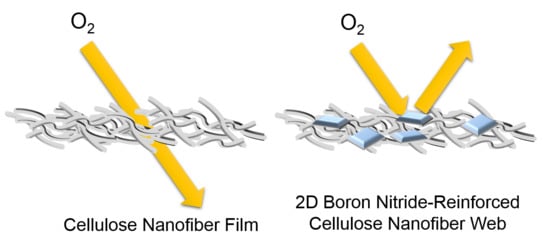
Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Boron Nitride Nanosheet (BNNS) Synthesis
2.3. Composite Film Preparation
2.4. Tensile Properties
2.5. Oxygen Transmission Rate (OTR)
2.6. Characterization
2.7. Cytotoxicity Test
3. Results and Discussion
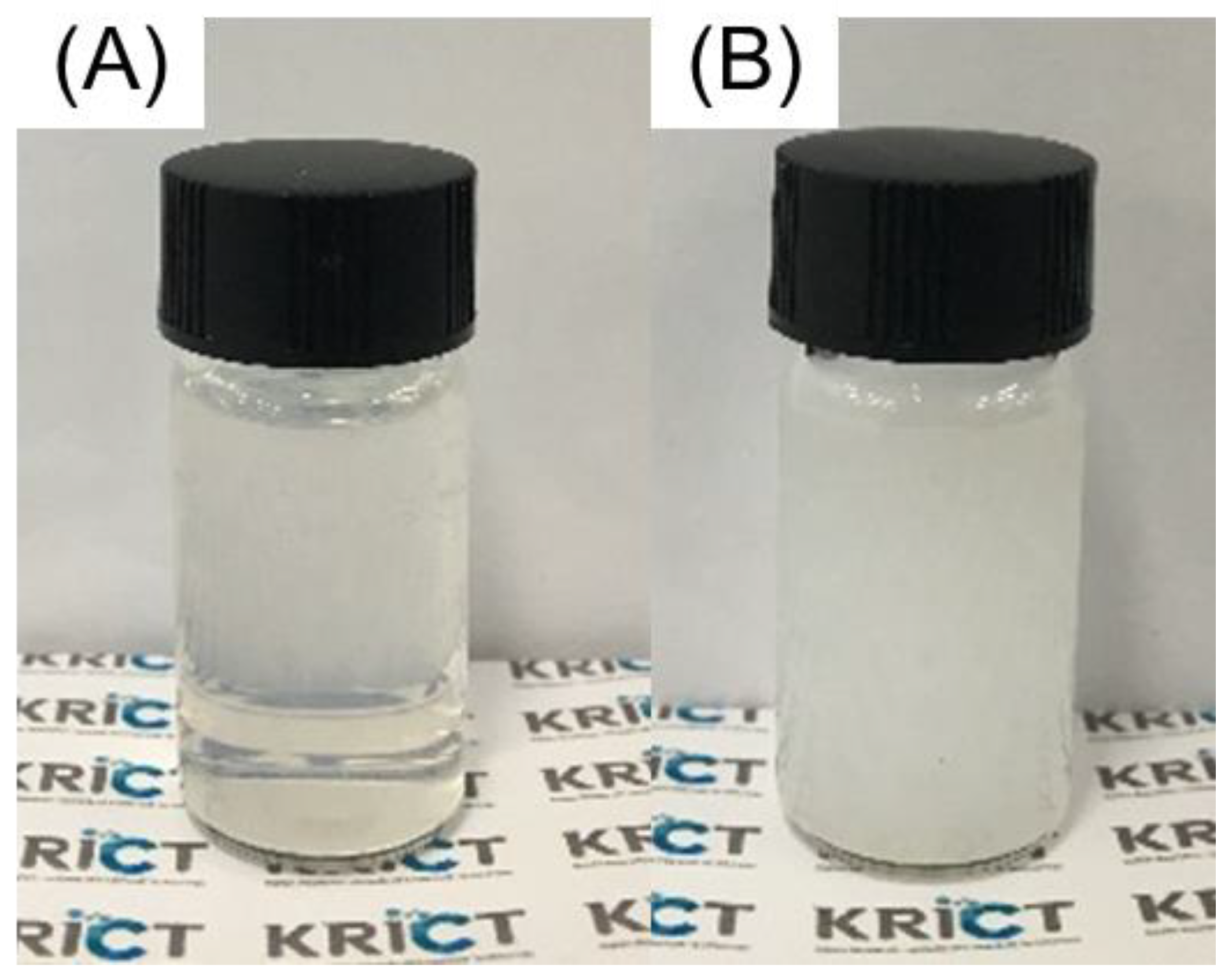
3.1. Appearance of Cellulose Nanofiber (CNF) and BNNS Solutions
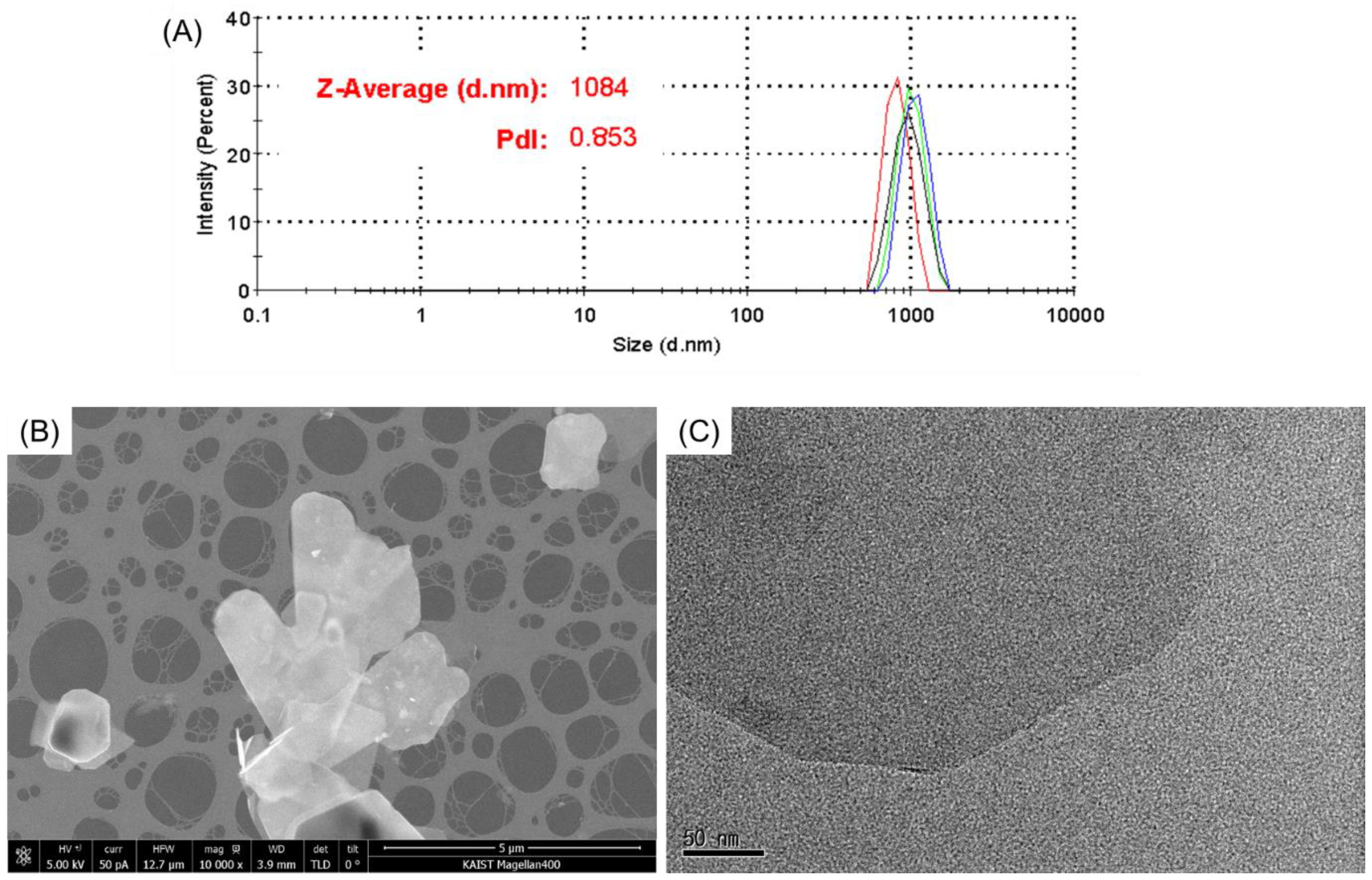
3.2. Analysis of BNNS Particles
3.3. Preparation of BNNS-Containing CNF Composites
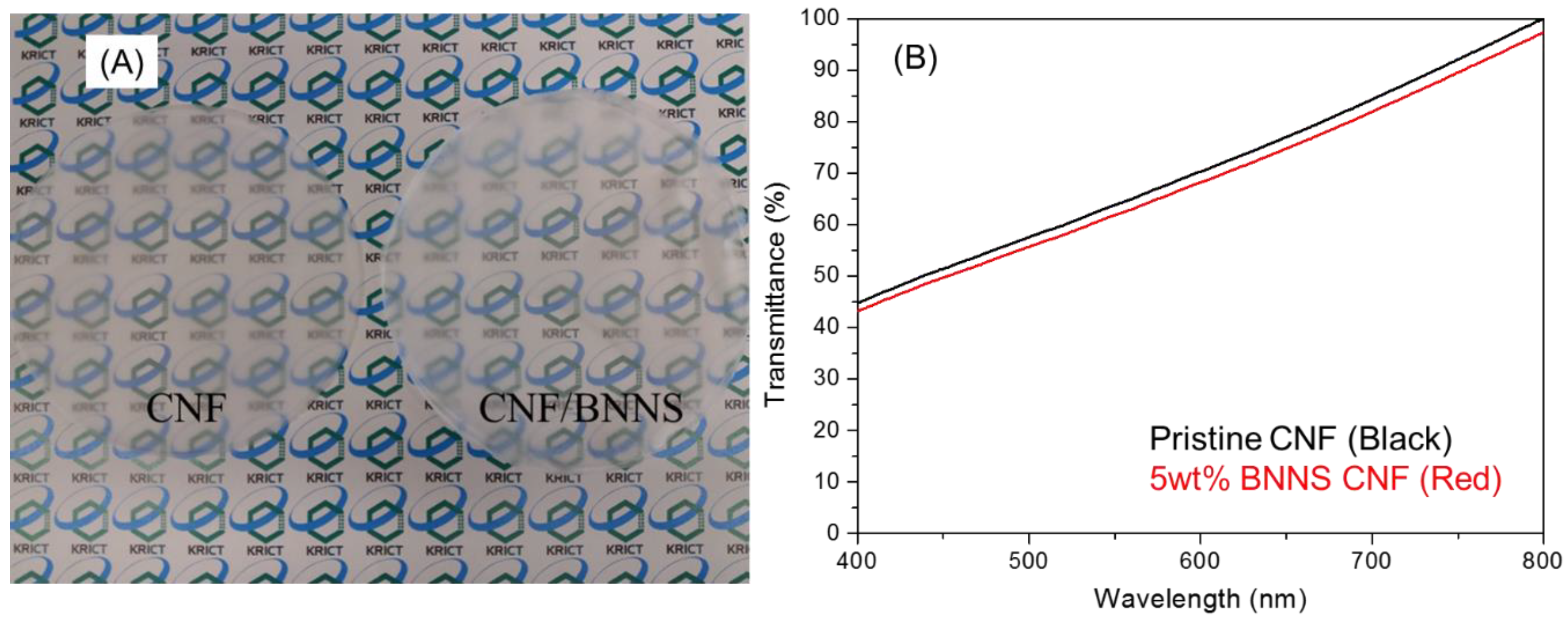
3.4. Optical Properties of the BNNS-Containing CNF Composite
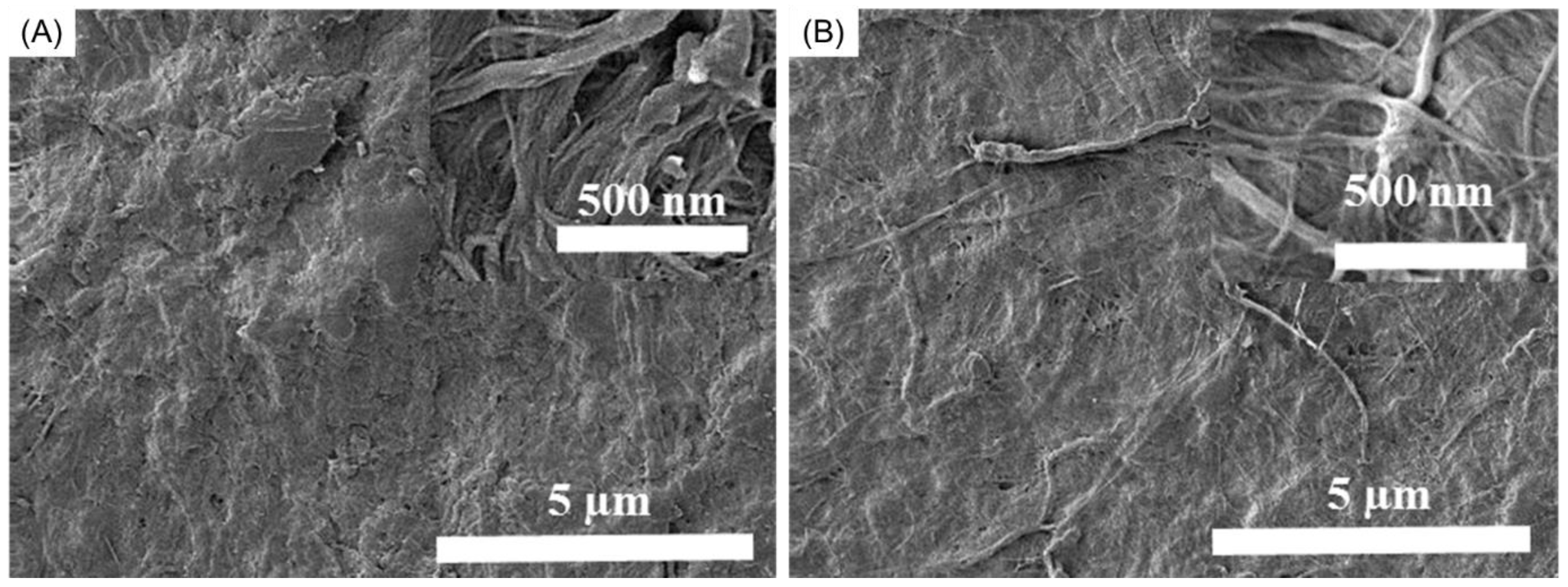
3.5. Morphology of the BNNS-Containing CNF Composite
