Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Polysaccharides and Reagents
2.2. Catalyst Preparation
2.3. Solubility
2.4. Reaction Procedure
2.5. Leaching Analysis
2.6. Catalyst Recycling
2.7. FTIR Analysis
2.8. Energy Dispersive X-Ray Spectrometry (EDS)
2.9. Surface Analysis by X-ray Photoelectron Spectroscopy (XPS)
2.10. SEM Analysis
2.11. High-Resolution Transmission Electron Microscopic (HRTEM) Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blaser, H.U.; Studer, M. The Role of catalysis for the clean production of fine chemicals. Appl. Catal. 1999, 189, 191–204. [Google Scholar] [CrossRef]
- Pignolet, L.H. Homogeneous Catalysis with Metal Phosphine Complexes; Plenume Press: New York, NY, USA, 1983; ISBN 9781461336235. [Google Scholar]
- Beller, M.; Bolm, C. Transition Metals for Organic Synthesis; John Wiley & Sons: New York, NY, USA, 1998; ISBN 9783527619399. [Google Scholar]
- Hegedus, L.S. Transition Metals in the Synthesis of Complex Organic Molecules; University Science Books: Sausalito, CA, USA, 1999; ISBN 1891389599. [Google Scholar]
- Pierluigi, B.; Liguori, F. Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Springer Science & Business Media: Heidelberg, Germany, 2010; ISBN 9789048136957. [Google Scholar]
- Giovanna, G.D.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; William Andrew Books: Norwich, CT, USA, 2015; ISBN 9780323353991. [Google Scholar]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780849317415. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper No. 441; FAO: Rome, Italy, 2003. [Google Scholar]
