Recyclable Choline Nicotinate and Ferulate Aqueous Solutions as Efficient Lignin Solvents
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Synthesis of [Ch][Na], [Ch][Fa], [Ch][Va] and [Ch][Sa]
2.3. Dissolution of Lignin
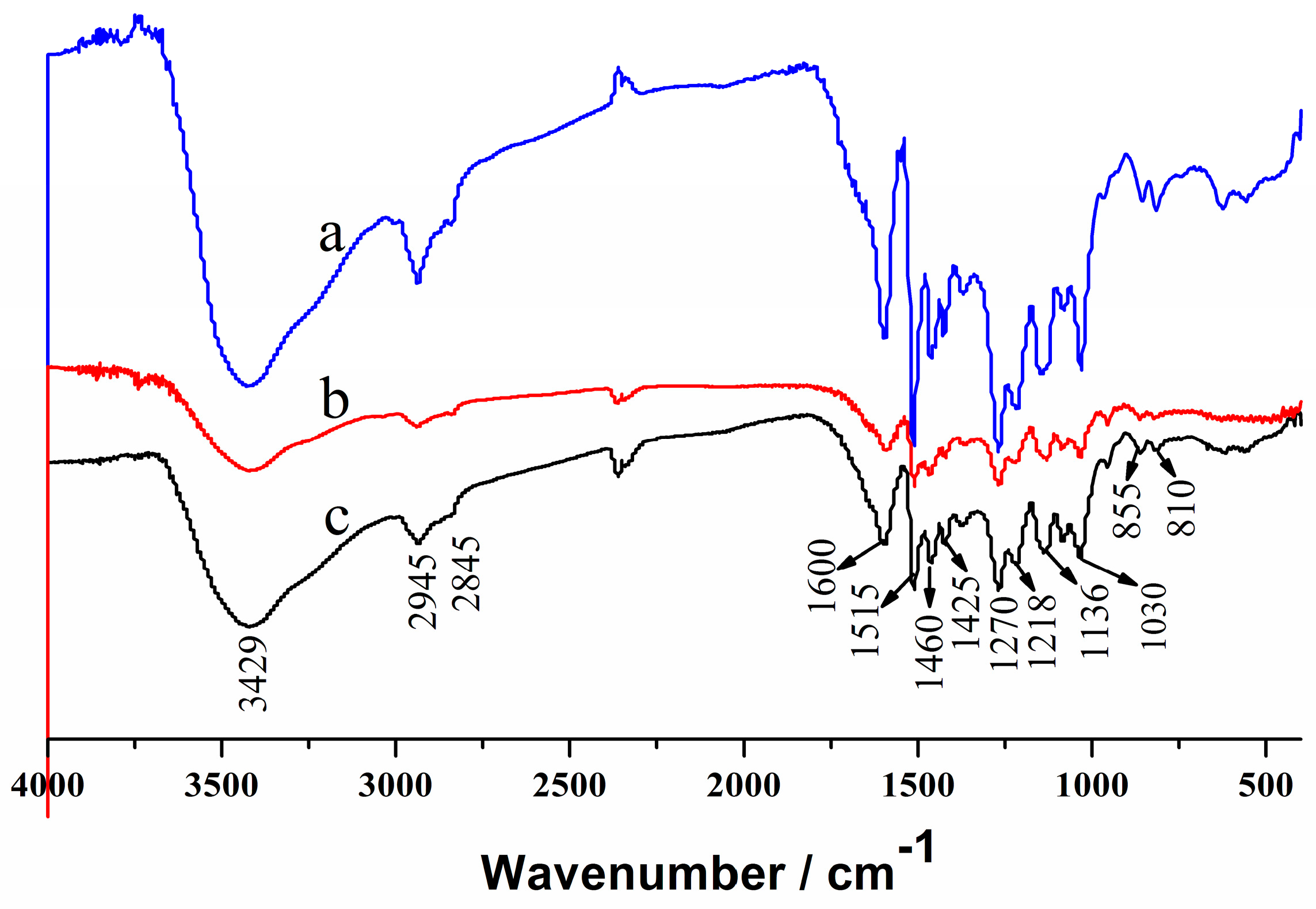
2.4. Characterization of the Regenerated Lignin
2.5. Measurements of 13C NMR Spectra
3. Results and Discussion
3.1. Thermal Properties of [Ch][Na], [Ch][Fa], [Ch][Va] and [Ch][Sa]
3.2. Effect of Anionic Structure
3.3. Effect of H2O Addition
3.4. Interaction between Lignin and [Ch][Na] in H2O/[Ch][Na] Solvent
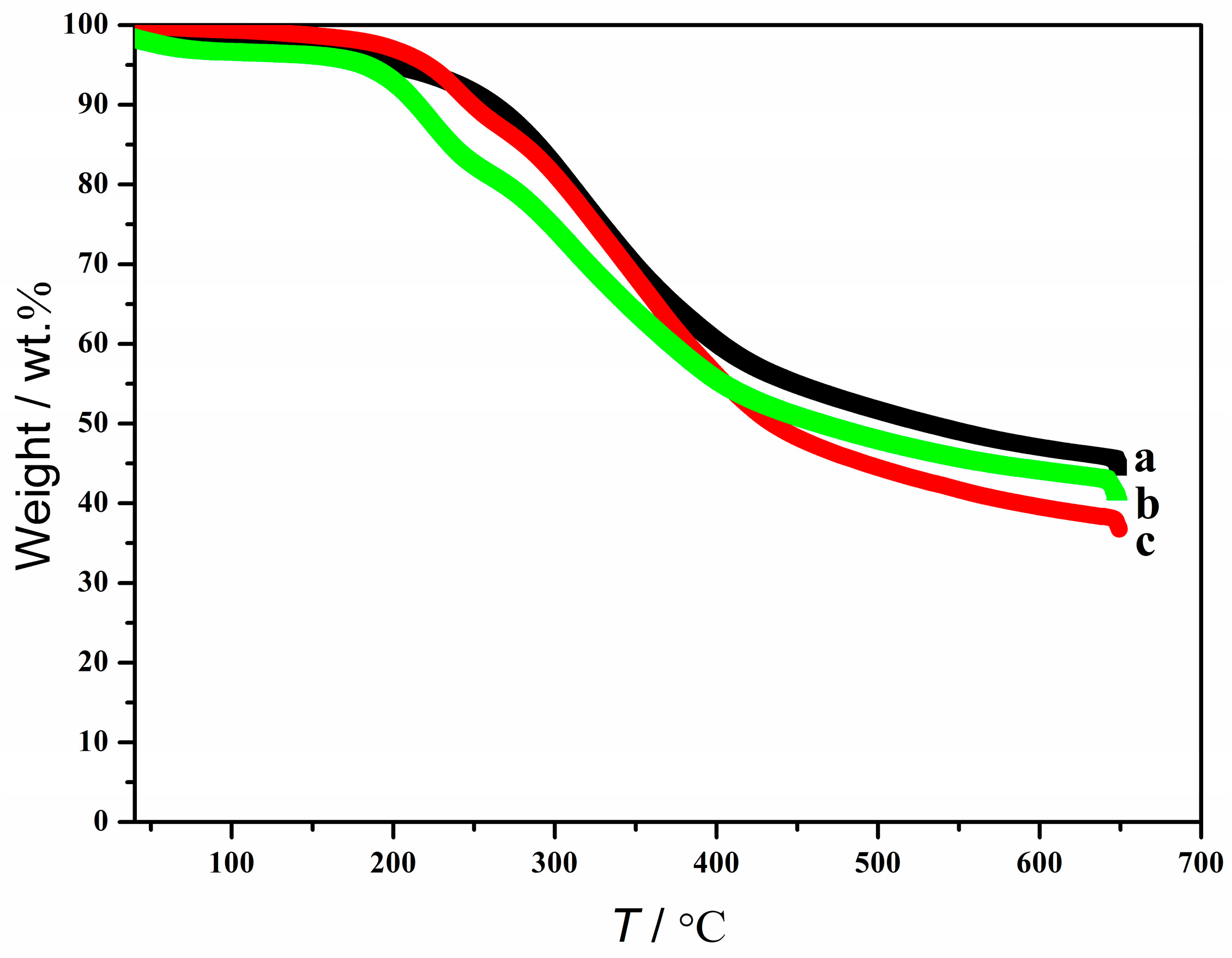
3.5. Recovery of Solvent and Structure and Thermal Properties of the Regenerated Lignin
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- George, W.H.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar]
- Metzger, J.O.; Bicke, C.; Faix, O.; Tuszynski, W.; Angermann, R.; Karas, M.; Strupat, K. Matrix assisted laser desorption mass spectrometry of lignins. J. Angew. Chem. Int. Ed. 1992, 31, 762–764. [Google Scholar]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuel sand biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Thielemans, W.; Can, E.; Morye, S.S.; Woo, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [PubMed]
- Xu, H.; Zhang, D.D.; Wu, F.M. Deep oxidative desulfurization of fuels based on [C4mimCl]CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Chen, F.F.; Huang, K.; Zhou, Y.; Tian, Z.Q.; Zhu, X.; Tao, D.J. Multi-molar absorption of CO2 by the activation of carboxylate groups in amino acid ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 7166–7170. