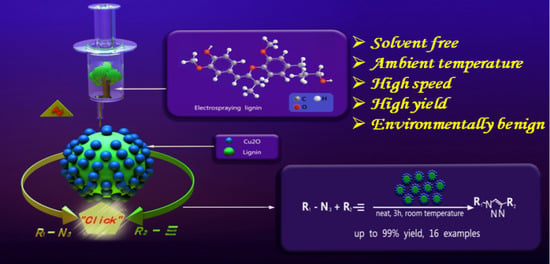
Lignin Nanosphere-Supported Cuprous Oxide as an Efficient Catalyst for Huisgen [3+2] Cycloadditions under Relatively Mild Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospraying Lignin Nanopheres
2.2. Preparation of Cu2O@L
2.3. Catalytic Performance Test
2.4. Analyses
3. Results
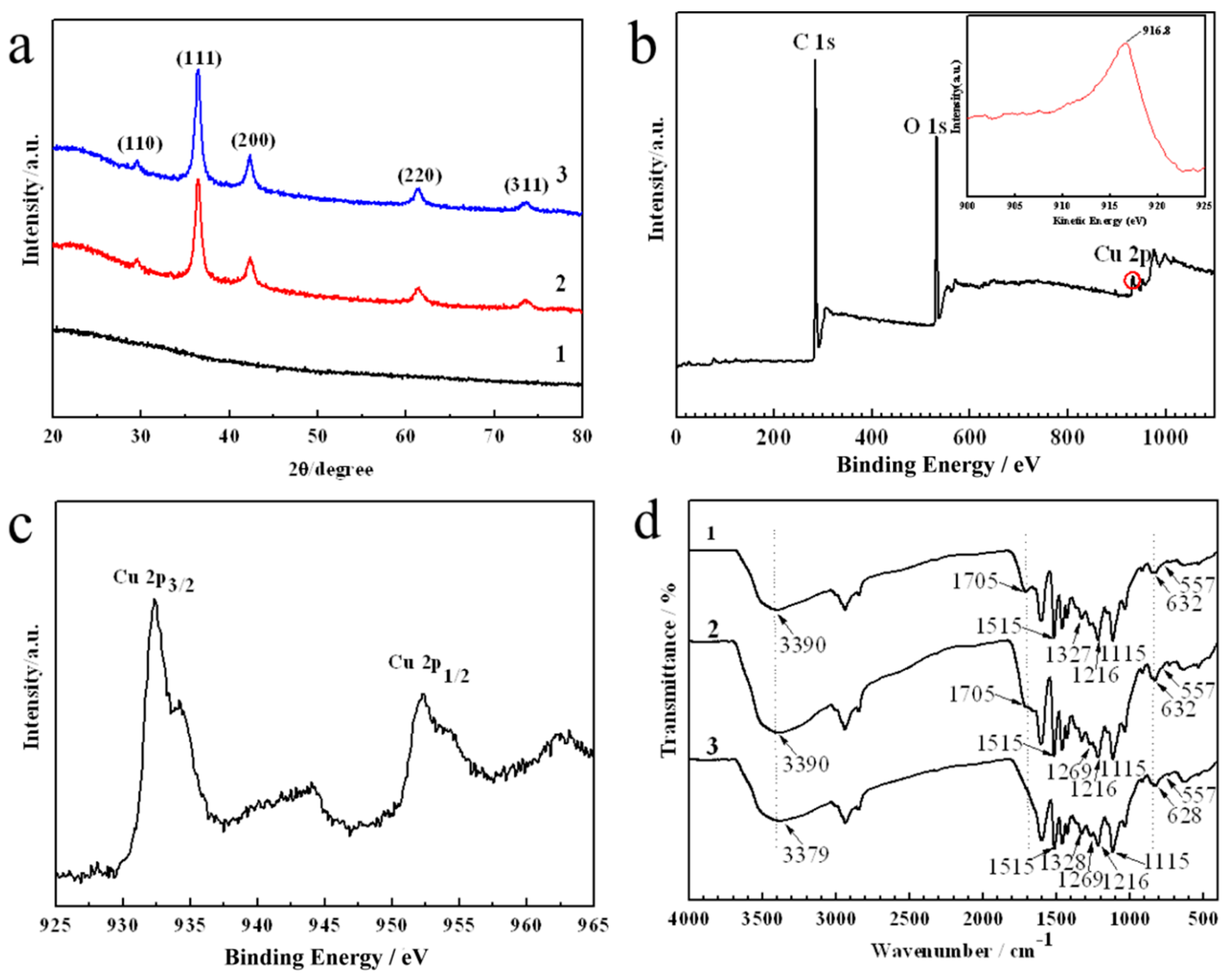
3.1. Characterization of the Electrosprayed Lignin Nanosphere and Cu2O@L
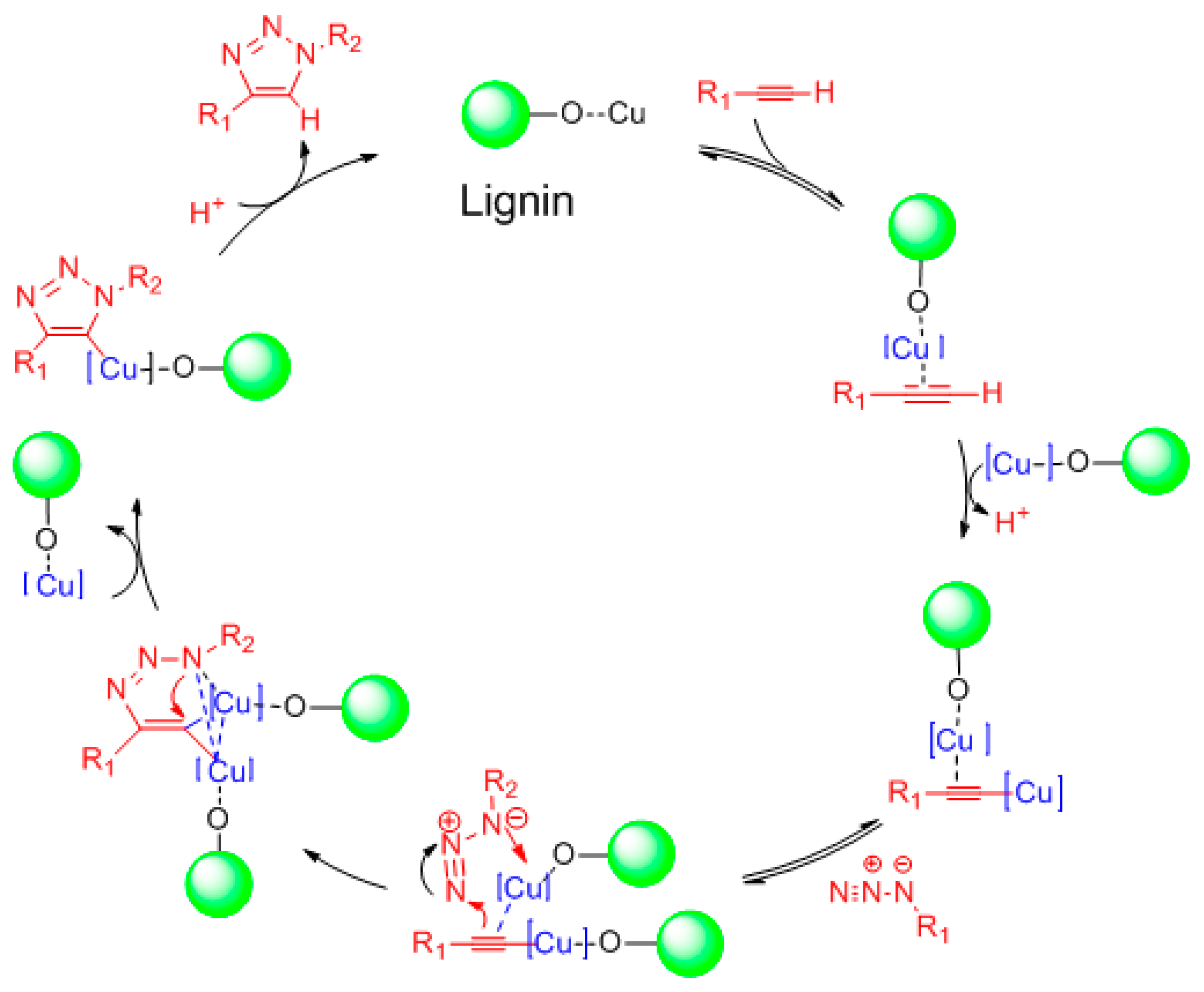
3.2. Catalytic Performance of Lignin Nanosphere-Supported Catalyst in the Huisgen “Click” Reaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray powder diffraction |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| AFM | Atomic force microscope |
| LSCM | Laser scanning confocal microscope |
| EDS | Energy dispersive analysis system of X-ray |
| TGA | Thermogravimetric analysis |
| FT-NMR | Fourier transform nuclear magnetic resonance |
| UV–vis–NIR | UV-visible-near infrared spectroscopy |
| FTIR | Flourier transformation infrared spectroscopy |
| AAS | Atomic absorption spectrophotometry |
| Cu2O@L | the lignin nanosphere-supported cuprous oxide |
| DMF | N,N-dimethylformamide |
| ID | inner diameter |
| FL | Fluorescence spectra |
| PMT | Photomultiplier tube |
| MW | weight-average molecular weight |
| TMS | tetramethylsilane |
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kumar, A.; Aerry, S.; Saxena, A.; Arnab, D.; Mozumdar, S. Copper nanoparticulates in Guar-gum: A recyclable catalytic system for the Huisgen [3+2]-cycloaddition of azides and alkynes without additives under ambient conditions. Green Chem. 2012, 14, 1298–1301. [Google Scholar] [CrossRef]
- Yao, B.; Hu, T.; Zhang, H.; Li, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. Multi-Functional Hyperbranched Poly(vinylene sulfide)s Constructed via Spontaneous Thiol–Yne Click Polymerization. Macromolecules 2015, 48, 7782–7791. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Ranganath, K.V.; Mahendar, K.; Sreedhar, B. Bifunctional Nanocrystalline MgO for Chiral Epoxy Ketones via Claisen−Schmidt Condensation−Asymmetric Epoxidation Reactions. J. Am. Chem. Soc. 2004, 126, 3396–3397. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Moglie, Y.; Radivoy, G. Copper Nanoparticles in Click Chemistry. Chem. Res. 2015, 48, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yu, S.; Shahzad, F.; Hong, J.P.; Kim, W.N.; Park, C.; Soon, M.H.; Koo, C.M. Highly anisotropic Cu oblate ellipsoids incorporated polymer composites with excellent performance for broadband electromagnetic interference shielding. Compos. Sci. Technol. 2017, 144, 57–62. [Google Scholar] [CrossRef]
- Jumde, R.P.; Evangelisti, C.; Mandoli, A.; Scotti, N.; Psaro, R. Aminopropyl-silica-supported Cu nanoparticles: An efficient catalyst for continuous-flow Huisgen azide-alkyne cycloaddition (CuAAC). J. Catal. 2015, 324, 25–31. [Google Scholar] [CrossRef]
- Gu, L.; Ma, D.; Yao, S.; Wang, C.; Shen, W.; Bao, X. Structured zeolitescatalysts with hierarchical channel structure. Chem. Commun. 2010, 46, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, W.; Li, J.; Zhou, X.; Qu, Y. Interfacial Effects of the CuO/GO Composite to Mediate the Side Reactions of N,N-Dimethylformamide Fragments. ACS Appl. Mater. Interfaces 2014, 6, 22174–22182. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, B.; Amali, A.J.; Pitchumani, K. Fabrication of Pd Nanoparticles Embedded C@Fe3O4 Core–Shell Hybrid Nanospheres: An Efficient Catalyst for Cyanation in Aryl Halides. ACS Appl. Mater. 2015, 7, 22907–22917. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, N.; Jones, C.W.; Weck, M. Rational Approach to Polymer-Supported Catalysts: Synergy between Catalytic Reaction Mechanism and Polymer Design. Acc. Chem. Res. 2008, 41, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Peng, X.; Zhong, L.; Wu, L.; Cao, X.; Sun, R.C. Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr. Polym. 2016, 136, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, L.; Contreras-Caceres, R.; Schellkopf, L.; Jehnichen, D.; Fischer, D.; Cai, C.; Stamm, M. Controlled growth of Ag nanoparticles decorated onto the surface of SiO2 spheres: A nanohybrid system with combined SERS and catalytic properties. RSC Adv. 2014, 4, 17846–17855. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Tzounis, L.; Logothetidis, S. Fe3O4@SiO2 core shell particles as platforms for the decoration of Ag nanoparticles. Mater. Today Proc. 2017, 4, 7076–7082. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A.J. Sorbent-Enhanced Methane Reforming over a Ni–Ca-Based, Bifunctional Catalyst Sorbent. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. J. Hydrog. Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrog. Energy 2018. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. Corrigendum to “A new method for preparing tannin-based foams”. Ind. Crops Prod. 2015, 67, 510. [Google Scholar] [CrossRef]
- Da Silva Souza, D.R.; de Mesquita, J.P.; Lago, R.M.; Caminhas, L.D.; Pereira, F.V. Cellulose nanocrystals: A versatile precursor for the preparation of different carbon structures and luminescent carbon dots. Ind. Crops Prod. 2016, 93, 121–128. [Google Scholar] [CrossRef]
- Si, H.; Lian, G.; Wang, J.; Li, L.; Wang, Q.; Cui, D.; Wong, C.P. Synthesis of Few-Atomic-Layer BN Hollow Nanospheres and Their Applications as Nanocontainers and Catalyst Support Materials. ACS Appl. Mater. Interfaces 2016, 8, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Carrott, P.J.M.; Carrott, M.R. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar]
- Wang, S.X.; Yang, L.; Stubbs, L.P.; Li, X.; He, C. Lignin-Derived Fused Electrospun Carbon Fibrous Mats as High Performance Anode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Graglia, M.; Pampel, J.; Hantke, T.; Fellinger, T.P.; Esposito, D. Nitro Lignin-Derived Nitrogen-Doped Carbon as an Efficient and Sustainable Electrocatalyst for Oxygen Reduction. ACS Nano 2016, 10, 4364–4371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Zeng, W.; Li, Y.; Xu, L.; Qiu, X.; Chen, R.F.; Huang, W. Improving Efficiency of Blue Organic Light-Emitting Diode with Sulfobutylated Lignin Doped PEDOT as Anode Buffer Layer. ACS Sustain. Chem. Eng. 2016, 4, 2004–2011. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials. Green Mater. 2013, 1, 137–160. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Jørgen, H.C.; Boerjan, W. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Jiang, S.; Low, Z.W.; Loh, X.J. Engineering highly stretchable lignin-based electrospun nanofibers for potential biomedical applications. J. Mater. Chem. B 2015, 3, 6194–6204. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X.Q. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.G.; Luo, Y.Q.; Wang, Q.; Wang, X.Y.; Sun, R.C. High-Value Utilization of Lignin to Synthesize Ag Nanoparticles with Detection Capacity For Hg2+. ACS Appl. Mater. Interfaces 2014, 6, 16147–16155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, K.; Sun, J.; Li, W.; Zhao, S.; Chen, Z.; Guan, J. Ultralow content silver densely-coated glass microsphere for high performance conducting polymer-matrix composites. Compos. Sci. Technol. 2017, 140, 89–98. [Google Scholar]
- Lu, C.; Qi, L.; Yang, J.; Wang, X.; Zhang, D.; Xie, J.; Ma, J. One-Pot Synthesis of Octahedral Cu2O Nanocages via a Catalytic Solution Route. Adv. Mater. 2005, 17, 2562–2567. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, X.; Wu, Y.; Qian, Y.; Zhou, M.; Deng, Y.; Li, Y. Aggregation-induced emission: The origin of lignin fluorescence. Polym. Chem. 2016, 7, 3502–3508. [Google Scholar] [CrossRef]
- Hong, N.; Xiao, J.; Li, Y.; Li, Y.; Wu, Y.; Yu, W.; Cao, Y. Unconventional fluorescence emission of non-conjugated lignin-SAF (GSL) was reported and GSL-doped PEDOT showed high performance in organic electronics. J. Mater. Chem. C 2016, 4, 5297–5306. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-Scale Synthesis of Cu2O Nanocubes and Subsequent Oxidation to CuO Hollow Nanostructures for Lithium-Ion Battery Anode Materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, L.; Jiang, K.; Xu, D. Well-Defined Non-spherical Copper Sulfide Mesocages with Single-Crystalline Shells by Shape-Controlled Cu2O Crystal Templating. Adv. Mater. 2006, 18, 1174–1177. [Google Scholar] [CrossRef]
- Yang, W.Y.; Rhee, S.W. Effect of electrode material on the resistance switching of Cu2O film. Appl. Phys. Lett. 2007, 91, 232907. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.F.; Sun, R.C. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Luo, R.; Zhou, X.; Fang, Y.; Ji, H. Metal-and solvent-free synthesis of cyclic carbonates from epoxides and CO2 in the presence of graphite oxide and ionic liquid under mild conditions: A kinetic study. Carbon 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Taft, B.R. Heterogeneous Copper-in-Charcoal-Catalyzed Click Chemistry. Angew. Chem. Int. Ed. 2006, 118, 8415–8418. [Google Scholar] [CrossRef]
