Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance
Abstract
1. Introduction
2. Materials and Methods
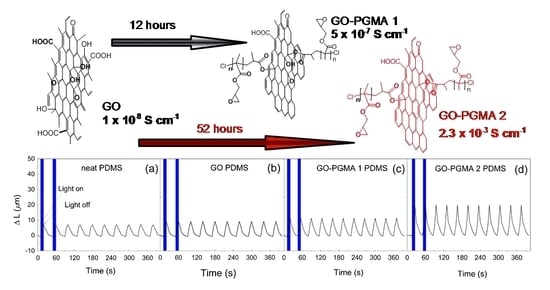
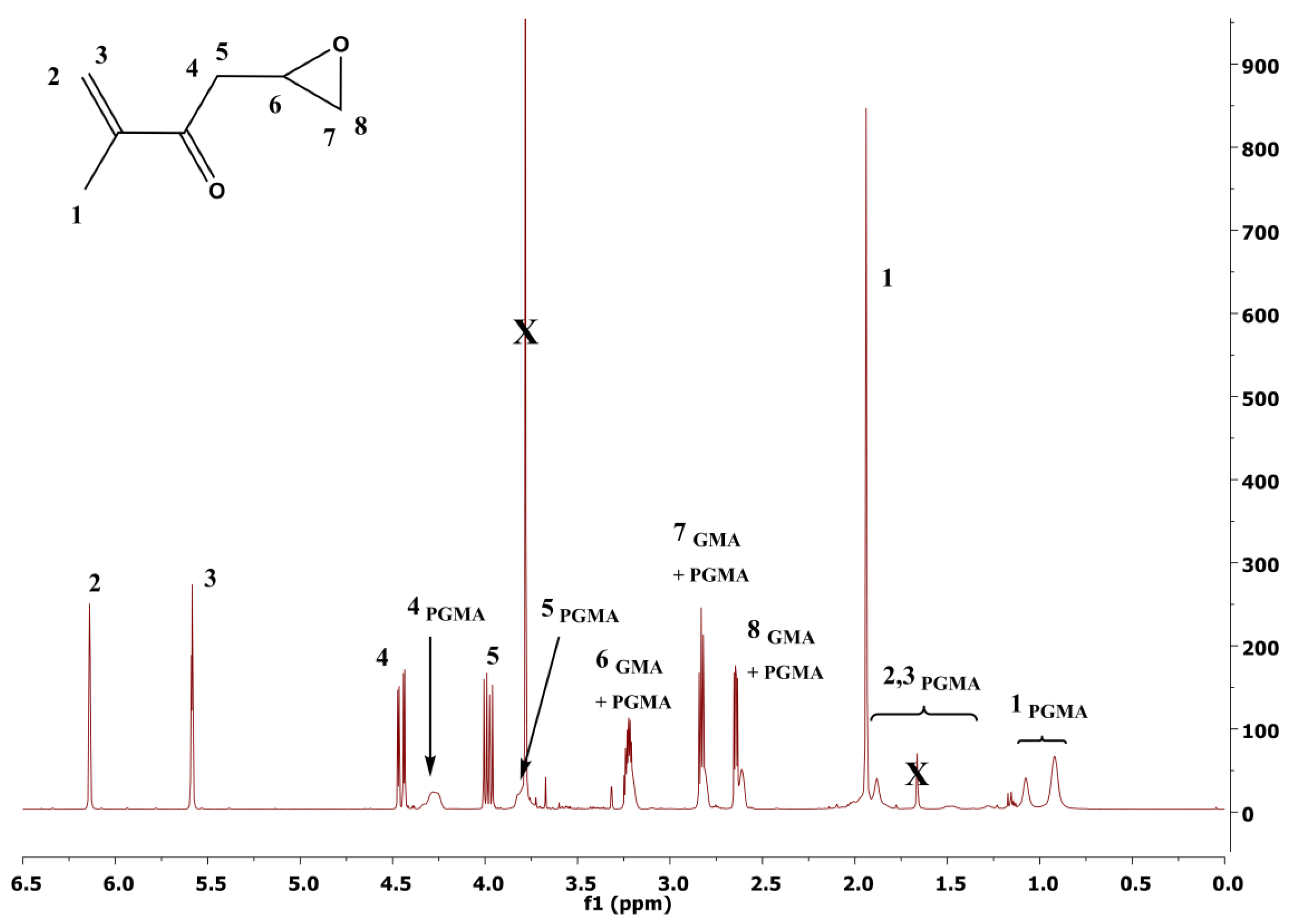
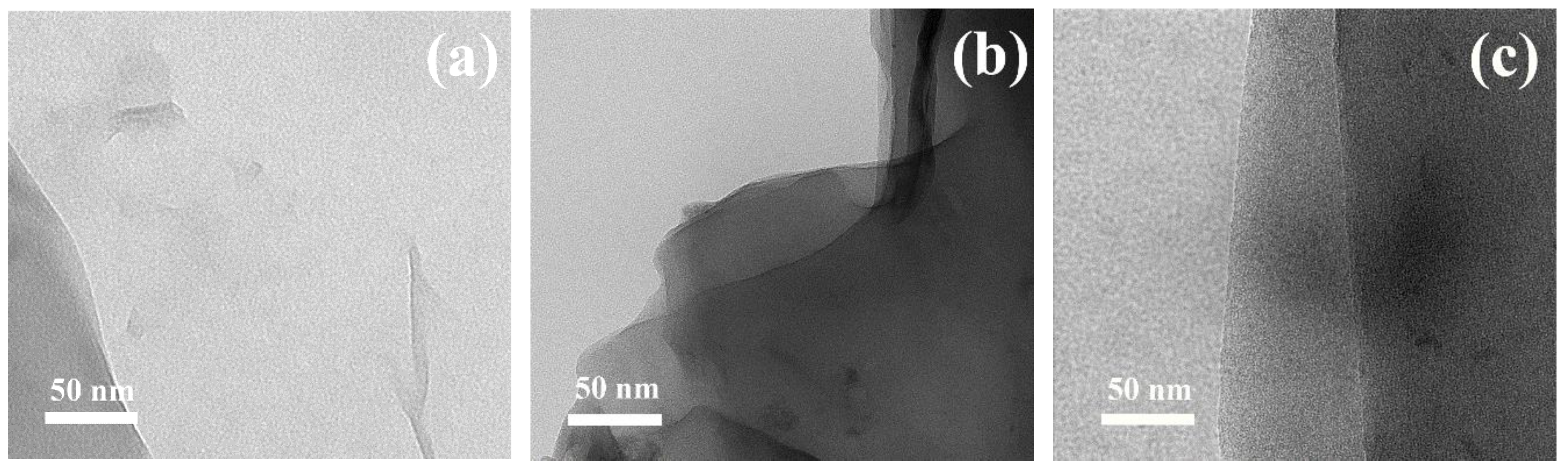
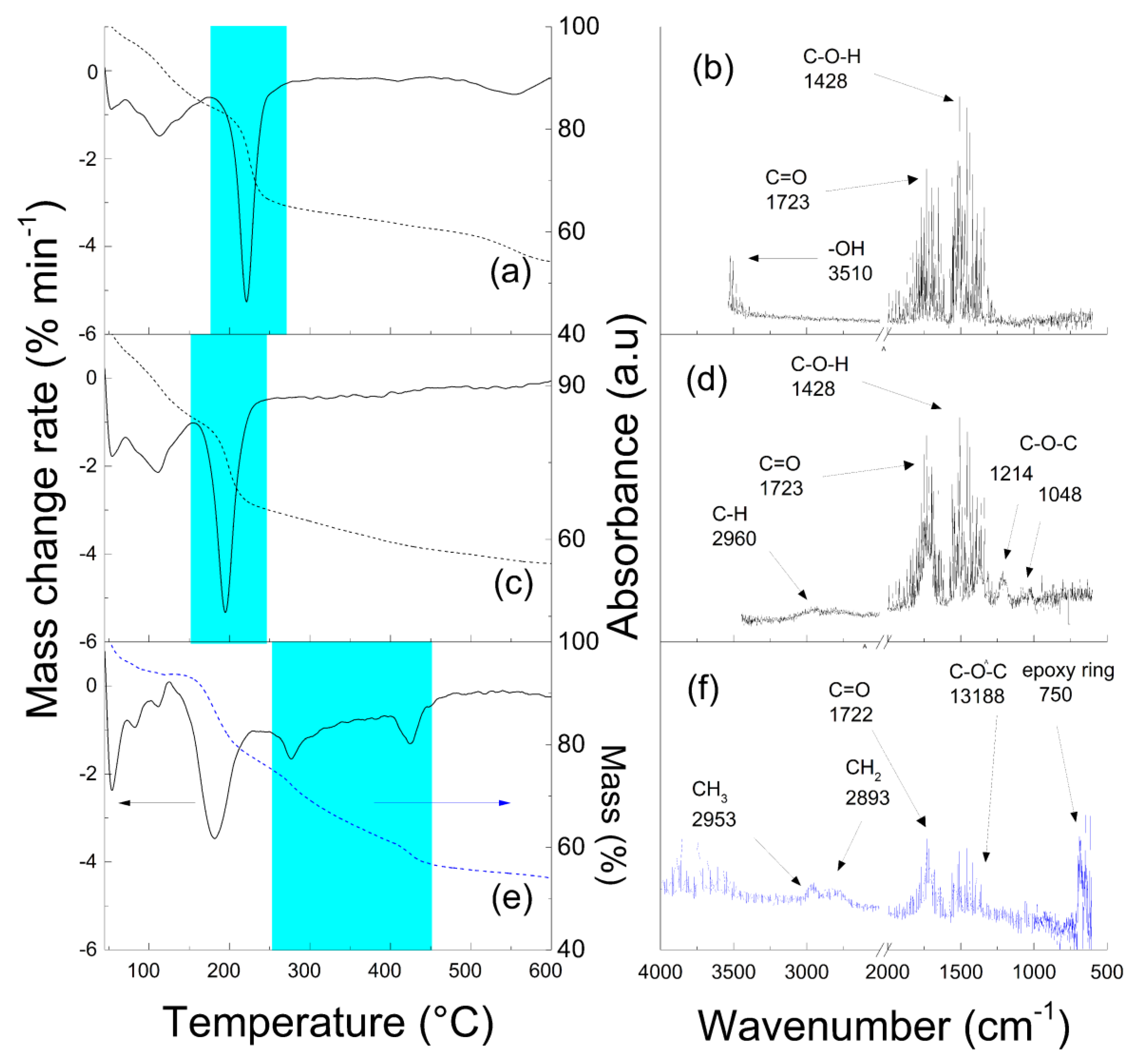
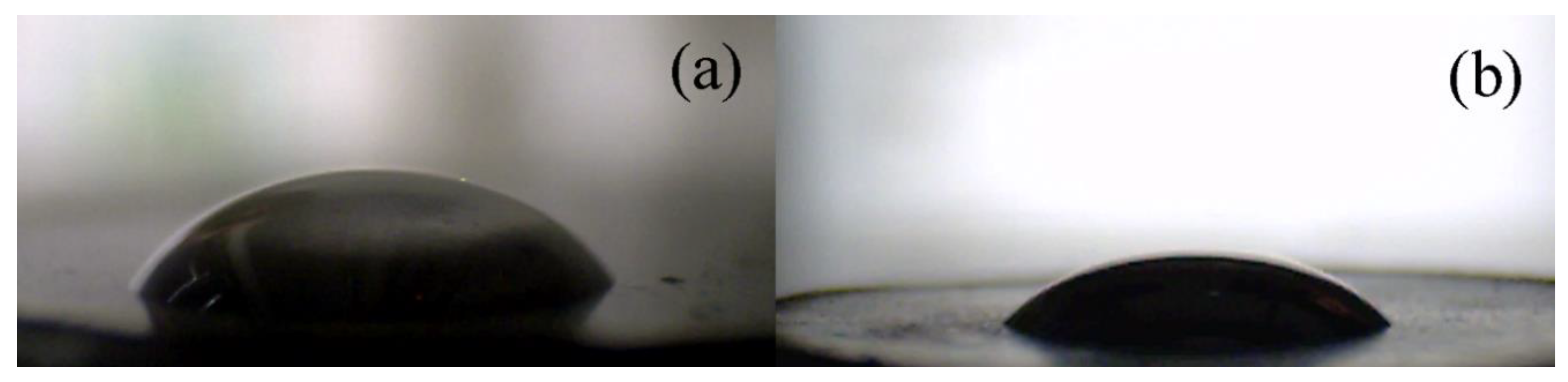
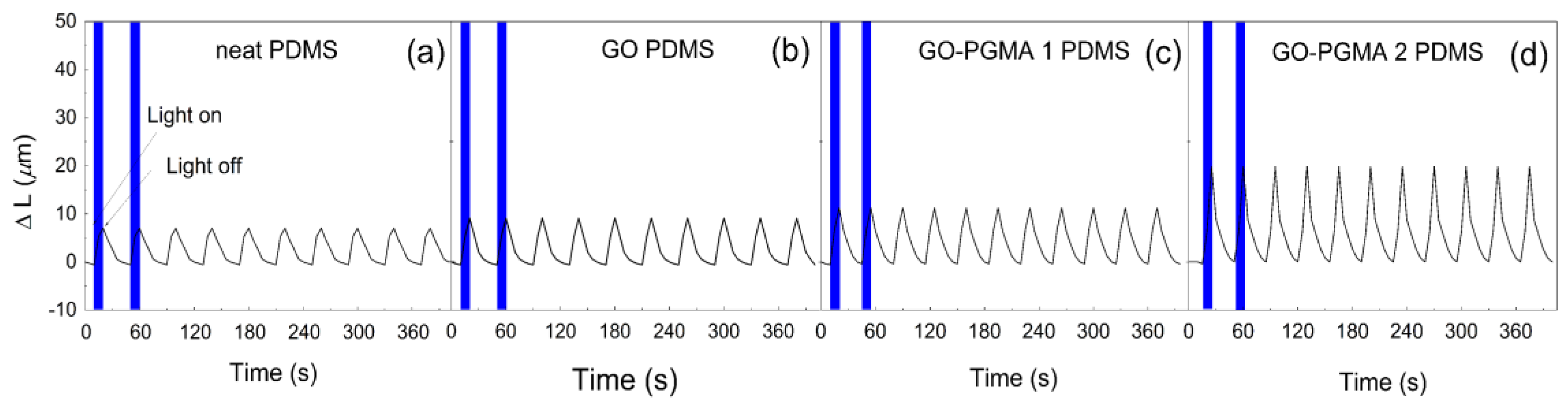
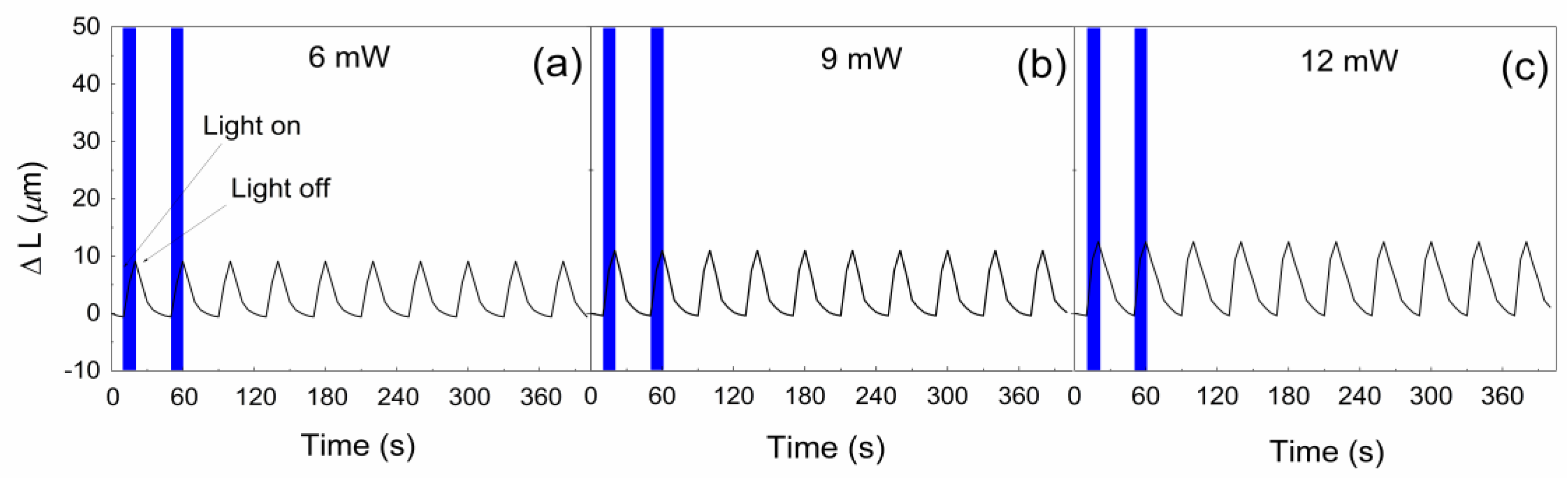
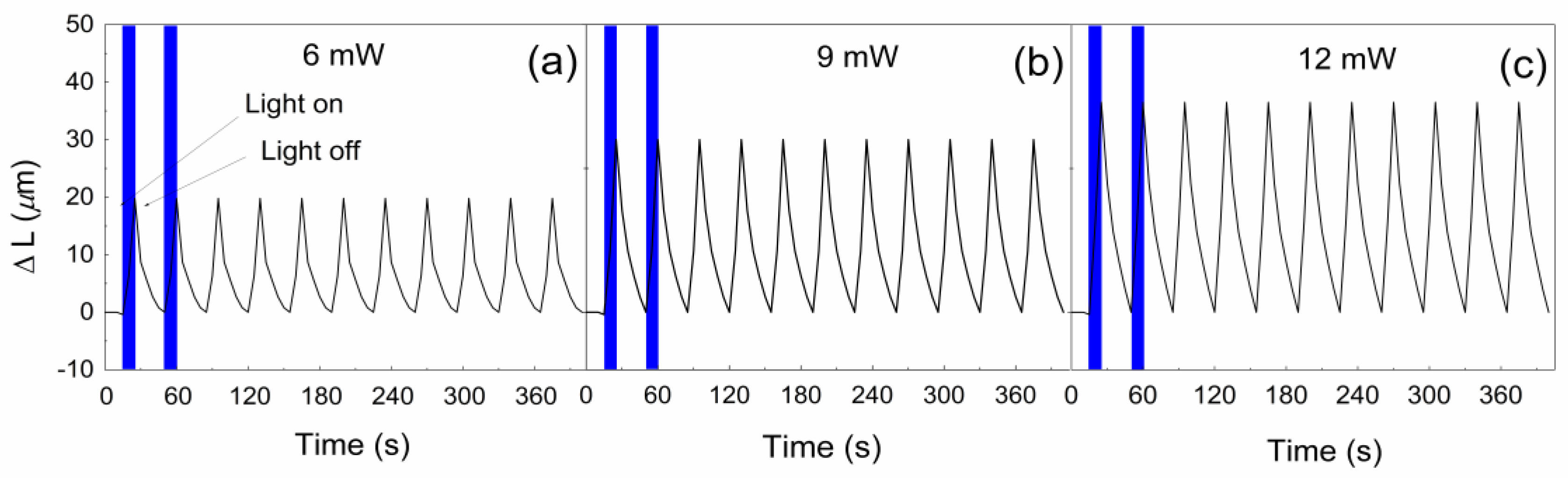
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ilcikova, M.; Mrlik, M.; Babayan, V.; Kasak, P. Graphene oxide modified by betaine moieties for improvement of electrorheological performance. RSC Adv. 2015, 5, 57820–57827. [Google Scholar] [CrossRef]
- Jun, C.S.; Kwon, S.H.; Choi, H.J.; Seo, Y. Polymeric Nanoparticle-Coated Pickering Emulsion-Synthesized Conducting Polyaniline Hybrid Particles and Their Electrorheological Study. ACS Appl. Mater. Interfaces 2017, 9, 44811–44819. [Google Scholar] [CrossRef] [PubMed]
- Mosse, A. Gossamer timescapes: A design-led investigation into electro-active and light responsive textiles for the home. Smart Mater. Struct. 2018, 27, 074009. [Google Scholar] [CrossRef]
- Peng, L.; Liu, Y.; Huang, J.N.; Li, J.H.; Gong, J.H.; Ma, J.H. Microfluidic fabrication of highly stretchable and fast electro-responsive graphene oxide/polyacrylamide/alginate hydrogel fibers. Eur. Polym. J. 2018, 103, 335–341. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Cvek, M.; Pavlinek, V.; Zahoranova, A.; Kronekova, Z.; Kasak, P. Carbonyl iron coated with a sulfobetaine moiety as a biocompatible system and the magnetorheological performance of its silicone oil suspensions. RSC Adv. 2016, 6, 32823–32830. [Google Scholar] [CrossRef]
