Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Current-Voltage Measurements
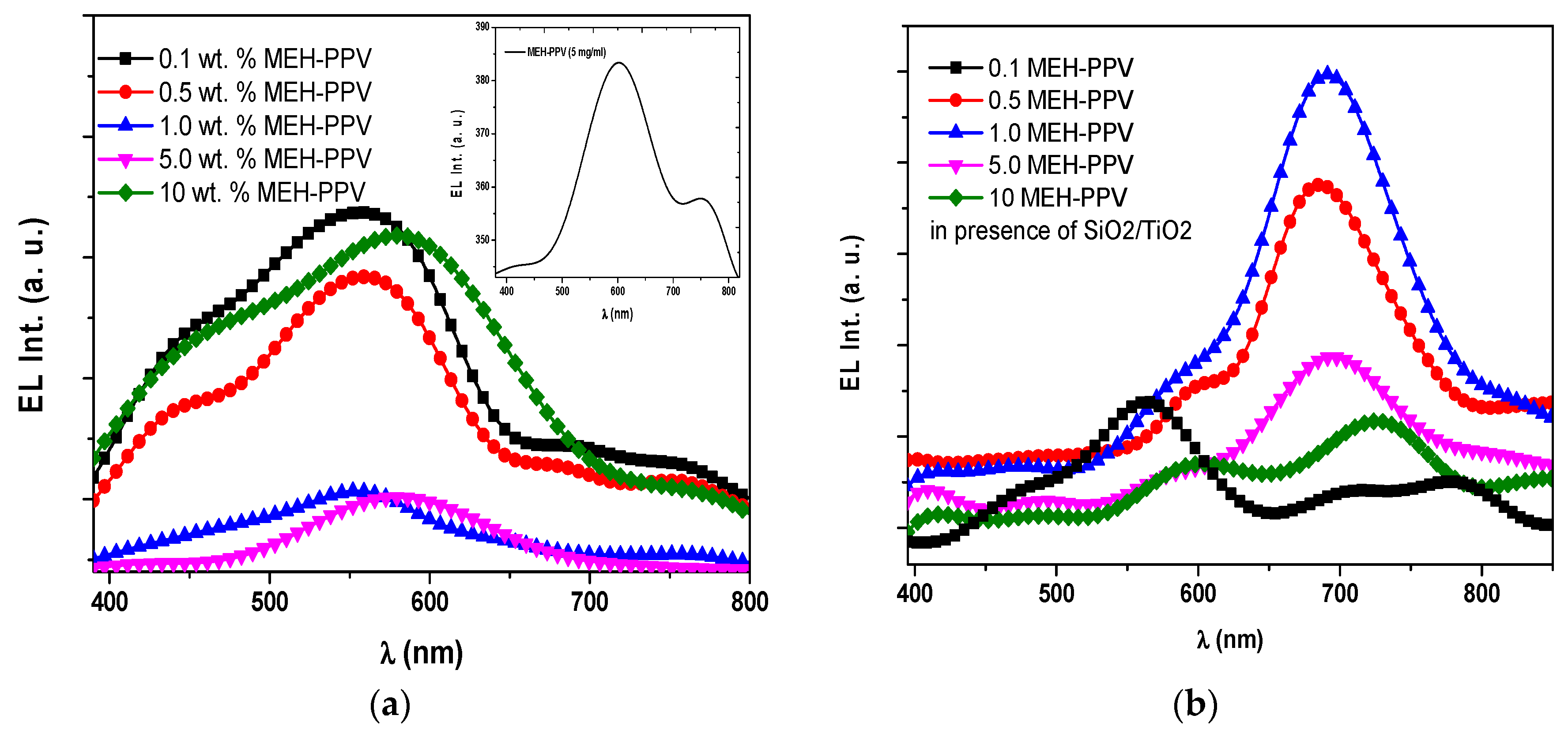
3.2. Electroluminescence Spectra
3.3. Color Measurements
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Assaka, A.M.; Rodrigues, P.C.; De Oliveira, A.R.; Ding, L.; Hu, B.; Karasz, F.E.; Akcelrud, L. Novel fluorine containing polyfluorenes with efficient blue electroluminescence. Polymer 2004, 45, 7071–7081. [Google Scholar] [CrossRef]
- Liu, S.P.; Chan, H.S.; Ng, S.C. Poly[2,7-(9,9-dihexylfluorene)-alt-pyridine] with donor-acceptor architectures: A new series of blue-light-emitting alternating copolymers. J. Polym. Sci. A Polym. Chem. 2004, 42, 4792–4801. [Google Scholar] [CrossRef]
- Jokinen, K.; Bykov, A.; Sliz, R.; Remes, K.; Fabritius, T.; Myllylä, R. Luminescence and spectrum variations caused by thermal annealing in undoped and doped polyfluorene OLEDs. Solid-State Electron. 2015, 103, 184–189. [Google Scholar] [CrossRef]
- Cuerva, C.; Campo, J.A.; Cano, M.; Arredondo, B.; Romero, B.; Otón, E.; Otón, J.M. Bis(pyridylpyrazolate)platinum(ii): A mechanochromic complex useful as a dopant for colour-tunable polymer OLEDs. New J. Chem. 2015, 39, 8467–8473. [Google Scholar] [CrossRef]
- De Azevedo, D.; Freitas, J.N.; Domingues, R.A.; Faleiros, M.M.; Atvars, T.D.Z. Correlation between the PL and EL emissions of polyfluorene-based diodes using bilayers or polymer blends. Synth. Met. 2017, 233, 28–34. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, H.; Miao, Y.; Wang, H.; Zhao, B.; Hao, Y.; Zhu, F.; Xu, B. A red tandem organic light-emitting diode based on organic photovoltaic-type charge generation layer. Org. Electron. 2016, 32, 1–6. [Google Scholar] [CrossRef]
- Nicolai, H.T.; Hof, A.; Blom, P.W. Device Physics of White Polymer Light-Emitting Diodes. Adv. Funct. Mater. 2012, 22, 2040–2047. [Google Scholar] [CrossRef]
- Reineke, S.; Thomschke, M.; Lüssem, B.; Leo, K. White organic light-emitting diodes: Status and perspective. Rev. Mod. Phys. 2013, 85, 1245. [Google Scholar] [CrossRef]
