and a 10-point calibration was performed using Agilent EasiCal PS standards. The data analysis was performed using the PSS WinGPC software.
3. Results
The results presented in this paper are focused not on assessing the yields or purity of the cyclic polymers, as the methods used have already been reported and proven capable of producing macrocycles [
5,
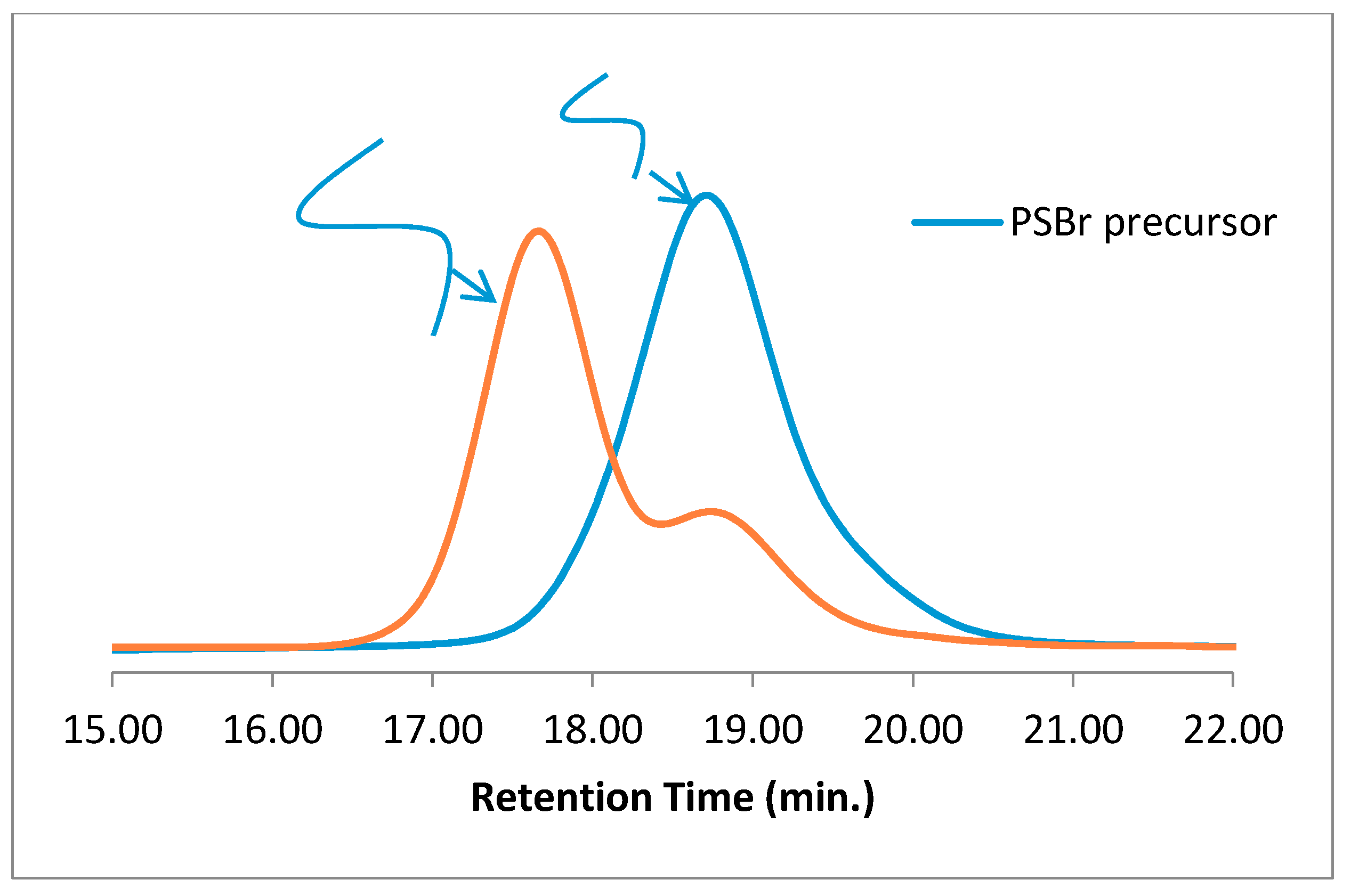
12]. Rather, the focus is on elucidating the identify of a low-molecular-weight species whose presence cannot be accounted for by the chemistry used to close the polymer chains into rings. To this end, linear polystyrene with diazide termini was prepared by a combination of ATRP and radical trap-assisted ARTC, and subjected to consecutive “click” reactions with a diyne, with the second reaction being intramolecular. The synthetic route is summarized in