- Huang, K.; Xue, L.; Hu, Y.C.; Huang, M.Y.; Jiang, Y.Y. Catalytic behaviors of silica-supported starch–polysulfosiloxane–Pt complexes in asymmetric hydrogenation of 4-methyl-2-pentanone. React. Funct. Polym. 2002, 50, 199–220. [Google Scholar] [CrossRef]
- Cirtiu, C.M.; Dunlop-Brie`re, A.F.; Moores, A. Cellulose nanocrystallites as an efficient support for nanoparticles of palladium: Application for catalytic hydrogenation and Heck coupling under mild conditions. Green Chem. 2011, 13, 288–291. [Google Scholar] [CrossRef]
- Zhou, P.P.; Wang, H.H.; Yang, J.Z.; Tang, J.; Sun, D.P.; Tang, W.H. Bacteria cellulose nanofibers supported palladium (0) nanocomposite and its catalysis evaluation in Heck reaction. Ind. Eng. Chem. Res. 2012, 51, 5743–5748. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L.; Peng, X.; Lin, J.; Sun, R. Xylan-type hemicelluloses supported terpyridine–palladium(II), complex as an efficient and recyclable catalyst for Suzuki–Miyaura reaction. Cellulose 2014, 21, 125–137. [Google Scholar] [CrossRef]
- Wu, C.Y.; Peng, X.W.; Zhong, L.X.; Li, X.H.; Sun, R.C. Green synthesis of palladium nanoparticles via branched polymers: A bio-based nanocomposite for C–C coupling reactions. RSC Adv. 2016, 6, 32202–32211. [Google Scholar] [CrossRef]
- Vyver, S.V.; Geboers, S.V.J.; Sels, B.F. Recent advances in the catalytic conversion of cellulose. ChemCatChem 2011, 3, 82–94. [Google Scholar] [CrossRef]
- Hardy, J.J.E.; Hubert, S.; Macquarrie, D.J.; Wilson, A.J. Chitosan-based heterogeneous catalysts for Suzuki andHeck reactions. Green Chem. 2004, 6, 53–56. [Google Scholar] [CrossRef]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized chitosan as agreen, recyclable, biopolymer-supported catalyst for the [3+2] Huisgen cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 5916–5920. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide–alkyne cycloadditionreactions in water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- Kumar, A.; Aerry, S.; Saxena, A.; Mozumdar, S. Copper nanoparticulates in Guar-gum: A recyclable catalytic system for the Huisgen [3+2]-cycloaddition of azides and alkynes without additives under ambient conditions. Green Chem. 2012, 14, 1298–1301. [Google Scholar] [CrossRef]
- Keshavarzipour, F.; Tavakol, H. Zinc cation supported on carrageenan magnetic nanoparticles: A novel, green, and efficient catalytic system for one-pot three-component synthesis of quinolone. Appl. Organomet. Chem. 2017, 31, e3682. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Baghban, A.; Zeynizadeh, B. Seaweed-derived κ-carrageenan: Modified κ-carrageenan as a recyclable green catalyst in the multicomponent synthesis of aminophosphonates and polyhydroquinolines. J. Appl. Polym. Sci. 2016, 133, 43190. [Google Scholar] [CrossRef]
- Quignard, F.; Di Renzo, F.; Guibal, E. From natural polysaccharides to materials for catalysis, adsorption, and remediation. Top. Curr. Chem. 2010, 294, 165–197. [Google Scholar] [PubMed]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; d’Alessandro, N. One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidationand reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Wu, Z.L.; Xie, H.B.; Yu, X.; Liu, E.H. Lignin-based green catalyst for the chemical fixation of carbon dioxide with epoxides to form cyclic carbonates under solvent-free conditions. ChemCatChem 2013, 5, 1328–1333. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Meier, H.; Kolshorn, H.; Elnagdi, M.H. Chitosan as heterogeneous catalyst in Michael additions: The reaction of cinnamonitriles with active methylene moieties and phenols. Arkivoc 2008, 16, 288–301. [Google Scholar]
- Vincent, T.; Guibal, E. Chitosan-Supported Palladium Catalyst. 3. Influence of experimental parameters on nitrophenol degradation. Langmuir 2003, 19, 8475–8483. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J.; White, R.J. Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C-C coupling reactions. Green Chem. 2008, 10, 382–387. [Google Scholar] [CrossRef]
- Primo, A.; Liebel, M.; Quignard, F. Palladium coordination biopolymer: A versatile access to highly porous dispersed catalyst for Suzuki reaction. Chem. Mater. 2009, 21, 621–627. [Google Scholar] [CrossRef]
- Xu, X.; Liu, P.; Li, S.H.; Zhang, P.; Wang, X.Y. Chitosan-supported imine palladacycle complex and its catalytic performance for heck reaction. React. Kinet. Catal. Lett. 2006, 88, 217–223. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. The use of polysaccharides and derivatives in palladium-catalyzed coupling reactions. Catal. Sci. Technol. 2014, 4, 295–310. [Google Scholar] [CrossRef]
- Suzuki, A.J. Cross-coupling reactions via organoboranes. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- De Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 2004; ISBN 9783527305186. [Google Scholar]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5052–5085. [Google Scholar] [CrossRef] [PubMed]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Whistler, R.L. Solubility of