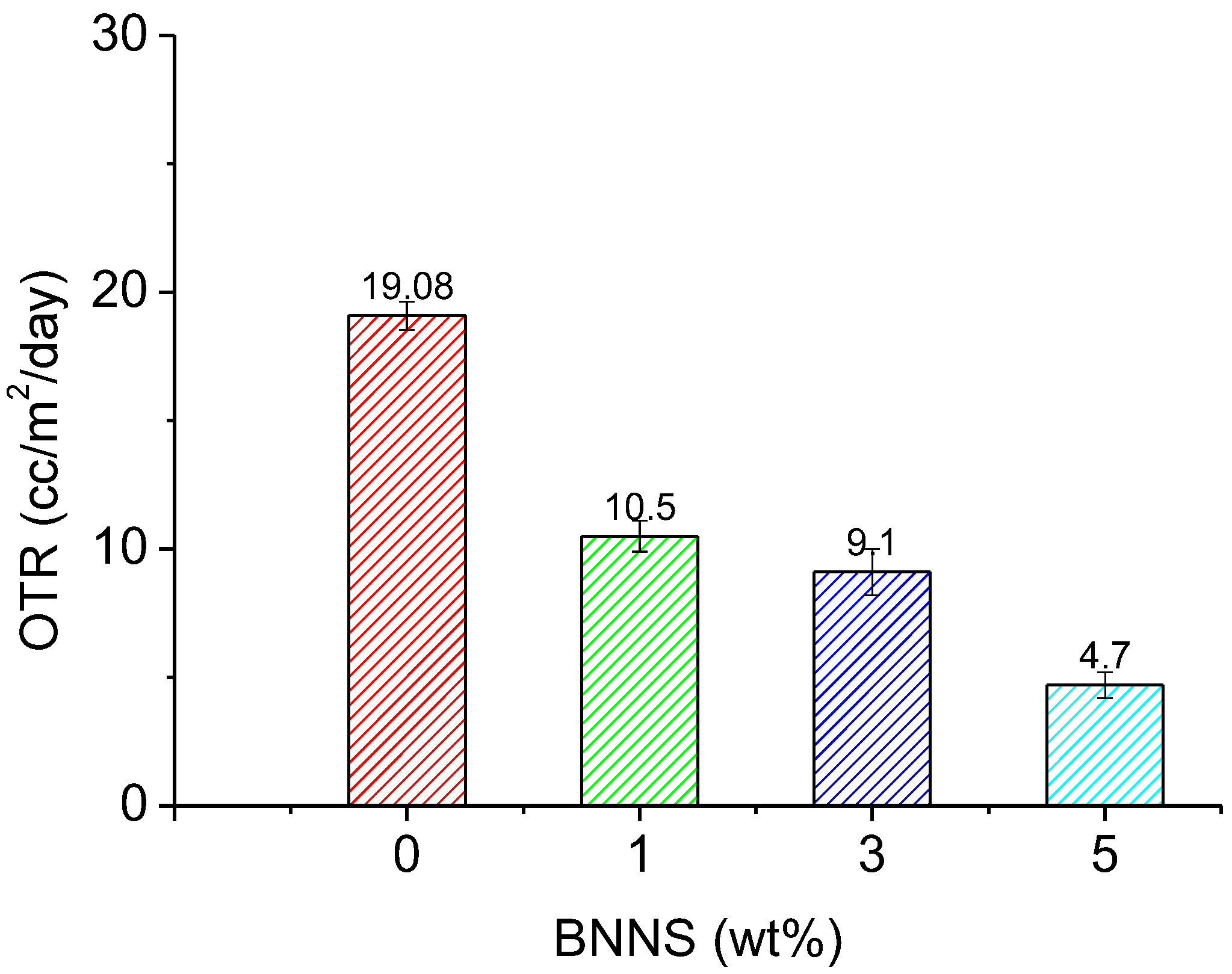
3.6. Oxygen Transmission Rate of BNNS-containing CNF Composite
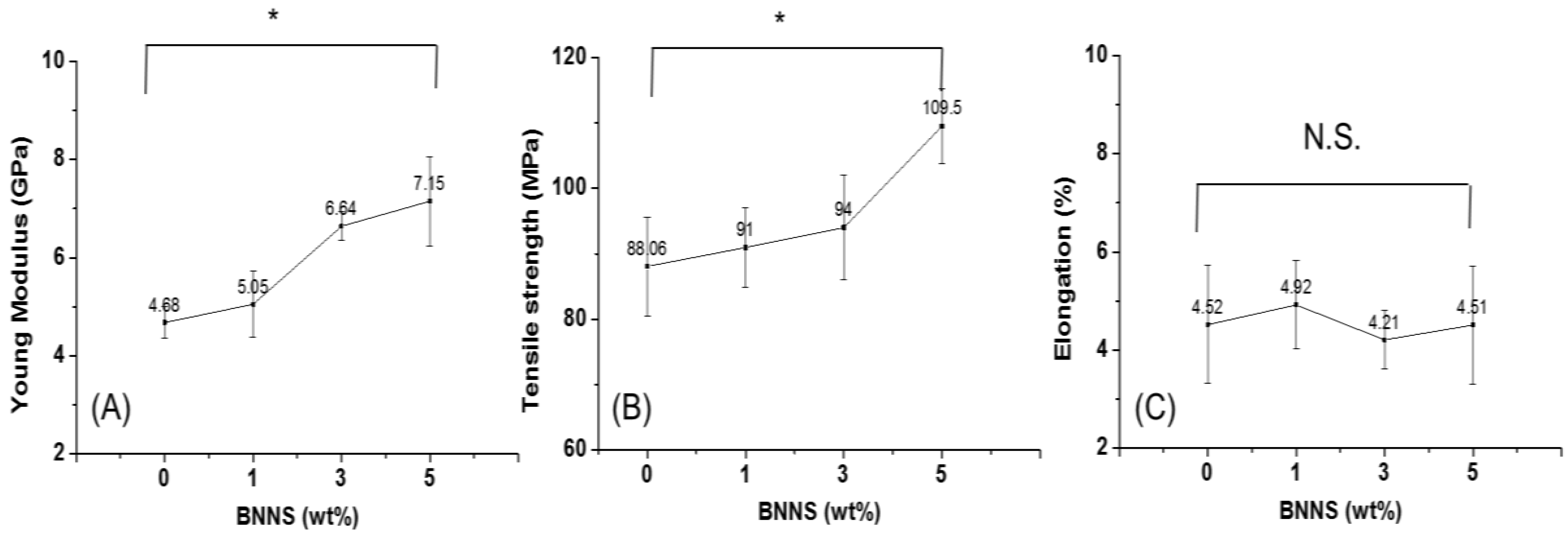
3.7. Tensile Properties of the BNNS-Containing CNF Composite
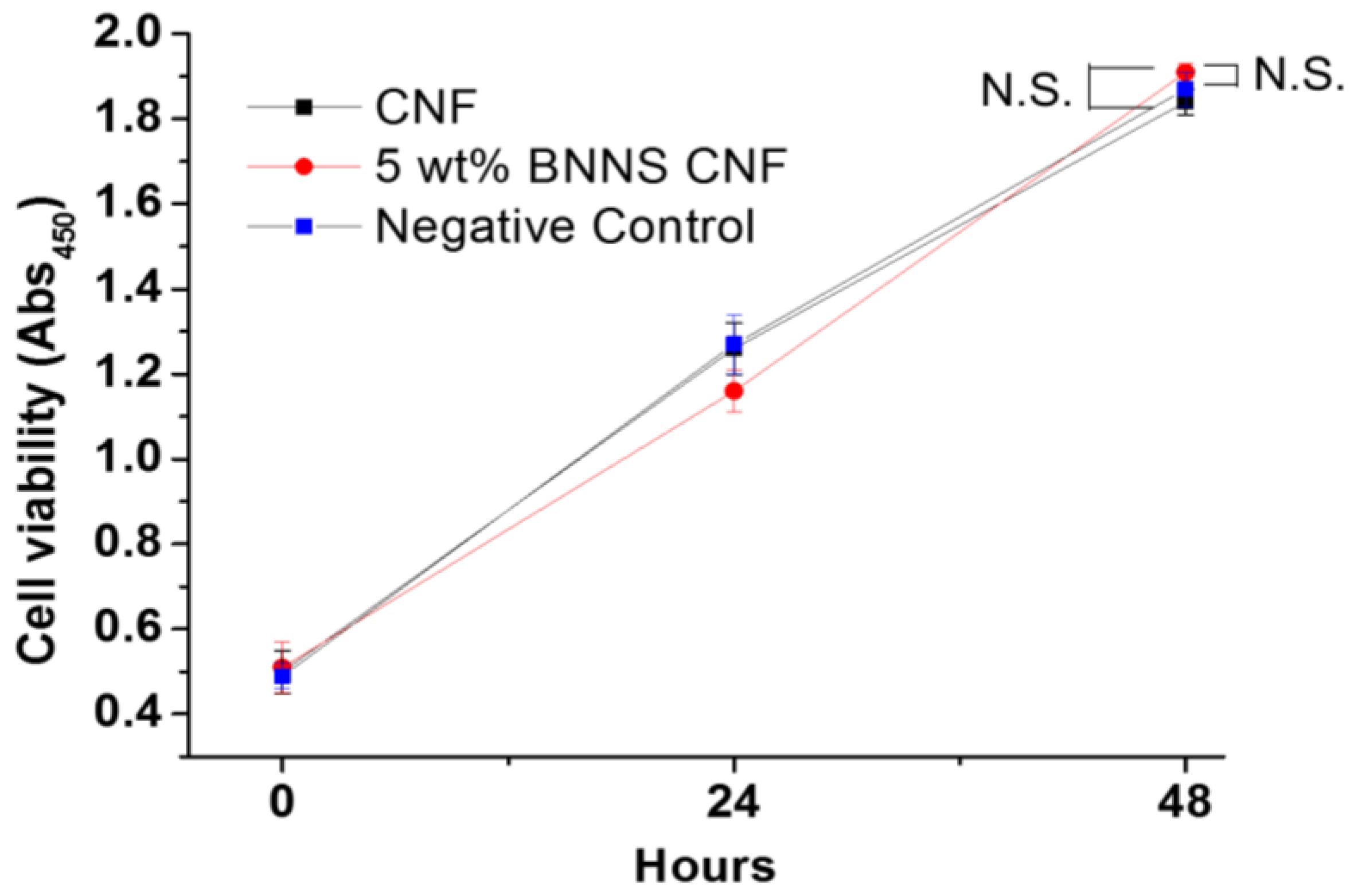
3.8. In Vitro Cytotoxicity Test of the BNNS-Containing CNF Composite
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nakaya, M.; Uedono, A.; Hotta, A. Recent progress in gas barrier thin film coatings on PET bottles in food and beverage applications. Coatings 2015, 5, 987–1001. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Liu, L.; Finkenstadt, V.; Liu, C.K.; Jin, T.; Fishman, M.; Hicks, K. Preparation of poly (lactic