Scheme 2 and detailed in Materials and Methods, and the GPC traces of both the precursor and cyclization reaction mixture are presented in
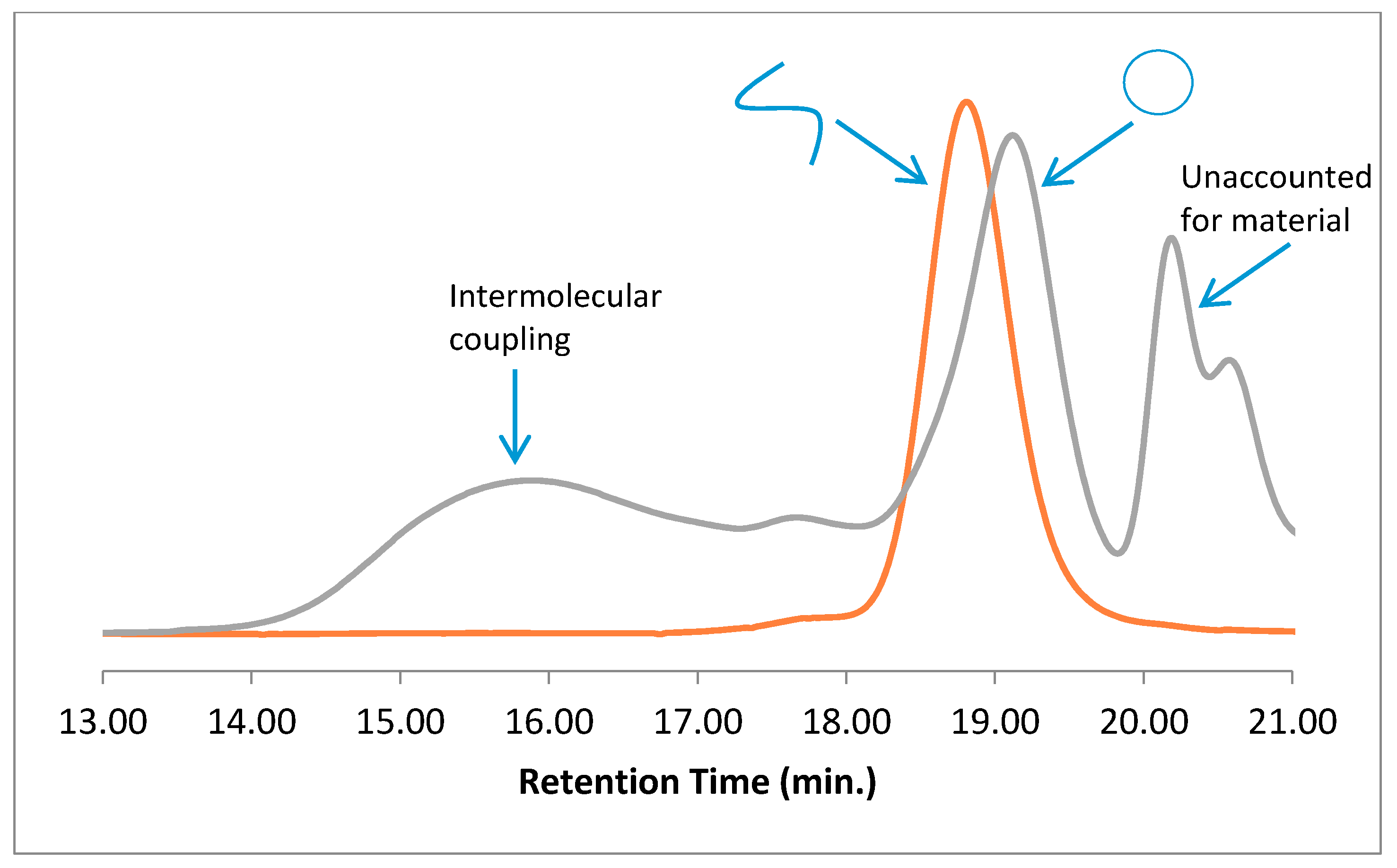
Figure 1 along with the characteristics of the polymers. Typically of intramolecular chain-end-joining reactions, a shift to longer elution times indicates the presence of cyclic material [
20], while the presence of material with a broad range of molecular weights shows that intermolecular coupling and “step” polymerization is competing with cyclization, although some cyclization occurring after step-growth cannot be ruled out [
23,
24]. The low-molecular-weight species that elute past 20 min and correspond to apparent molecular weights of near 1100 Da are too high to be residual monomer or ligand/catalyst complexes, yet cannot be explained by any expected “click” chemistry.