- Han, S.; Choi, J.; Seo, Y.P.; Park, I.J.; Choi, H.J.; Seo, Y. High-Performance Magnetorheological Suspensions of Pickering-Emulsion-Polymerized Polystyrene/Fe3O4 Particles with Enhanced Stability. Langmuir 2018, 34, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jia, W.P.; Zheng, W.; Liu, H.; Jiang, D.G.; Li, Z.M.; Tian, Y.; Zhang, W.L.; Liu, J.Q. Hierarchically magnetic Ni-Al binary layered double hydroxides: Towards tunable dual electro/magneto-stimuli performances. J. Ind. Eng. Chem. 2018, 58, 163–171. [Google Scholar] [CrossRef]
- Nakayama, M.; Kajiyama, S.; Kumamoto, A.; Nishimura, T.; Ikuhara, Y.; Yamato, M.; Kato, T. Stimuli-responsive hydroxyapatite liquid crystal with macroscopically controllable ordering and magneto-optical functions. Nat. Commun. 2018, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Mrlík, M.; Špírek, M.; Al-Khori, J.; Ahmad, A.A.; Mosnaček, J.; AlMaadeed, M.A.; Kasák, P. Mussel-mimicking sulfobetaine-based copolymer with metal tunable gelation, self-healing and antibacterial capability. Arabian J. Chem. 2017. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.; Pan, J.M.; Meng, Z.H.; Pan, G.Q.; Sellergren, B. Molecularly Imprinted Polymers with Stimuli-Responsive Affinity: Progress and Perspectives. Polymers 2015, 7, 1689–1715. [Google Scholar] [CrossRef]