polysaccharides and their behavior in solution. In Carbohydrate in Solution; Isbell, H.S., Ed.; American Chemical Society Press: Washington, DC, USA, 1973; pp. 242–255. ISBN 9780841201781. [Google Scholar]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Lianzhong, A. Solubility of Polysaccharides. In Polysaccharides: Structure and Solubility; Xu, Z., Ed.; InTech: Winchester, UK, 2017; pp. 7–21. ISBN 9789535136507. [Google Scholar]
- Arad, S.; Levy-Ontman, O. Red microalgal cell wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotech. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Dubinsky, O.; Arad, M.S.; Christiaen, D.; Glaser, R. Structure of 3-O-(alpha-d-glucopyranosyluronic acid)-l-galactopyranose, an aldobiouronic acid isolated from the polysaccharides of various unicellular red algae. Carbohydr. Res. 1990, 208, 301–305. [Google Scholar] [CrossRef]
- Sabu, T.; Dominique, D.; Christophe, C.; Jyotishkumar, P. Sulphated polysaccharides in the cell wall of red microalgae. In Handbook of Biopolymer-Based Materials: From Blends and Composites to Gels and Complex Networks; John Wiley & Sons Ltd: Hamburg, Germany, 2013; pp. 351–370. ISBN 9783527328840. [Google Scholar]
- Bullock, K.M.; Mitchell, M.B.; Toczko, J.F. Optimization and and scale-up of a Suzuki-Miyaura coupling reaction: Development of an efficient palladium removal technique. Org. Process. Res. Dev. 2008, 12, 896–899. [Google Scholar] [CrossRef]
- Butters, M.; Harvey, J.N.; Jover, J.; Lennox, A.J.J.; Lloyd-Jones, G.C.; Murray, P.M. Aryl trifluoroborates in Suzuki–Miyaura coupling: The roles of endogenous Aryl Boronic acid and Fluoride. Angew. Chem. Int. Ed. 2010, 49, 5156–5160. [Google Scholar] [CrossRef] [PubMed]