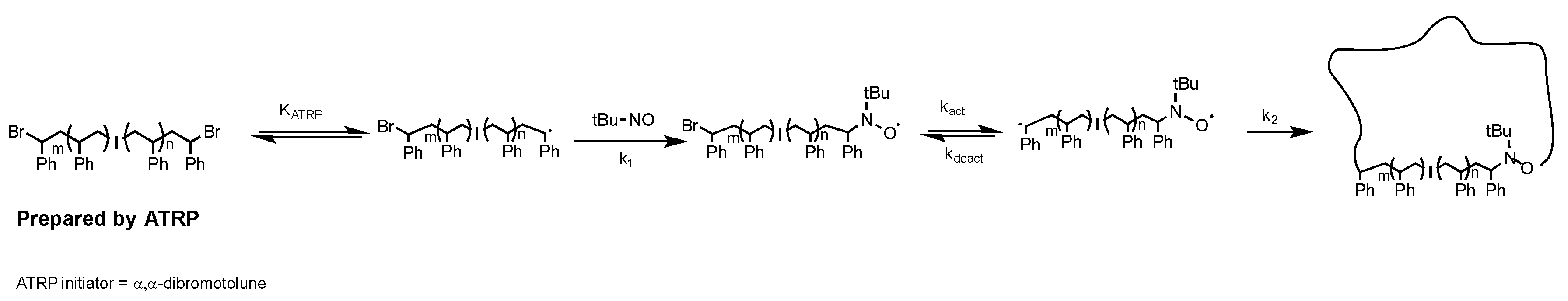
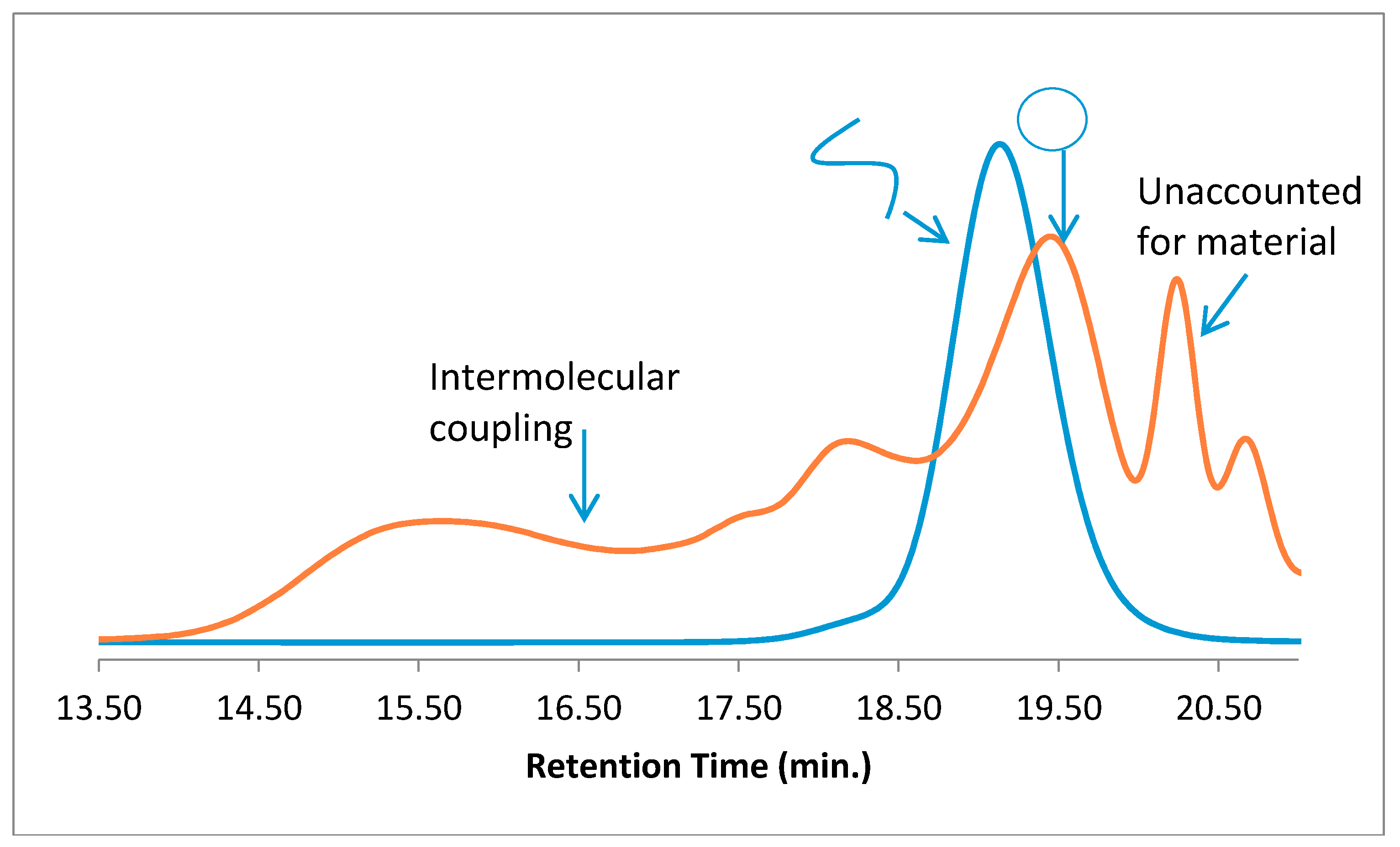
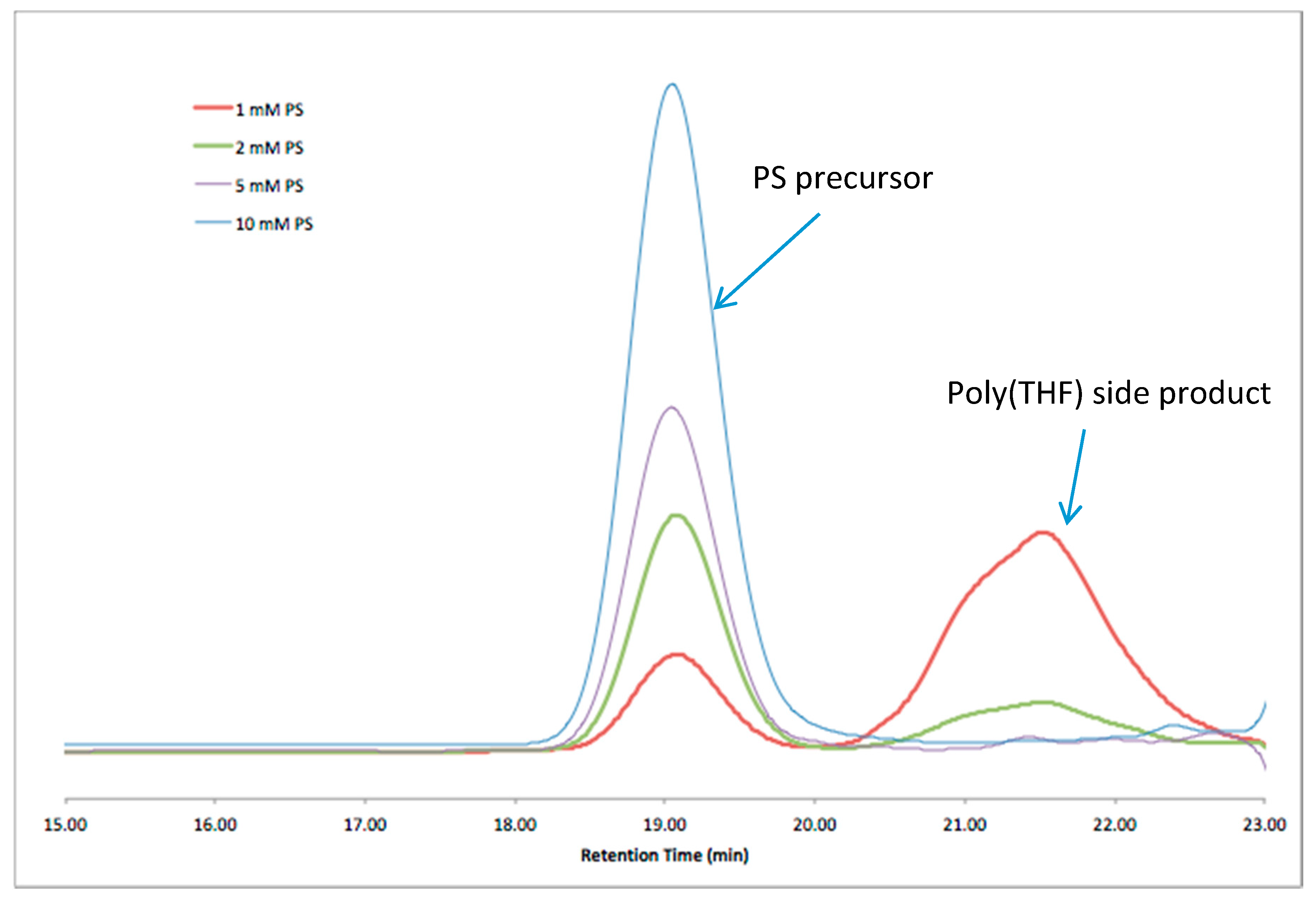
When cyclic polymers were prepared by a different route, using intramolecular RTA-ATRC (
Scheme 3), similar, unaccounted-for material was observed: the expected signals attributed to cyclic material and “step” products, but also the low-molecular-weight material at nearly the exact same elution volume. The cyclization reaction mixture contained many of the same reactants as the “click” cyclization reaction, and this led us to further consider if some combination of ligand/catalyst and polymer was leading to these low-molecular-weight impurities. Interestingly, when an intermolecular chain-end-joining reaction of RTA-ATRC was carried out with monobrominated PS under much higher reactant concentrations, this low-molecular-weight