- Quites, F.J.N.; Faria, G.R.C.; Germino, J.C.; Atvars, T.D.Z. Tuning emission colors from blue to green in polymeric light-emitting diodes fabricated using polyfluorene blends. J. Polym. Chem. 2014, 118, 10380–10390. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Chang, H.J. Fabrication and characterization of white polymer light emitting diodes using PFO: MDMO-PPV. J. Nanosci. Nanotechnol. 2012, 12, 3606–3610. [Google Scholar] [CrossRef] [PubMed]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Salleh, M.M. Influence of TiO2 nanoparticles on enhancement of optoelectronic properties of PFO-based light emitting diode. J. Nanomater. 2013, 2013, 561534. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Pei, Q. Flexible and stretchable electrodes for next generation polymer electronics: A review. Sci. China Chem. 2016, 59, 659–671. [Google Scholar] [CrossRef]
- Borriello, C.; Prontera, C.; Mansour, S.A.; Aprano, S.; Maglione, M.; Bruno, A.; Luccio, T.D.; Minarini, C. Optoelectronic properties of OLEDs based on CdSe/ZnS quantum dots and F8BT. Phys. Status Solidi C 2015, 12, 1416–1420. [Google Scholar] [CrossRef]
- Kang, B.-H.; You, T.-Y.; Yeom, S.-H.; Kim, K.-J.; Kim, S.-H.; Lee, S.-W.; Yuan, H.; Kwon, D.-H.; Kang, S.-W. Highly efficient white light-emitting diodes based on quantum dots and polymer interface. IEEE Photon. Technol. Lett. 2012, 24, 1594–1596. [Google Scholar] [CrossRef]
- Ikawa, S.; Yagi, S.; Maeda, T.; Nakazumi, H. White polymer light-emitting diodes co-doped with phosphorescent iridium complexes bearing the same cyclometalated ligand. Phys. Status Solidi C 2012, 9, 2553–2556. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, X.-L.; Bai, Y.; Mi, D.; Ge, Z.; Su, S.-J. Highly-efficient hybrid white organic light-emitting diodes based on a high radiative exciton ratio deep-blue emitter with improved concentration of phosphorescent dopant. RSC Adv. 2015, 5, 32298–32306. [Google Scholar] [CrossRef]