- Dallas, A.S.; Gothelf, K.V. Effect of water on the palladium-catalyzed amidation of Aryl Bromides. J. Org. Chem. 2005, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Tokunaga, M.; Obora, Y.; Tsujii, Y. Homogeneous palladium catalyst suppressing Pd black formation in air oxidation of alcohols. J. Am. Chem. Soc. 2004, 126, 6554–6555. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.; Dlugy, C. Palladium catalyzed heck and Suzuki coupling in glycerol. Chem. Pap. 2007, 61, 228–232. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Bettigeri, S.V.; Phopase, J. Palladium catalyzed ligand-free Suzuki cross-coupling reactions of benzylic halides with aryl boronic acids under mild conditions. Tetrahedron Lett. 2004, 45, 6959–6962. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchly, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Indicative evidence for a conformational transition in ι-carrageenan. Carbohydr. Res. 1987, 161, 247–255. [Google Scholar] [CrossRef]
- Janaswamy, S.; Chandrasekaran, R. Effect of calcium ions on the organization of iota-carrageenan helices: An X-ray investigation. Carbohydr. Res. 2002, 337, 523–535. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: Various types of counter ions. Food Hydrocoll. 2003, 17, 481–485. [Google Scholar] [CrossRef]
- Pekcan, S.; Kara, J. Biomater. Cation effect on thermal transition of ι-carrageenan: A photon transmission study. Sci. Polym. Ed. 2005, 16, 317–333. [Google Scholar]