material was not observed in the unpurified reaction mixture. This is consistent with our previous studies that used ATRC-type reactions to prepare polymeric dimers [
12]. The GPC traces of the precursor and reaction mixture are shown in
Figure 3, with signals corresponding to only the coupled product and residual precursor found in the GPC traces of the product.
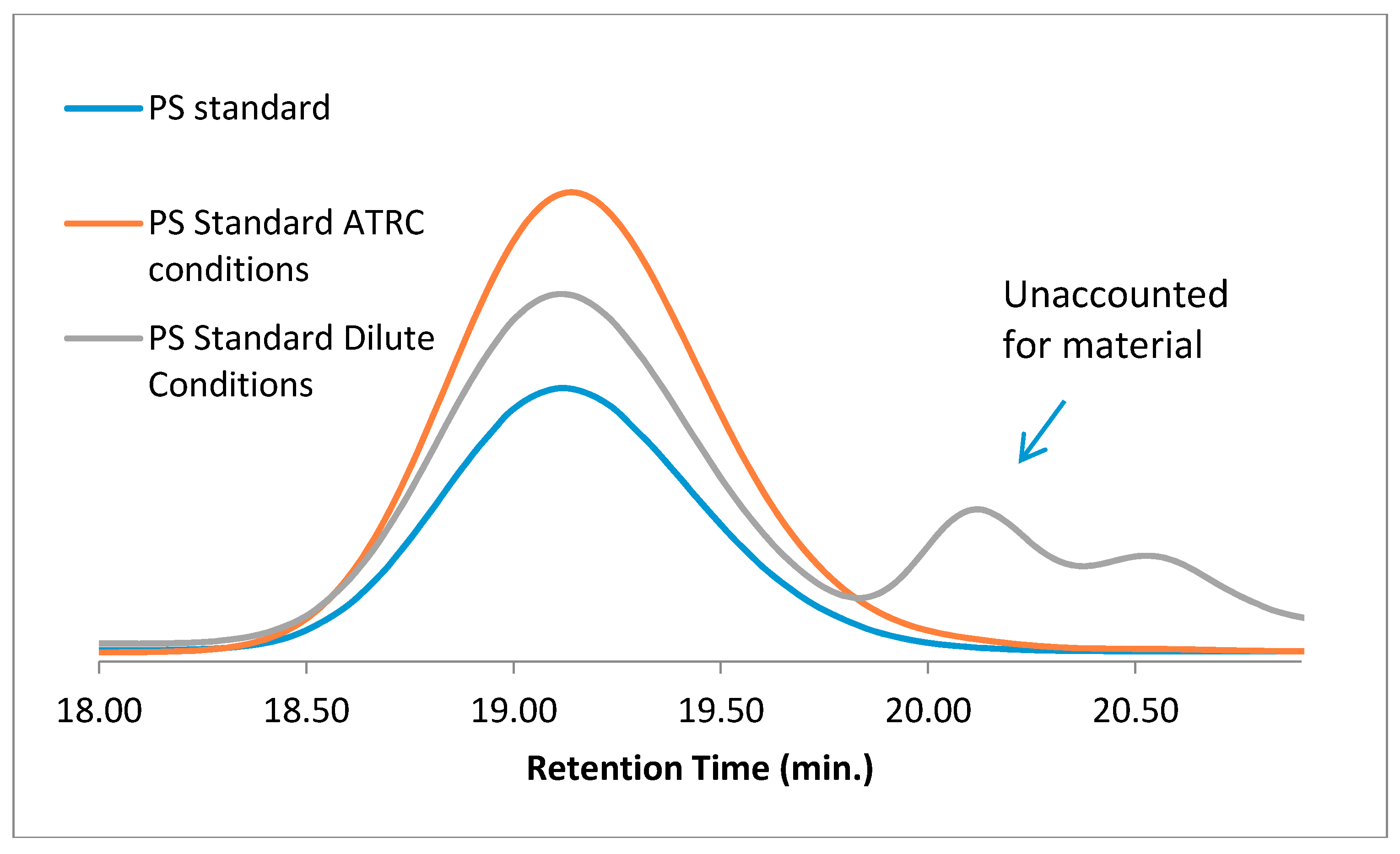
To remove the possibility of having reactive polymer chain ends undergoing some sort of depolymerization under highly dilute conditions, PS analytical standards were used as a “linear precursor” and subjected to cyclization reaction conditions and more concentrated dimerization conditions. As shown in
Figure 4, under more concentrated ATRC-type conditions, the only signal observed was that of the original PS standard, while under highly dilute conditions