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.J.; Chen, F.F.; Tian, Z.Q.; Huang, K.; Mahurin, S.M.; Jiang, D.E.; Dai, S. Highly efficient carbon monoxide capture by carbanion-functionalized ionic liquids through c-site interactions. Angew. Chem. Int. Ed. 2017, 56, 6843–6847. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.J.; Cheng, Z.; Chen, F.F.; Li, Z.M.; Hu, N.; Chen, X.S. Synthesis and thermophysical properties of biocompatible cholinium-based amino acid ionic liquids. J. Chem. Eng. Data. 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Tao, D.J.; Dong, Y.; Cao, Z.J.; Chen, F.F.; Chen, X.S.; Huang, K. Tuning the acidity of sulfonic functionalized ionic liquids for highly efficient and selective synthesis of terpene esters. J. Ind. Eng. Chem. 2016, 41, 122–129. [Google Scholar] [CrossRef]
- Xu, A.R.; Li, Q. Sustainable and low viscous 1-allyl-3-methylimidazolium acetate + PEG solvent for cellulose processing. Polymers 2017, 9, 54–62. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, W.; Zhang, X.Q.; Wei, Y.; Zhang, A.P.; Liu, S.J.; Wang, X.Y.; Liu, F. Structural changes of bagasse during the homogeneous esterification with maleic anhydride in ionic liquid 1-allyl-3-methylimidazolium chloride. Polymers 2018, 10, 433–449. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.J.; Liu, X.M.; Zhang, S.J. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [PubMed]
- Cao, Y.J.; Zhang, R.R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H.Z. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [PubMed]
- Ji, W.Y.; Ding, Z.D.; Liu, J.H.; Song, Q.X.; Xia, X.L.; Gao, H.Y.; Wang, H.J.; Gu, W.X. Mechanism of lignin dissolution and regeneration in ionic liquid. J. Energy Fuels 2012, 26, 6393–6403. [Google Scholar] [CrossRef]