- Curcio, M.; Mauro, L.; Naimo, G.D.; Amantea, D.; Cirillo, G.; Tavano, L.; Casaburi, I.; Nicoletta, F.P.; Alvarez-Lorenzo, C.; Iemma, F. Facile synthesis of pH-responsive polymersomes based on lipidized PEG for intracellular co-delivery of curcumin and methotrexate. Colloid Surf. B Biointerfaces 2018, 167, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.L.; Zhao, Y.L.; Wang, P.X.; Li, Z.; Hou, X.Y.; Huang, Z.T.; Yang, H.B. Rheological behavior and mechanism of pH-responsive wormlike micelle variations induced by isomers of phthalic acid. Soft Matter 2018, 14, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Zahoranova, A.; Mrlik, M.; Tomanova, K.; Kronek, J.; Luxenhofer, R. ABA and BAB Triblock Copolymers Based on 2-Methyl-2-oxazoline and 2-n-Propyl-2-oxazoline: Synthesis and Thermoresponsive Behavior in Water. Macromol. Chem. Phys. 2017, 218, 1700031. [Google Scholar] [CrossRef]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive Poly(2-Oxazoline) Molecular Brushes by Living Ionic Polymerization: Modulation of the Cloud Point by Random and Block Copolymer Pendant Chains. Macromol. Chem. Phys. 2012, 213, 1963–1969. [Google Scholar] [CrossRef]
- Xiu, M.M.; Kang, Q.; Tao, M.L.; Chen, Y.; Wang, Y. Thermoresponsive AIE supramolecular complexes in dilute solution: Sensitively probing the phase transition from two different temperature-dependent emission responses. J. Mater. Chem. C 2018, 6, 5926–5936. [Google Scholar] [CrossRef]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboo, R. Poly(2-isopropenyl-2-oxazoline) as a versatile platform towards thermoresponsive copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mosnacek, J.; Mrlik, M.; Sedlacek, T.; Csomorova, K.; Czanikova, K.; Krupa, I. Influence of surface modification of carbon nanotubes on interactions with polystyrene-b-polyisoprene-b-polystyrene matrix and its photo-actuation properties. Polym. Adv. Technol. 