mimicking cyclization conditions, the unaccounted for, low-molecular-weight material was also observed. This rules out the reactive functional groups on the PS chain ends being responsible for creating low-molecular-weight materials.
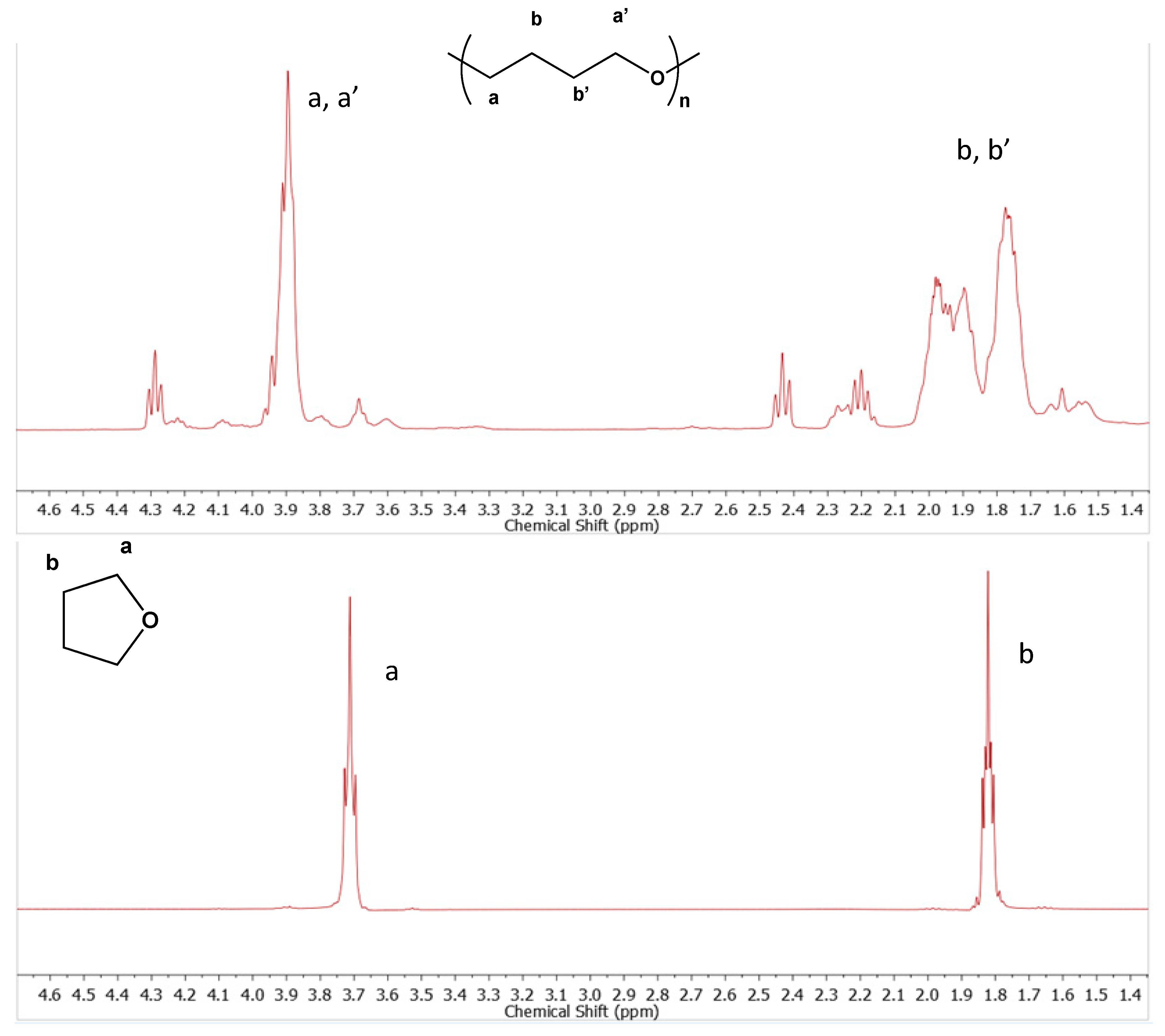
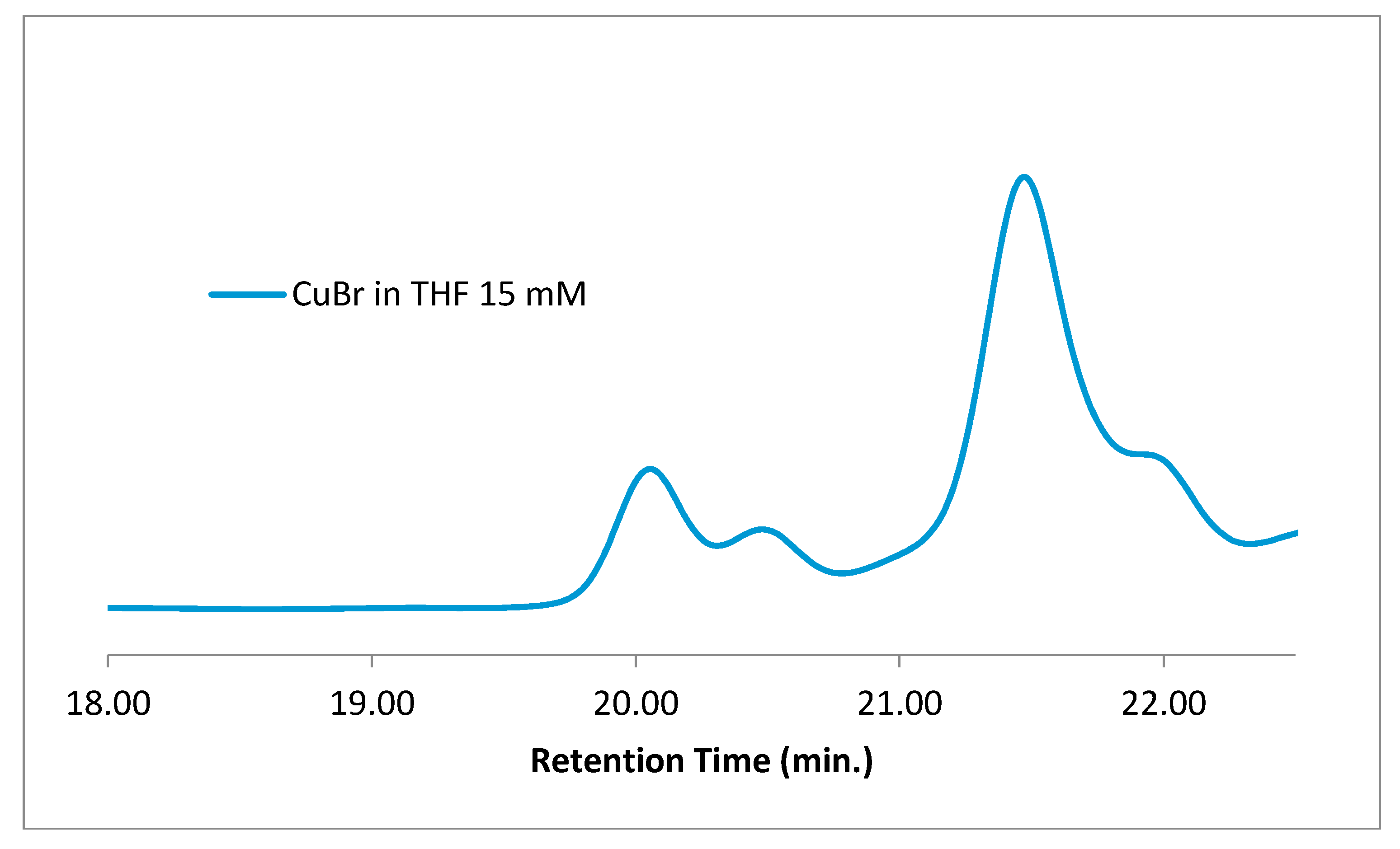
Removing the polymer, ligand, and radical trap and only having CuBr in THF at the concentration and temperature used for cyclization reactions, perhaps surprisingly, resulted in identical signals to those observed in the earlier cyclization reactions. It should be noted that, as a test, when the same components were prepared and immediately analyzed by GPC, no such signals were observed; only after the reaction mixture was stirred at cyclization temperature (60 °C) for the allotted time did these signals appear on GPC. This low-molecular-weight material was isolated as a sticky gel by rotary evaporation of the reaction mixture and analyzed by