- Pu, Y.Q.; Jiang, N.; Ragauskas, A.J. Ionic liquid as a green solvent for lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Hart, W.E.S.; Harper, J.B.; Aldous, L. The effect of changing the components of an ionic liquid upon the solubility of lignin. Green Chem. 2015, 17, 214–218. [Google Scholar] [Green Version]
- Glas, D.; Doorslaer, C.V.; Depuydt, D.; Liebner, F.; Rosenau, T.; Binnemans, K.; Vosa, D.E.D. Lignin solubility in non-imidazolium ionic liquids. J. Chem. Technol. Biotechnol. 2015, 90, 1821–1826. [Google Scholar]
- Rashid, T.; Kait, C.F.; Regupathi, I.; Murugesan, T. Dissolution of kraft lignin using protic ionic liquids and characterization. Ind. Crops Prod. 2016, 84, 284–293. [Google Scholar] [CrossRef]
- Hou, X.D.; Xu, J.; Li, N.; Zong, M.H. Effect of anion structures on cholinium ionic liquids pretreatment of rice straw and the subsequent enzymatic hydrolysis. Biotechnol. Bioeng. 2015, 112, 65–73. [Google Scholar] [PubMed]
- Liu, Q.P.; Hou, X.D.; Li, N.; Zong, M.H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar]
- Wang, Y.T.; Wei, L.G.; Li, K.L.; Ma, Y.C.; Ma, N.N.; Ding, S.; Wang, L.L.; Zhao, D.Y.; Yan, B.; Wan, W.Y.; et al. Lignin dissolution in dialkylimidazolium-based ionic liquid–water mixtures. Bioresour. Technol. 2014, 170, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.M.; Zhao, X.H.; Sun, R.C.; Mu, T.C. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Xu, A.R.; Li, W.J.; Zhang, Y.B.; Xu, H. Eco-friendly polysorbate aqueous solvents for efficient dissolution of lignin. RSC Adv. 2016, 6, 8377–8379. [Google Scholar] [CrossRef]
- Xu, A.R.; Guo, X.; Ma, J.Y.; Liu, C.Y.; Li, Q.; Wen, S. Novel and efficient diethylene glycol/H2O solvent for lignin dissolution. BioResources 2016, 11, 6532–6539. [Google Scholar] [CrossRef]