2014, 25, 1293–1300. [Google Scholar] [CrossRef]
- Kobatake, S.; Takami, S.; Muto, H.; Ishikawa, T.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sheng, L.; Liu, J.N.; Ju, L.; Li, J.H.; Du, Z.; Zhang, W.R.; Li, M.J.; Zhang, S.X.A. Photoinduced Proton Transfer between Photoacid and pH-Sensitive Dyes: Influence Factors and Application for Visible-Light-Responsive Rewritable Paper. Adv. Funct. Mater. 2018, 28, 1705532. [Google Scholar] [CrossRef]
- Du, L.; Xu, Z.Y.; Fan, C.J.; Xiang, G.; Yang, K.K.; Wang, Y.Z. A Fascinating Metallo-Supramolecular Polymer Network with Thermal/Magnetic/Light-Responsive Shape-Memory Effects Anchored by Fe3O4 Nanoparticles. Macromolecules 2018, 51, 705–715. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, J.C.; Li, W.H.; Samali, B. Development and characterization of a magnetorheological elastomer based adaptive seismic isolator. Smart Mater. Struct. 2013, 22, 035005. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z.N. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Godman, N.P.; Kowalski, B.A.; Auguste, A.D.; Koerner, H.; White, T.J. Synthesis of Elastomeric Liquid Crystalline Polymer Networks via Chain Transfer. ACS Macro Lett. 2017, 6, 1290–1295. [Google Scholar] [CrossRef]