- Nazarudin, M.F.; Shamsurf, A.A.; Shamsudin, M.N. Preparation and physicochemical evaluation of biodegradable magnetic k-carrageenan beads and application for chromium ions pre-concentration. J. Chil. Chem. Soc. 2011, 56, 891–894. [Google Scholar] [CrossRef]
- Ferguson, G.; McCrindle, R.; McAlees, A.J.; Parvez, M. Trans Dichlorobis(triphenylphosphine)palladium(II). Acta Cryst. 1982, B38, 2679–2681. [Google Scholar] [CrossRef]
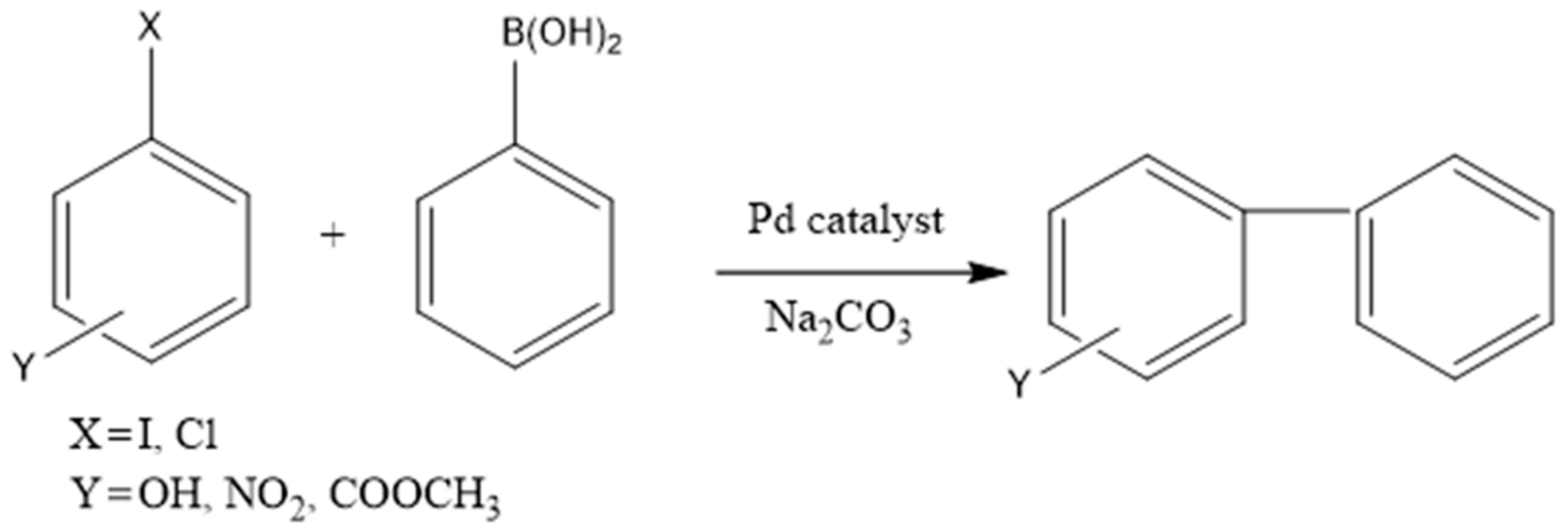
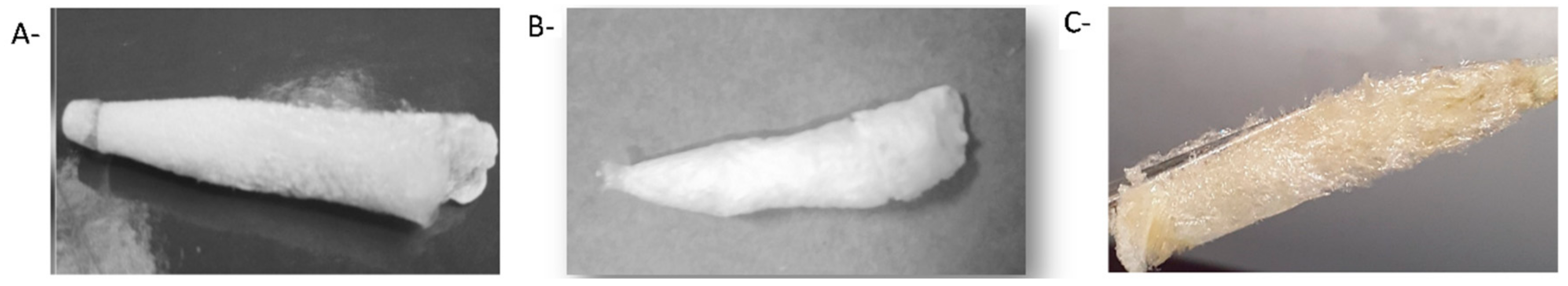

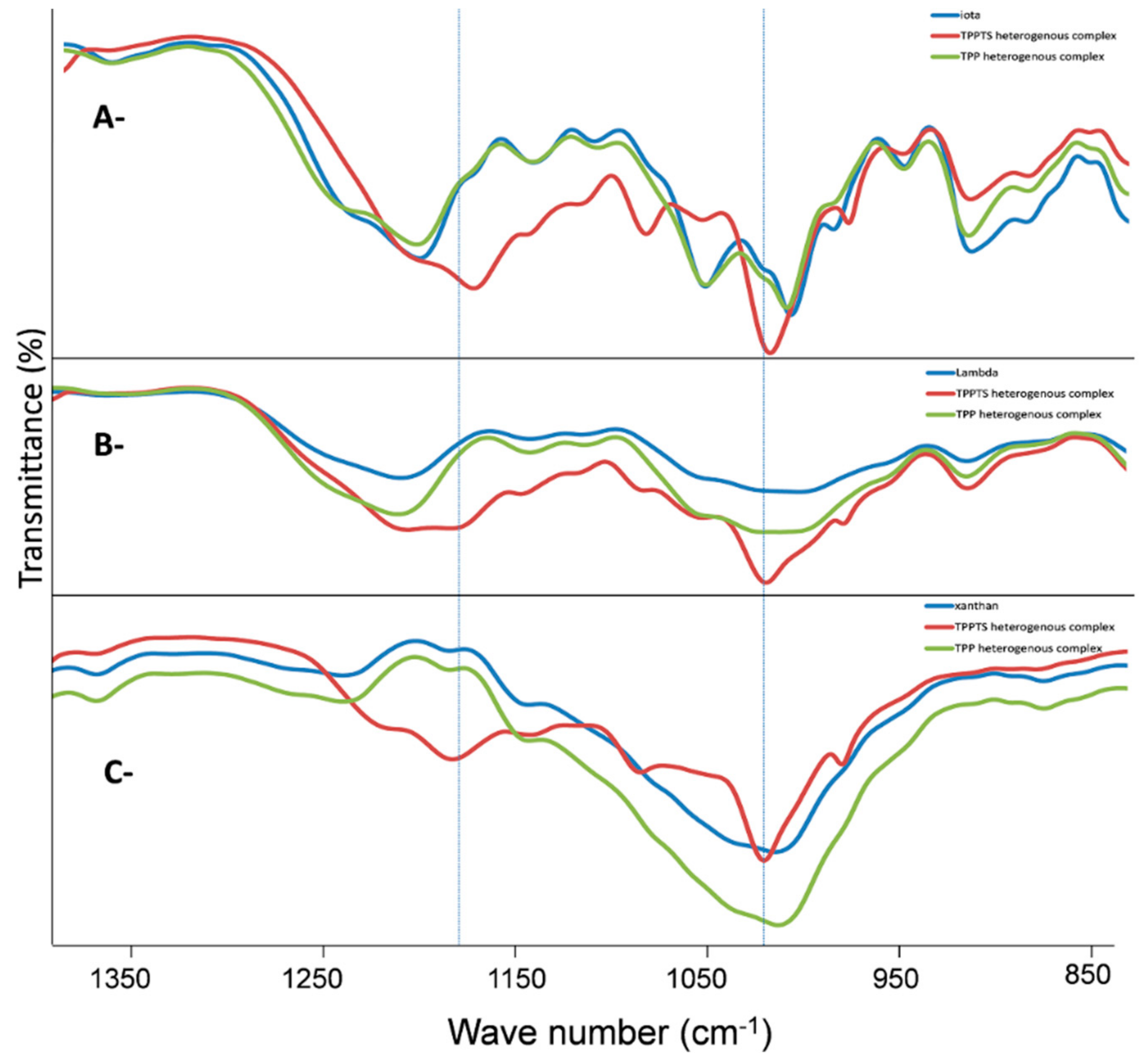
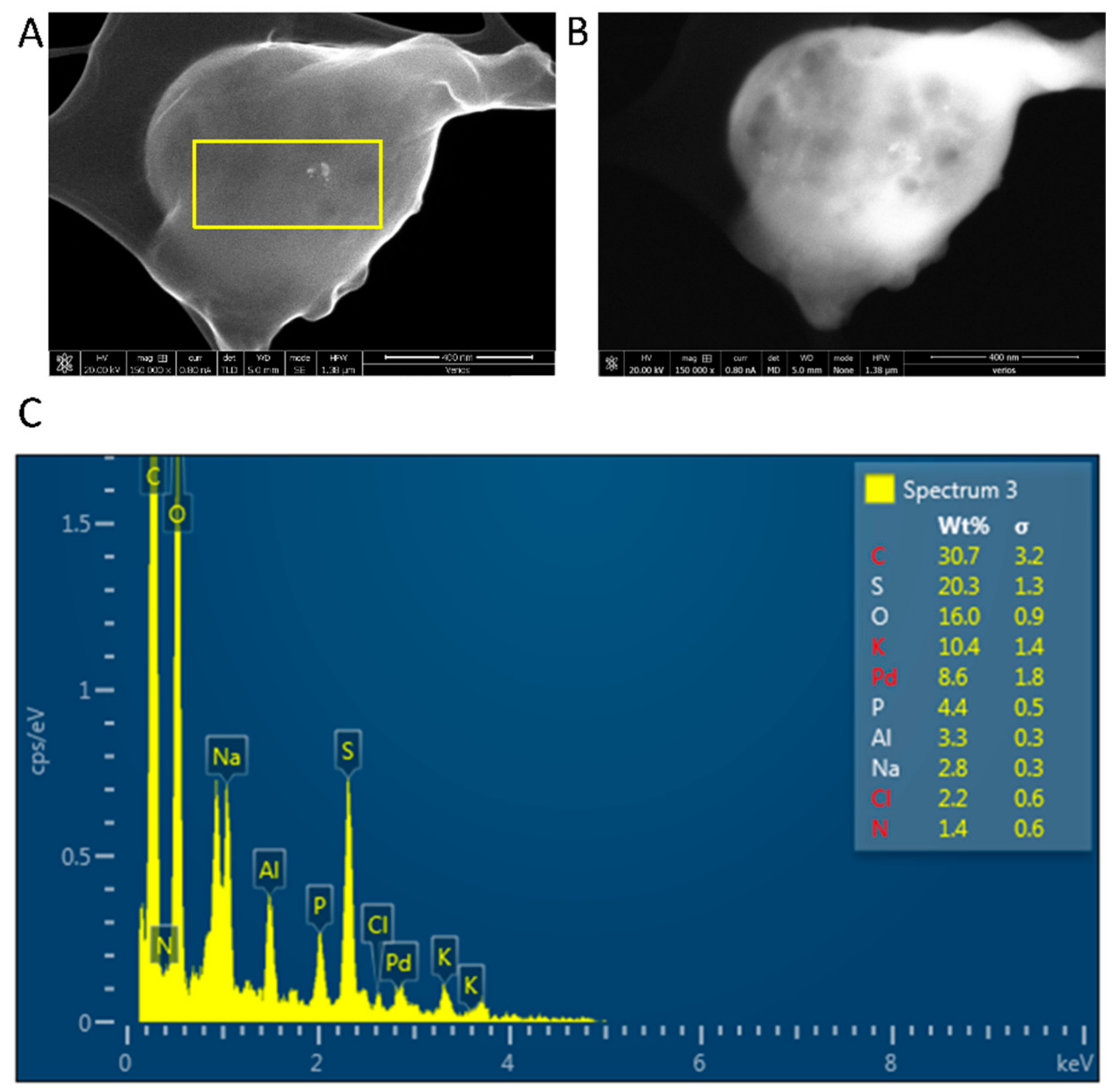
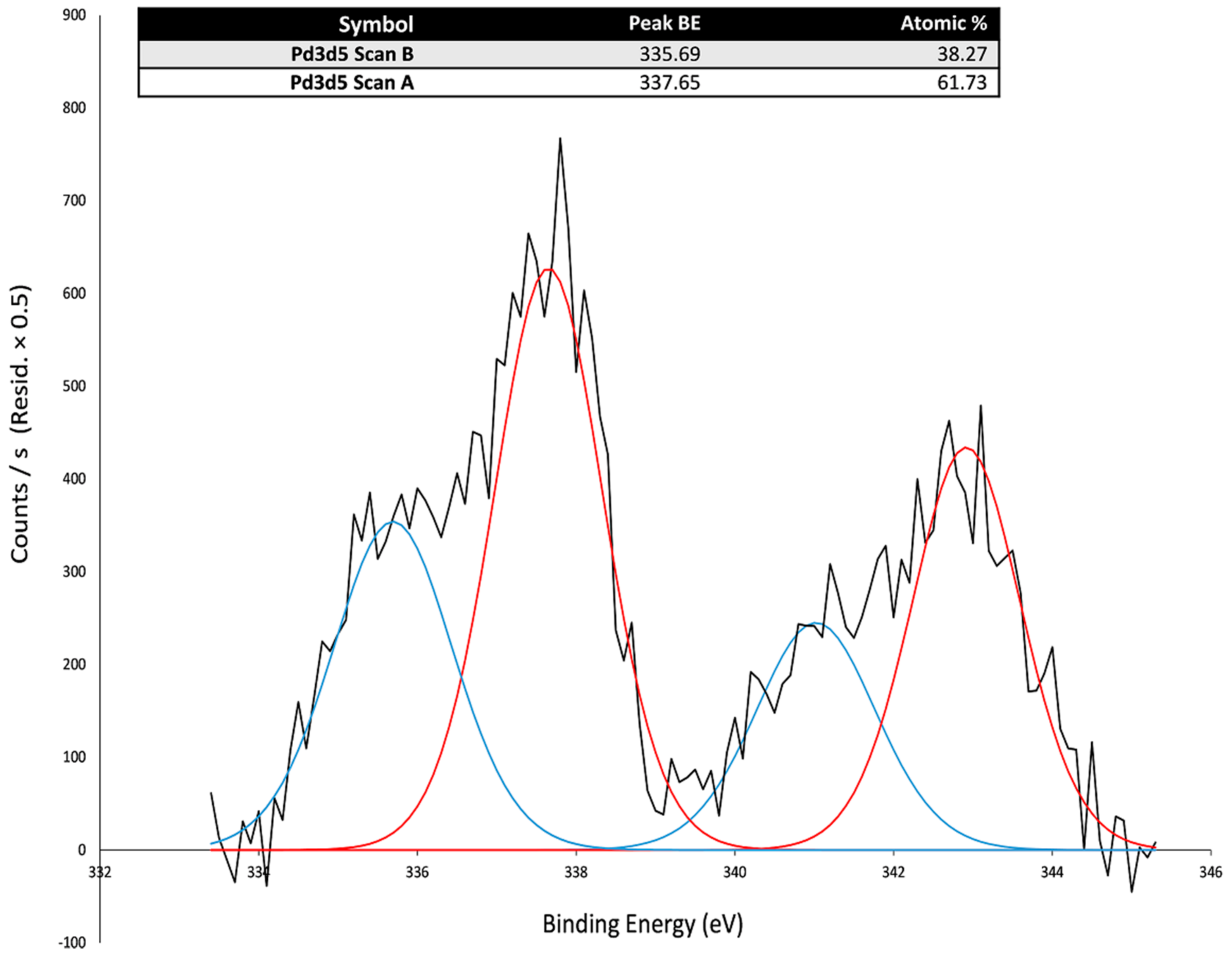
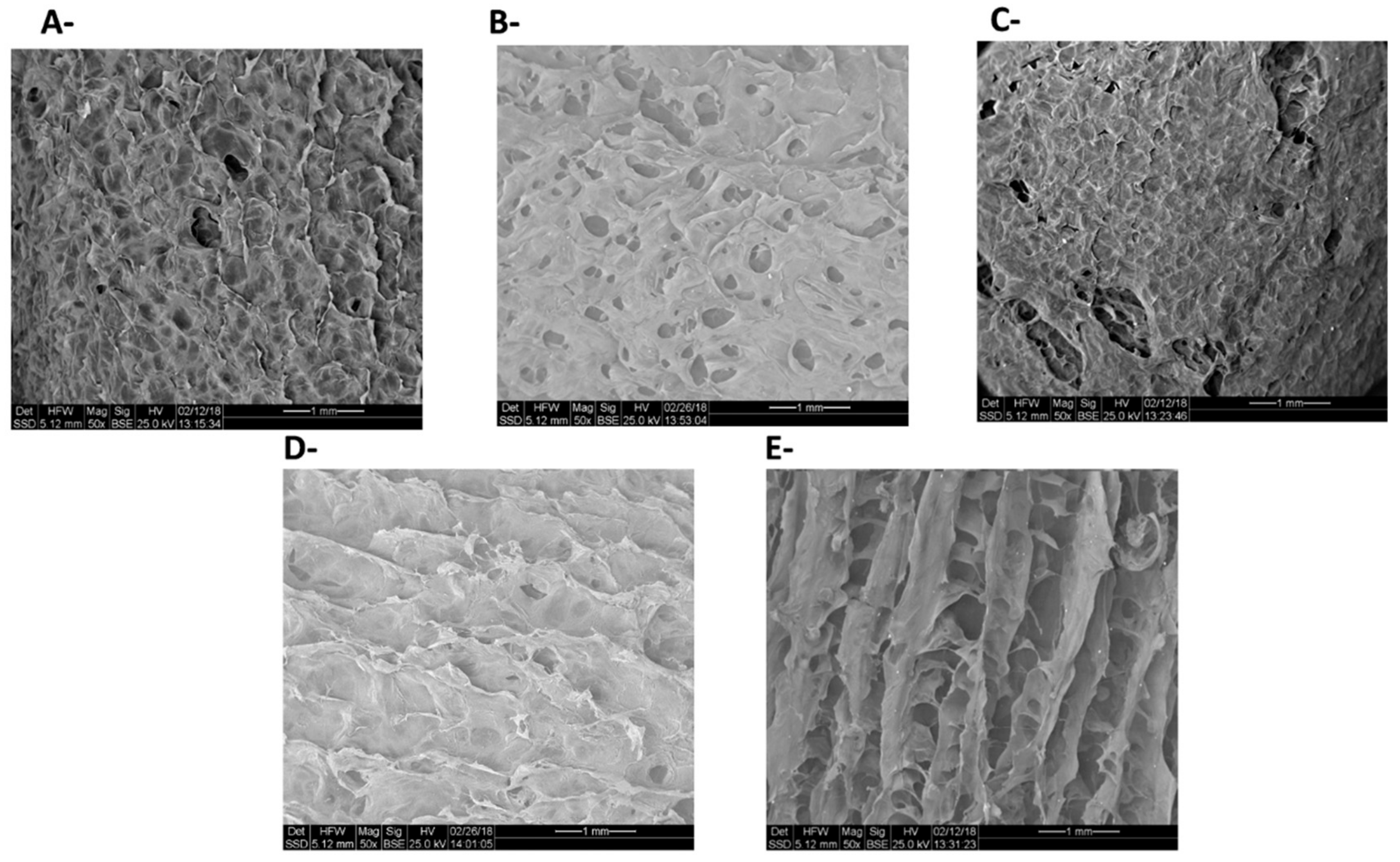
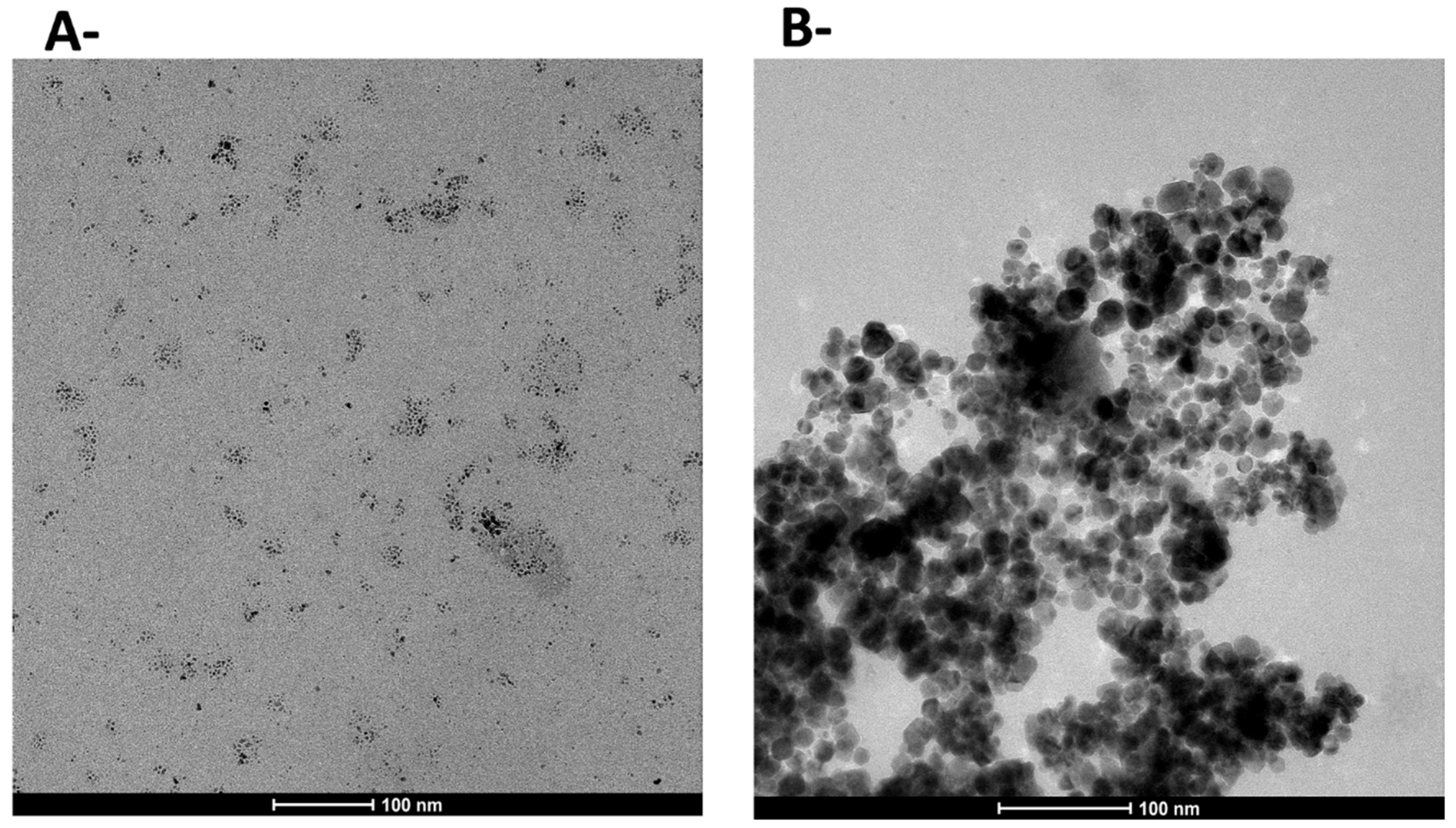
| Polysaccharide | Functional Groups | Branched/Linear | Building Block | Miscibility in Ethanol |
|---|---|---|---|---|
| i | –OH,–OSO3− | Linear | d-Gal-4-sulfate,3,6-anhydro-d-Gal-2-sulfate | NoNo b |
| κ | –OH,–OSO3− | Linear | d-Gal-4-sulfate,3,6-anhydro-d-Gal | NoNo b |
| λ | –OH,–OSO3− | Linear | d-Gal-2-sulfate, d-Gal-2,6 disulfate | NoNo b |
| P | –OH,–OSO3−–COO− | Branched | Not defined | NoNo b |
| C | –OH,–NH2 | Linear | β-(1 → 4)-linked d-glucosamine and N-acetyl-d-glucosamine | NoNo b |
| X | –OH–CH2OCOCH3–COO− | Branched | β-(1 → 4)-d-glucopyranose | NoYes b |
| G | –OH | Branched | β-(1 → 4)-linked mannose | YesYes b |
| LB | –OH | Branched | β-(1 → 4)-linked mannose | YesYes b |
| Entry | Polysaccharide | TOF (h−1) |
|---|---|---|
| 1 | Non | 0.846 |
| 2 | i | 1.319 |
| 3 | κ | 1.621 |
| 4 | λ | 1.150 |
| 5 | P | 1.319 |
| 6 | C | 0.119 |
| 7 | G | 2.073 |
| 8 | X | 2.063 |
| 9 | LB | 2.077 |
| 10 | Cb | 2.056 |
| 11 | Non c | 2.065 |
| Entry/Cycle | TOF (h−1)b |
|---|---|
| 1 | 1.063 |
| 2 | 0.966 |
| 3 | 0.955 |