- Chassaing, S.; Kumarraja, M.; Sani Souna Sido, A.; Pale, P.; Sommer, J. Click Chemistry in CuI-zeolites: The Huisgen [3+2]-Cycloaddition. Org. Lett. 2007, 9, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, C.; Srivastava, A.K.; Gupta, P.; Boukherroub, R.; Jain, S.L. Visible Light Assisted Photocatalytic [3+2] Azide–Alkyne “Click” Reaction for the Synthesis of 1,4-Substituted 1,2,3-Triazoles Using a Novel Bimetallic Ru–Mn Complex. ACS Sustain. Chem. Eng. 2015, 4, 69–75. [Google Scholar] [CrossRef]
- Deraedt, C.; Pinaud, N.; Astruc, D. Recyclable Catalytic Dendrimer Nanoreactor for Part-Per-Million CuI Catalysis of “Click” Chemistry in Water. J. Am. Chem. Soc. 2014, 136, 12092–12098. [Google Scholar] [PubMed]
- Monguchi, Y.; Nozaki, K.; Maejima, T.; Shimoda, Y.; Sawama, Y.; Kitamura, Y.; Kitade, Y.; Sajiki, H. Solvent-free Huisgen cyclization using heterogeneous copper catalysts supported on chelate resins. Green Chem. 2013, 15, 490–495. [Google Scholar]
- Rodríguez-Rodríguez, M.; Gras, E.; Pericàs, M.A.; Gómez, M. Metal-Free Intermolecular Azide–Alkyne Cycloaddition Promoted by Glycerol. Chemistry 2015, 21, 18706–18710. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, C.; Cao, L.; Zhang, D.; Liu, S.; Zhang, Q. Solvent-free 1,3-dipolar cycloaddition of azomethine imines with terminal alkynes promoted by calcium fluoride under the ball milling condition. Chem. Res. Chin. Univ. 2015, 31, 543–548. [Google Scholar] [CrossRef]
- Wang, B.; Durantini, J.; Nie, J.; Lanterna, A.E.; Scaiano, J.C. Heterogeneous Photocatalytic Click Chemistry. J. Am. Chem. Soc. 2016, 138, 13127–13130. [Google Scholar] [CrossRef] [PubMed]
- Chanda, K.; Rej, S.; Huang, M.H. Facet-Dependent Catalytic Activity of Cu. Chemistry 2013, 19, 16036–16043. [Google Scholar] [CrossRef] [PubMed]



| Reaction | Alkyl Halide | Alkyne | Product a,b | Yield (%) c |
|---|---|---|---|---|
| 1 |  |  |  | 99 |
| 2 |  |  |  | 98 |