1H NMR after removal of residual copper. At this point, it was clear that the material must arise from THF itself, and a
1H NMR spectrum of THF was compared to that of the isolated low-molecular-weight material. As shown in
Figure 5, the material is consistent with low-molecular-weight poly(THF) [
25] and, by process of elimination, must be poly(THF). Smaller, sharper signals around 4.4 ppm and between 2.2–2.5 ppm may be due to smaller, oligomeric chains and/or end groups. The GPC data shows some variety in the length and apparent size of these side products, and future work needs to be done for a more thorough structural assignment and mechanistic explanation. Interestingly, this side product is only formed under highly dilute conditions, and while the exact mechanism is not clear, it could result from trace amounts of a protic source or simply complexation with CuBr and another THF molecule acting as a nucleophile.
A series of mock cyclizations were performed, using various concentrations of PS standards (
Mn = 2970 Da) and reactants to mimic the polymer precursor and commonly used molar ratios of copper bromide, copper, and PMDETA ligand. The goal of these series of reactions was to relate reactant concentrations to the evolution and the extent to which the unwanted poly(THF) was observed. As shown in
Figure 6, greater relative amounts of poly(THF) side products, compared to PS, were found at lower concentrations of reactants overall. Note that these reaction protocols differed when compared to the standard intermolecular ATRC-type conditions (
Figure 4) in that they were allowed to proceed for 24 h to match a time length applicable to cyclization conditions. The results of
Figure 6 are consistent with the side product being formed in highly dilute conditions, but its production was absent or minimized when reactant concentrations were increased.
Lastly, to demonstrate that the low-molecular-weight species is formed from THF and is not somehow related to the other species in the mock cyclization reactions, removing the polymer, ligand, and radical trap and only having CuBr in THF, surprisingly, resulted in the identical signals being observed (
Figure 7). It should be noted that, as a test, when the same components were prepared and immediately analyzed by GPC, no such signals were observed; only after the reaction mixture was stirred at cyclization temperature (60 °C) for the allotted time did these signals appear on GPC.