- Derue, L.; Olivier, S.; Tondelier, D.; Maindron, T.; Geffroy, B.; Ishow, E. All-solution-processed organic light-emitting diodes based on photostable photo-cross-linkable fluorescent small molecules. ACS Appl. Mater. Interfaces 2016, 8, 16207–16217. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Cavallari, M.; Santos, G.; Fonseca, F.J.; Andrade, A.; Mergulhão, S. Determination of carrier mobility in MEH-PPV thin-films by stationary and transient current techniques. J. Non-Cryst. Solids. 2012, 358, 484–491. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J. Semiconducting polyfluorenes—Towards reliable structure–property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Bajpai, M.; Srivastava, R.; Kamalasanan, M.; Tiwari, R.; Chand, S. Charge transport and microstructure in PFO: MEH-PPV polymer blend thin films. Synth. Met. 2010, 160, 1740–1744. [Google Scholar] [CrossRef]
- Chen, H.C.; Wang, C.T.; Liu, C.L.; Liu, Y.C.; Chen, W.C. Full color light-emitting electrospun nanofibers prepared from PFO/MEH-PPV/PMMA ternary blends. J. Polym. Sci. B Polym. Phys. 2009, 47, 463–470. [Google Scholar] [CrossRef]
- Buckley, A.; Rahn, M.; Hill, J.; Cabanillas-Gonzalez, J.; Fox, A.; Bradley, D. Energy transfer dynamics in polyfluorene-based polymer blends. Chem. Phys. Lett. 2001, 339, 331–336. [Google Scholar] [CrossRef]
- Grice, A.; Bradley, D.; Bernius, M.; Inbasekaran, M.; Wu, W.; Woo, E. High brightness and efficiency blue light-emitting polymer diodes. Appl. Phys. Lett. 1998, 73, 629–631. [Google Scholar] [CrossRef]
- Sahoo, H. Förster resonance energy transfer–A spectroscopic nanoruler: Principle and applications. J. Photochem. Photobiol. C 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Főrster, T. 10th Spiers Memorial Lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Gupta, V.; Bharti, V.; Kumar, M.; Chand, S.; Heeger, A.J. Polymer–Polymer Förster Resonance Energy Transfer Significantly Boosts the Power Conversion Efficiency of Bulk-Heterojunction Solar Cells. Adv. Mater. 2015, 27, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Salleh, M.M.; AlSalhi, M.S. Inhibition of dark quenching by TiO2 nanoparticles content in novel PFO/fluorol 7GA hybrid: A new role to improve OLED performance. Chem. Phys. Lett. 2013, 570, 109–112. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Haji Jumali, M.H.; AlSalhi, M.S. Enhanced optoelectronic properties of PFO/Fluorol 7GA hybrid light emitting diodes via additions of TiO2 nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Influence of anatase titania nanoparticles content on optical and structural properties of amorphous silica. Mater. Res. Bull. 2017, 89, 286–291. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Energy transfer mechanism and optoelectronic properties of (PFO/TiO2)/Fluorol 7GA nanocomposite thin films. Opt. Mater. 2017, 72, 644–649. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsieh, M.-T.; Chen, J.-F.; Hwang, S.-W.; Chen, C.H. Highly power efficient organic light-emitting diodes with ap-doping layer. Appl. Phys. Lett. 2006, 89, 253504. [Google Scholar] [CrossRef]
- Jumali, M.H.H.; Al-Asbahi, B.A.; Yap, C.C.; Salleh, M.M.; Alsalhi, M.S. Optoelectronic property enhancement of conjugated polymer in poly (9, 9′-di-n-octylfluorenyl-2.7-diyl)/titania nanocomposites. Thin Solid Films 2012, 524, 257–262. [Google Scholar] [CrossRef]
- Zhang, Q.; Chambers, D.K.; Selmic, S. Polymer Light-Emitting Diodes Based on Poly [9, 9-di-(2′-ethylhexyl) fluorenyl-2.7-diyl]. J. Nanoelectron. Optoe. 2006, 1, 219–223. [Google Scholar] [CrossRef]
- Hsieh, S.-N.; Kuo, T.-Y.; Hsu, P.-C.; Wen, T.-C.; Guo, T.-F. Study of polymer blends on polymer light-emitting diodes. Mater. Chem. Phys. 2007, 106, 70–73. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Flaifel, M.H.; Salleh, M.M. Photophysical properties and energy transfer mechanism of PFO/Fluorol 7GA hybrid thin films. J. Lumin. 2013, 142, 57–65. [Google Scholar] [CrossRef]
- De Deus, J.F.; Faria, G.C.; Iamazaki, E.T.; Faria, R.M.; Atvars, T.D.; Akcelrud, L. Polyfluorene based blends for white light emission. Org. Electron. 2011, 12, 1493–1504. [Google Scholar] [CrossRef]