- Anderson, I.A.; Gisby, T.A.; McKay, T.G.; O’Brien, B.M.; Calius, E.P. Multi-functional dielectric elastomer artificial muscles for soft and smart machines. J. Appl. Phys. 2012, 112, 041101. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, J.C.; Tian, T.F.; Li, W.H. A highly adjustable magnetorheological elastomer base isolator for applications of real-time adaptive control. Smart Mater. Struct. 2013, 22, 095020. [Google Scholar] [CrossRef]
- Robinson, S.S.; O’Brien, K.W.; Zhaob, H.; Peele, B.N.; Larson, C.M.; Murray, B.C.M.; van Meerbeek, I.M.; Dunham, S.N.; Shepherd, R.F. Integrated soft sensors and elastomeric actuators for tactile machines with kinesthetic sense. Extreme Mech. Lett. 2015, 5, 47–53. [Google Scholar] [CrossRef]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Pavlinek, V.; Spitalsky, Z.; Mosnacek, J. Graphene oxide reduction during surface-initiated atom transfer radical polymerization of glycidyl methacrylate: Controlling electro-responsive properties. Chem. Eng. J. 2016, 283, 717–720. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mulhaupt, R. Functionalized Graphenes and Thermoplastic Nanocomposites Based upon Expanded Graphite Oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Pei, S.F.; Zhao, J.P.; Du, J.H.; Ren, W.C.; Cheng, H.M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Spitalsky, Z.; Micusik, M.; Csomorova, K.; Sasinkova, V.; Kleinova, A.; Mosnacek, J. A tertiary amine in two competitive processes: Reduction of graphene oxide vs. catalysis of atom transfer radical polymerization. RSC Adv. 2015, 5, 3370–3376. [Google Scholar] [CrossRef]