- Xu, A.R.; Guo, X.; Zhang, Y.B.; Li, Z.Y.; Wang, J.J. Efficient and sustainable solvents for lignin dissolution: Aqueous choline carboxylate solutions. Green Chem. 2017, 19, 4067–4073. [Google Scholar] [CrossRef]
- Jordana, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, K.; Wei, L.; Ma, Y.; Shao, G.; Zhao, D.; Wan, W.; Song, L. Understanding lignin treatment in dialkylimidazolium-based ionic liquid−water mixtures. Bioresour. Technol. 2015, 196, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod. Bioref. 2014, 8, 836–856. [Google Scholar]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X.F.; Sun, R.C. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S.S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136. [Google Scholar]
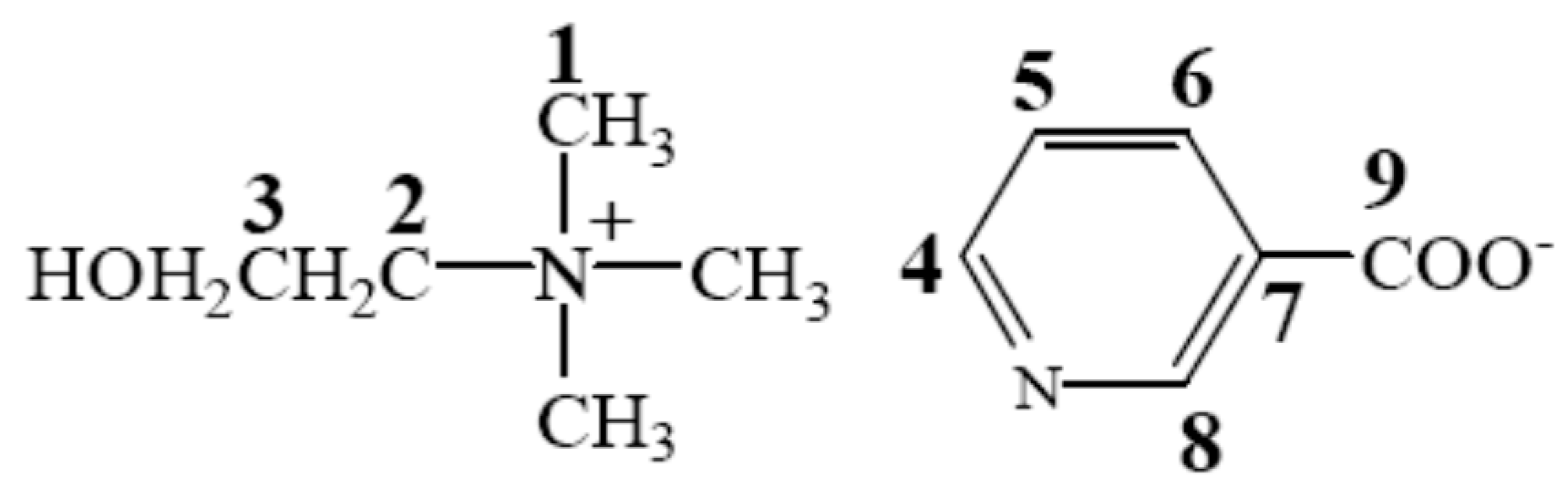
| IL | Abbreviation | Schematic Stucture | Tm (°C) | Tg (°C) | Td (°C) | |
|---|---|---|---|---|---|---|
| Cation | Anion | |||||
| Choline nicotinate | [Ch][Na] | 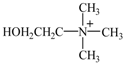 | 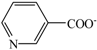 | 76.9 | — a | 219.0 |
| Choline ferulate | [Ch][Fa] | 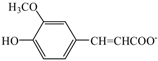 | — a | −7.2 | 163.1 | |
| Choline vanillate | [Ch][Va] | 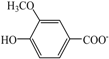 | — a | 102.9 | 174.8 | |
| Choline syringate | [Ch][Sa] | 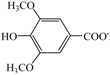 | — a | −1.6 | 170.6 | |
| R | Solubility (g/100 g Solvent) | ||
|---|---|---|---|
| [Ch][Na] | [Ch][Fa] | [Ch][Va] and [Ch][Sa] | |
| 0 | — a | — a | — a |
| 1 | — a | — a | — a |
| 2 | 0.4 | — a | — a |
| 3 | >57 | — a | — a |
| 4 | >72 | 29 | — a |
| 6 | — b | >42 | — a |
| 7 | >72 | — b | — a |
| 8 | >69 | — b | — a |
| 10 | >69 | >51 | — a |
| 15 | — a | >50 | — a |
| 16 | — a | — a | — a |
| 17 | — a | — a | — a |
| Entry | Solvent | Solubility (Gram Per 100 g of Solvent) |
|---|---|---|