- Voigt, M.; Chappell, J.; Rowson, T.; Cadby, A.; Geoghegan, M.; Jones, R.A.; Lidzey, D.G. The interplay between the optical and electronic properties of light-emitting-diode applicable conjugated polymer blends and their phase-separated morphology. Org. Electron. 2005, 6, 35–45. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Gao, J.; Wang, D.; Yu, G.; Heeger, A.J. Electrochemical properties of luminescent polymers and polymer light-emitting electrochemical cells. Synth. Met. 1999, 99, 243–248. [Google Scholar] [CrossRef]
- Berggren, M.; Inganäs, O.; Gustafsson, G.; Andersson, M.R.; Hjertberg, T.; Wennerström, O. Controlling colour by voltage in polymer light emitting diodes. Synth. Met. 1995, 71, 2185–2186. [Google Scholar] [CrossRef]
- Berggren, M.; Inganäs, O.; Gustafsson, G.; Rasmusson, J.; Andersson, M.R.; Hjertberg, T.; Wennerström, O. Light-emitting diodes with variable colours from polymer blends. Nature 1994, 372, 444–446. [Google Scholar] [CrossRef]

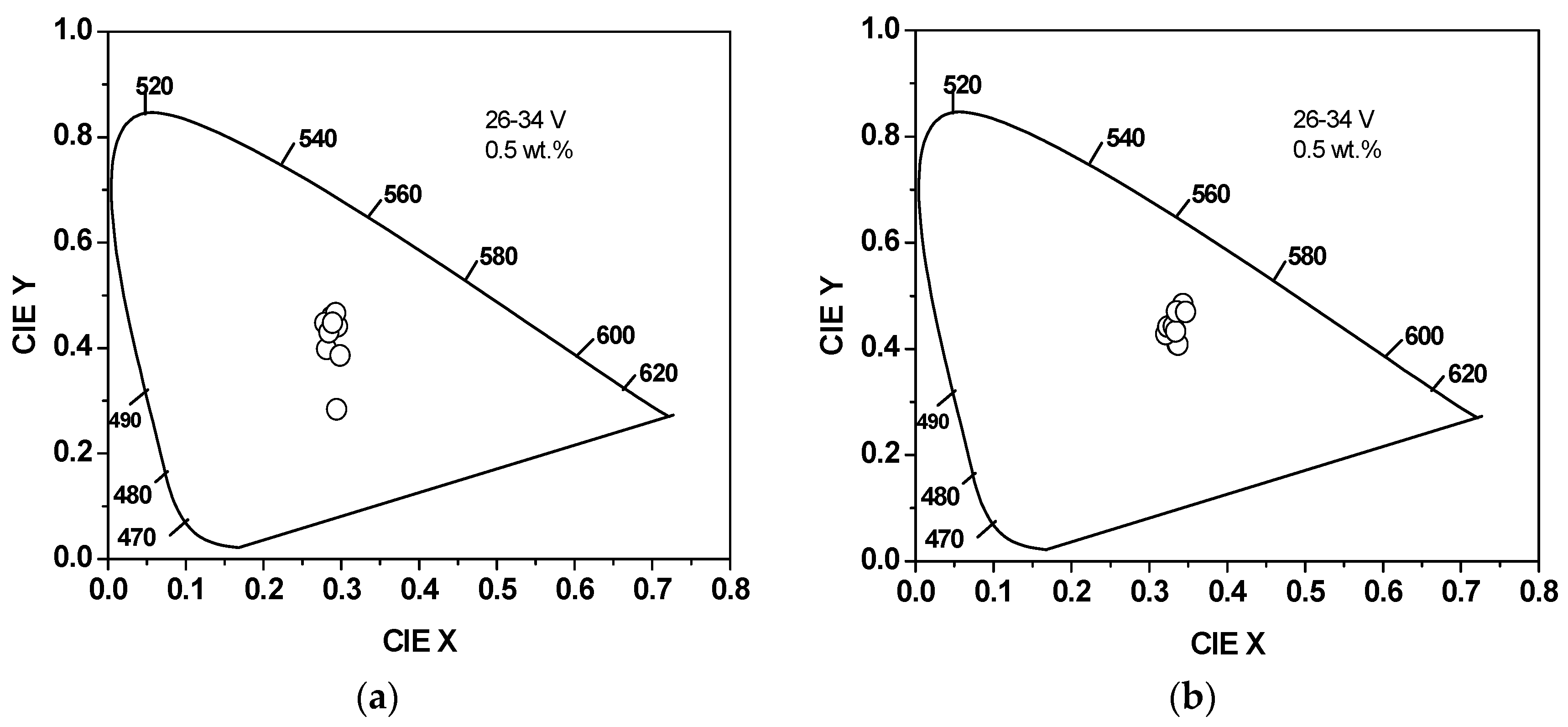
| Acceptor Content in the Blend (wt. %) | In the Presence of SiO2/TiO2 Nanocomposite | In the Absence of SiO2/TiO2 Nanocomposite | ||||
|---|---|---|---|---|---|---|
| CIE Coordinates | V (Volt) | CIE Coordinates | V (Volt) | |||
| X | Y | X | Y | |||
| 0.1 | 0.313 | 0.395 | 31.5 | 0.294 | 0.284 | 36 |
| 0.5 | 0.337 | 0.447 | 30.5 | 0.298 | 0.305 | 34 |
| 1 | 0.333 | 0.413 | 29.5 | 0.285 | 0.37 | 39.5 |
| 5 | 0.318 | 0.279 | 34 | 0.374 | 0.346 | 34.5 |
| 10 | 0.307 | 0.226 | 39.5 | 0.319 | 0.243 | 38 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asbahi, B.A. Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices. Polymers 2018, 10, 800. https://doi.org/10.3390/polym10070800
Al-Asbahi BA. Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices. Polymers. 2018; 10(7):800. https://doi.org/10.3390/polym10070800
Chicago/Turabian StyleAl-Asbahi, Bandar Ali. 2018. "Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices" Polymers 10, no. 7: 800. https://doi.org/10.3390/polym10070800
APA StyleAl-Asbahi, B. A. (2018). Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices. Polymers, 10(7), 800. https://doi.org/10.3390/polym10070800