- Peraza-Hernandez, E.A.; Hartl, D.J.; Malak, R.J.; Lagoudas, D.C. Origami-inspired active structures: A synthesis and review. Smart Mater. Struct. 2014, 23, 094001. [Google Scholar] [CrossRef]
- Czanikova, K.; Krupa, I.; Ilcikova, M.; Kasak, P.; Chorvat, D.; Valentin, M.; Slouf, M.; Mosnacek, J.; Micusik, M.; Omastova, M. Photo-actuating materials based on elastomers and modified carbon nanotubes. J. Nanophotonics 2012, 6, 063522. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Osicka, J.; Ilcikova, M.; Mrlik, M.; Minarik, A.; Pavlinek, V.; Mosnacek, J. The Impact of Polymer Grafting from a Graphene Oxide Surface on Its Compatibility with a PDMS Matrix and the Light-Induced Actuation of the Composites. Polymers 2017, 9, 264. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Chorvat, D.; Krupa, I.; Slouf, M.; Koynov, K.; Mosnacek, J. Viscoelastic and photo-actuation studies of composites based on polystyrene-grafted carbon nanotubes and styrene-b-isoprene-b-styrene block copolymer. Polymer 2014, 55, 211–218. [Google Scholar] [CrossRef]
- Ilcikova, M.; Mrlik, M.; Sedlacek, T.; Slouf, M.; Zhigunov, A.; Koynov, K.; Mosnacek, J. Synthesis of Photoactuating Acrylic Thermoplastic Elastomers Containing Diblock Copolymer-Grafted Carbon Nanotubes. ACS Macro Lett. 2014, 3, 999–1003. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J.; Kim, S.G. Electrorheology of Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Mrlik, M.; Moucka, R.; Ilcikova, M.; Bober, P.; Kazantseva, N.; Spitalsky, Z.; Trchova, M.; Stejskal, J. Charge transport and dielectric relaxation processes in aniline-based oligomers. Synth. Met. 2014, 192, 37–42. [Google Scholar] [CrossRef]