| 3 |  |  |  | 98 |
| 4 |  |  |  | 98 |
| 5 |  |  |  | 99 |
| 6 |  |  |  | 98 |
| 7 |  |  |  | 97 |
| 8 |  |  |  | 96 |
| 9 |  |  |  | 99 |
| 10 |  |  |  | 97 |
| 11 |  |  |  | 98 |
| 12 |  |  |  | 98 |
| 13 |  |  |  | 99 |
| 14 |  |  |  | 96 |
| 15 |  |  |  | 98 |
| 16 |  |  |  | 99 |
| Entry | Catalyst | mol % a | Solvent | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Copper in charcoal | 5 | dioxane | 25 | 10 | 84 | [47] |
| 2 | Copper in zeolite | 10 | toluene | 25 | 15 | 94 | [48] |
| 3 | CuSO4·5H2O | 1 | H2O/t-BuOH | 90 | 8 | 89 | [49] |
| 4 | Photocatalytic-Cu(II) | 5 | ethanol | 25 | 5 | 96 | [50] |
| 5 | Dendrimer-CuI | 0.5 | H2O | 25 | 3 | 98 | [51] |
| 6 | Chitosan-CuSO4 | 0.4 | H2O | 25 | 4–6 | 99 | [8] |
| 7 | Cu on chelated resins | 1 | solvent-free | 50/80 | 24/4 | 100 | [52] |
| 8 | CuCl/microwaves | 2.5 | glycerol | 100 | 0.5 | 82 | [53] |
| 9 | CuOAc/ball-milling | 10 | solvent-free | - | 1 | 90 | [54] |
| 10 | CuO@Nb2O5/UV | 1–1.2 | THF | 25 | 6 | 99 | [55] |
| 11 | Cu2O Nanocrystals | 6.25 | EtOH | 55 | 1 | 96 | [56] |
| 12 | Cu2O@L | 1.2 | solvent-free | 25 | 3 | 99 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Peng, X.; Zhong, L.; Li, X.; Sun, R. Lignin Nanosphere-Supported Cuprous Oxide as an Efficient Catalyst for Huisgen [3+2] Cycloadditions under Relatively Mild Conditions. Polymers 2018, 10, 724. https://doi.org/10.3390/polym10070724
Zhou Z, Peng X, Zhong L, Li X, Sun R. Lignin Nanosphere-Supported Cuprous Oxide as an Efficient Catalyst for Huisgen [3+2] Cycloadditions under Relatively Mild Conditions. Polymers. 2018; 10(7):724. https://doi.org/10.3390/polym10070724
Chicago/Turabian StyleZhou, Zidan, Xinwen Peng, Linxin Zhong, Xuehui Li, and Runcang Sun. 2018. "Lignin Nanosphere-Supported Cuprous Oxide as an Efficient Catalyst for Huisgen [3+2] Cycloadditions under Relatively Mild Conditions" Polymers 10, no. 7: 724. https://doi.org/10.3390/polym10070724
APA StyleZhou, Z., Peng, X., Zhong, L., Li, X., & Sun, R. (2018). Lignin Nanosphere-Supported Cuprous Oxide as an Efficient Catalyst for Huisgen [3+2] Cycloadditions under Relatively Mild Conditions. Polymers, 10(7), 724. https://doi.org/10.3390/polym10070724