| 4 | 0.900 |
| 5 | 0.863 |
| 6 | 0.722 |
| 7 | 0.700 |
| Entry | Halobenzene | Homogeneous TOF (h−1) | Heterogeneous TOF (h−1) b |
|---|---|---|---|
| 1 | Iodobenzene | 0.846 | 1.319 |
| 2 | Chlorobenzene | 0.538 | 0.440 |
| 3 | 4-Chlorobenzyl alcohol | 1.098 | 1.283 |
| 4 | 4-Chloroacetophenone | 0.479 | 0.313 |
| 5 | 1-Chloro-3-nitrobenzene | 0.106 | 0.223 |
| i | i-PdCl2(TPPTS)2 | |||
|---|---|---|---|---|
| Name | Peak BE (eV) | Atomic % | Peak BE (eV) | Atomic % |
| P2p | - | - | 130.13 | 1.09 |
| S2p | 166.97 | 5.55 | 166.58 | 7.96 |
| Cl2s | - | - | 266.22 | 0.78 |
| C1s | 283.11 | 51.51 | 282.85 | 47.87 |
| Pd3d | - | - | 334.27 | 0.30 |
| K2s | - | - | 375.55 | 0.79 |
| N1s | 396.93 | 3.37 | 397.46 | 1.86 |
| Ca2s | 345.19 | 2.34 | 436.79 | 0.81 |
| O1s | 529.89 | 36.53 | 529.64 | 35.41 |
| Na1s | 1070.56 | 0.70 | 1069.79 | 3.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy-Ontman, O.; Biton, S.; Shlomov, B.; Wolfson, A. Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts. Polymers 2018, 10, 659. https://doi.org/10.3390/polym10060659
Levy-Ontman O, Biton S, Shlomov B, Wolfson A. Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts. Polymers. 2018; 10(6):659. https://doi.org/10.3390/polym10060659
Chicago/Turabian StyleLevy-Ontman, Oshrat, Shira Biton, Boris Shlomov, and Adi Wolfson. 2018. "Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts" Polymers 10, no. 6: 659. https://doi.org/10.3390/polym10060659
APA StyleLevy-Ontman, O., Biton, S., Shlomov, B., & Wolfson, A. (2018). Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts. Polymers, 10(6), 659. https://doi.org/10.3390/polym10060659