- Mrlik, M.; Cvek, M.; Osicka, J.; Moucka, R.; Sedlacik, M.; Pavlinek, V. Surface-initiated atom transfer radical polymerization from graphene oxide: A way towards fine tuning of electric conductivity and electro-responsive capabilities. Mater. Lett. 2018, 211, 138–141. [Google Scholar] [CrossRef]
- Cvek, M.; Mrlik, M.; Ilcikova, M.; Plachy, T.; Sedlacik, M.; Mosnacek, J.; Pavlinek, V. A facile controllable coating of carbonyl iron particles with poly(glycidyl methacrylate): A tool for adjusting MR response and stability properties. J. Mater. Chem. C 2015, 3, 4646–4656. [Google Scholar] [CrossRef]
- Georgousis, G.; Pandis, C.; Kalamiotis, A.; Georgiopoulos, P.; Kyritsis, A.; Kontou, E.; Pissis, P.; Micusik, M.; Czanikova, K.; Kulicek, J.; et al. Strain sensing in polymer/carbon nanotube composites by electrical resistance measurement. Compos. Part B Eng. 2015, 68, 162–169. [Google Scholar] [CrossRef]
- Rabindranath, R.; Bose, H. On the mobility of iron particles embedded in elastomeric silicone matrix. In Proceedings of the 13th International Conference on Electrorheological Fluids and Magnetorheological Suspensions, Ankara, Turkey, 2–6 July 2012; Unal, H.I., Ed.; Iop Publishing Ltd.: Bristol, UK, 2013. [Google Scholar]
- Feng, Y.Y.; Qin, M.M.; Guo, H.Q.; Yoshino, K.; Feng, W. Infrared-Actuated Recovery of Polyurethane Filled by Reduced Graphene Oxide/Carbon Nanotube Hybrids with High Energy Density. ACS Appl. Mater. Interfaces 2013, 5, 10882–10888. [Google Scholar] [CrossRef] [PubMed]


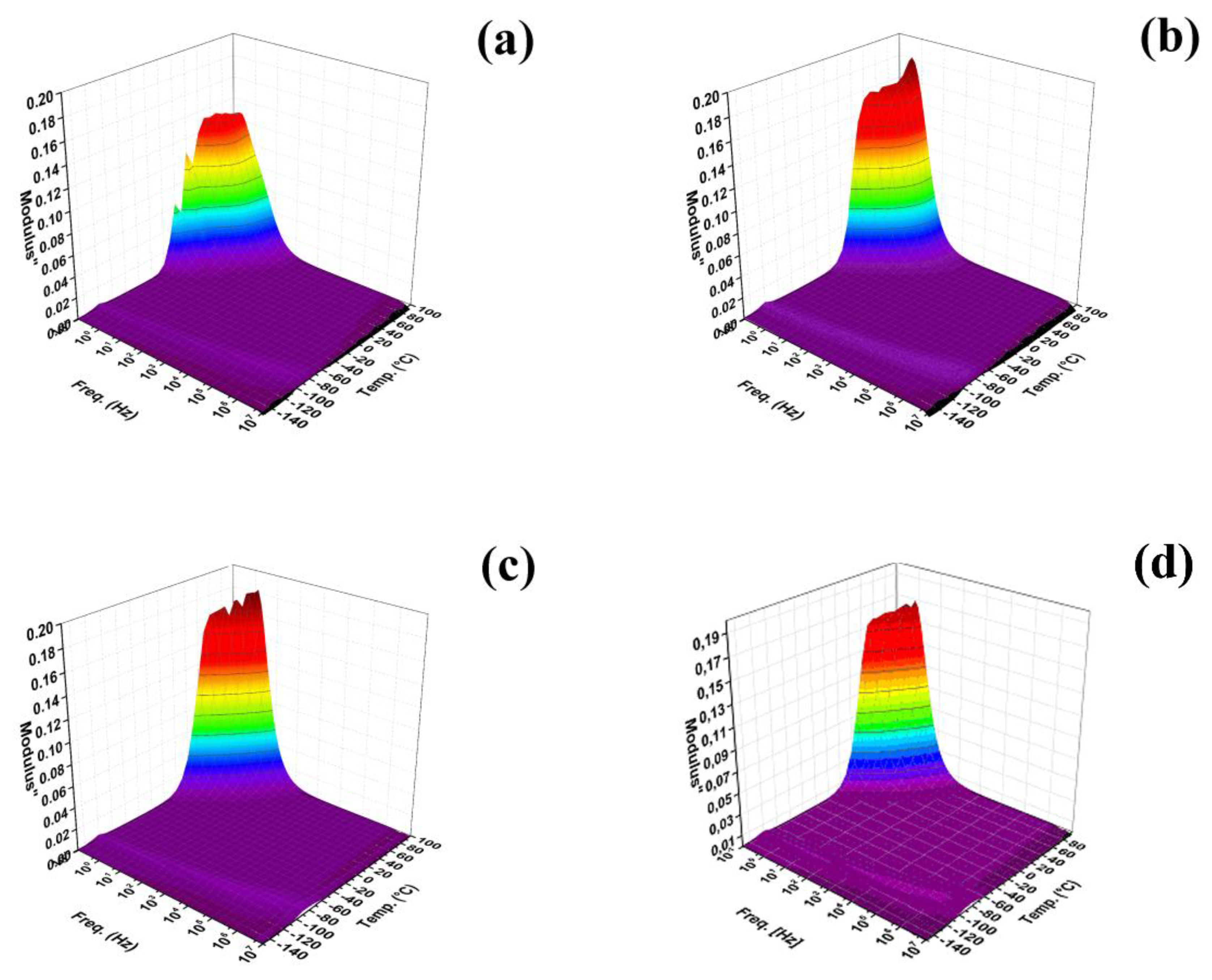