| 1 | [Ch][Fa]/H2O (R = 4:1) | 31.0 |
| 2 | [Ch][Fa]/H2O (R = 6:1) | >45.0 |
| 3 | [Ch][Fa]/H2O (R = 10:1) | >51.0 |
| 4 | [Ch][Fa]/H2O (R = 15:1) | >50.0 |
| Lignin Concentration (wt %) | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 53.43 | 55.17 | 67.10 | 148.83 | 123.62 | 132.52 | 137.19 | 149.80 | 171.08 |
| 8.0 | 53.38 | 55.14 | 67.06 | 148.78 | 123.58 | 132.49 | 137.20 | 149.71 | 171.05 |
| Δδ | −0.05 | −0.03 | −0.04 | −0.05 | −0.04 | −0.03 | 0.01 | −0.09 | −0.03 |
| Sample | Mn | Mw |
|---|---|---|
| Regenerated lignin from [Ch][Fa]/H2O (R = 10:1)/lignin solution | 2,062,903 | 2,738,281 |
| Regenerated lignin from [Ch][Na] (R = 8:1)/H2O/lignin solution | 2,244,528 | 2,842,232 |
| Original lignin | 2,056,690 | 2,796,590 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Chen, L.; Xu, X.; Xiao, Z.; Liu, R.; Gao, R.; Yuan, M.; Zhang, L. Recyclable Choline Nicotinate and Ferulate Aqueous Solutions as Efficient Lignin Solvents. Polymers 2018, 10, 840. https://doi.org/10.3390/polym10080840
Xu A, Chen L, Xu X, Xiao Z, Liu R, Gao R, Yuan M, Zhang L. Recyclable Choline Nicotinate and Ferulate Aqueous Solutions as Efficient Lignin Solvents. Polymers. 2018; 10(8):840. https://doi.org/10.3390/polym10080840
Chicago/Turabian StyleXu, Airong, Lin Chen, Xingmin Xu, Zhihong Xiao, Rukuan Liu, Ruixue Gao, Mengzhen Yuan, and Luwei Zhang. 2018. "Recyclable Choline Nicotinate and Ferulate Aqueous Solutions as Efficient Lignin Solvents" Polymers 10, no. 8: 840. https://doi.org/10.3390/polym10080840
APA StyleXu, A., Chen, L., Xu, X., Xiao, Z., Liu, R., Gao, R., Yuan, M., & Zhang, L. (2018). Recyclable Choline Nicotinate and Ferulate Aqueous Solutions as Efficient Lignin Solvents. Polymers, 10(8), 840. https://doi.org/10.3390/polym10080840