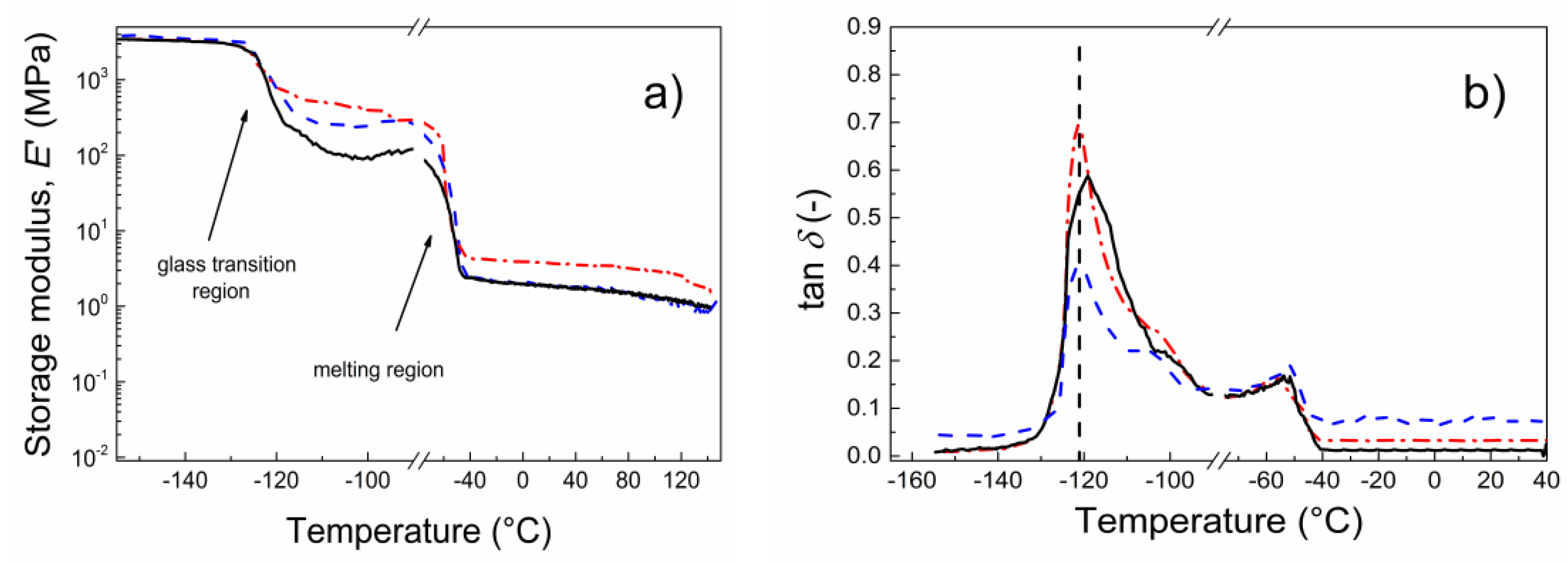

| Sample Code | Ea (kJ·mol−1) of Tg (−120 °C) |
|---|---|
| pure PDMS | 45.70 |
| 0.1 vol % neat GO/PDMS | 36.57 |
| 0.1 vol % GO-PGMA 1/PDMS | 31.32 |
| 0.1 vol % GO-PGMA 2/PDMS | 23.80 |
| Sample Code | Thermal Conductivity (W·m·K−1) |
|---|---|
| pure PDMS | 0.071 |
| 0.1 vol % neat GO/PDMS | 0.144 |
| 0.1 vol % GO-PGMA-1/PDMS | 0.152 |
| 0.1 vol % GO-PGMA-2/PDMS | 0.161 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osicka, J.; Mrlik, M.; Ilcikova, M.; Hanulikova, B.; Urbanek, P.; Sedlacik, M.; Mosnacek, J. Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance. Polymers 2018, 10, 832. https://doi.org/10.3390/polym10080832
Osicka J, Mrlik M, Ilcikova M, Hanulikova B, Urbanek P, Sedlacik M, Mosnacek J. Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance. Polymers. 2018; 10(8):832. https://doi.org/10.3390/polym10080832
Chicago/Turabian StyleOsicka, Josef, Miroslav Mrlik, Marketa Ilcikova, Barbora Hanulikova, Pavel Urbanek, Michal Sedlacik, and Jaroslav Mosnacek. 2018. "Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance" Polymers 10, no. 8: 832. https://doi.org/10.3390/polym10080832
APA StyleOsicka, J., Mrlik, M., Ilcikova, M., Hanulikova, B., Urbanek, P., Sedlacik, M., & Mosnacek, J. (2018). Reversible Actuation Ability upon Light Stimulation of the Smart Systems with Controllably Grafted Graphene Oxide with Poly (Glycidyl Methacrylate) and PDMS Elastomer: Effect of Compatibility and Graphene Oxide Reduction on the Photo-Actuation Performance. Polymers, 10(8), 832. https://doi.org/10.3390/polym10080832