acid) and pectin composite films intended for applications in antimicrobial packaging. J. Appl. Polym. Sci. 2007, 106, 801–810. [Google Scholar] [CrossRef]
- Henry, B.; Erlat, A.; McGuigan, A.; Grovenor, C.; Briggs, G.; Tsukahara, Y.; Miyamoto, T.; Noguchi, N.; Niijima, T. Characterization of transparent aluminium oxide and indium tin oxide layers on polymer substrates. Thin Solid Films 2001, 382, 194–201. [Google Scholar] [CrossRef]
- Farris, S.; Introzzi, L.; Fuentes-Alventosa, J.M.; Santo, N.; Rocca, R.; Piergiovanni, L. Self-assembled pullulan–silica oxygen barrier hybrid coatings for food packaging applications. J. Agric. Food Chem. 2012, 60, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, A.; Katami, T.; Shibamoto, T. Formation of dioxins from combustion of polyvinylidene chloride in a well-controlled incinerator. Chemosphere 2006, 62, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W., Jr.; Berger, T.; Borrelli, F.; Garrity, P.; Jacobs, R.; Ledvina, J.; Lewis, J.; McCreedy, R.; Smith, T.; Tuhovak, D. Characterization of emissions of dioxins and furans from ethylene dichloride, vinyl chloride monomer and polyvinyl chloride facilities in the United States. Consolidated report. Chemosphere 2001, 43, 689–700. [Google Scholar] [CrossRef]
- Tartakowski, Z. Recycling of packaging multilayer films: New materials for technical products. Resour. Conserv. Recycl. 2010, 55, 167–170. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-L.; Jo, Y.K.; Cha, M.; Cha, Y.J.; Yoon, D.K.; Sanandiya, N.D.; Prajatelistia, E.; Oh, D.X.; Hwang, D.S. Mussel-inspired anisotropic nanocellulose and silver nanoparticle composite with improved mechanical properties, electrical conductivity and antibacterial activity. Polymers 2016, 8, 102–114. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, G.; Saito, T.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø.; Fukuzumi, H.; Isogai, A. Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 2012, 19, 705–711. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2017, 6, 49–70. [Google Scholar] [CrossRef]
- Oinonen, P.; Krawczyk, H.; Ek, M.; Henriksson, G.; Moriana, R. Bioinspired composites from cross-linked galactoglucomannan and microfibrillated cellulose: Thermal, mechanical and oxygen barrier properties. Carbohydr. Polym. 2016, 136, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Lindström, T. Aspects on nanofibrillated cellulose (NFC) processing, rheology and NFC-film properties. Curr. Opin. Colloid Interface Sci. 2017, 29, 68–75. [Google Scholar] [CrossRef]
- Galland, S.; Leterrier, Y.; Nardi, T.; Plummer, C.J.; Månson, J.A.E.; Berglund, L.A. UV-cured cellulose nanofiber composites with moisture durable oxygen barrier properties. J. Appl. Polym. Sci. 2014, 131, 40604–40618. [Google Scholar] [CrossRef]
- Visanko, M.; Liimatainen, H.; Sirviö, J.A.; Mikkonen, K.S.; Tenkanen, M.; Sliz, R.; Hormi, O.; Niinimäki, J. Butylamino-functionalized cellulose nanocrystal films: Barrier properties and mechanical strength. RSC Adv. 2015, 5, 15140–15146. [Google Scholar] [CrossRef]
- Kulomaa, T.; Matikainen, J.; Karhunen, P.; Heikkilä, M.; Fiskari, J.; Kilpeläinen, I. Cellulose fatty acid esters as sustainable film materials–effect of side chain structure on barrier and mechanical properties. RSC Adv. 2015, 5, 80702–80708. [Google Scholar] [CrossRef]
- Hanif, Z.; Jeon, H.; Tran, T.H.; Jegal, J.; Park, S.-A.; Kim, S.-M.; Park, J.; Hwang, S.Y.; Oh, D.X. Butanol-mediated oven-drying of nanocellulose with enhanced dehydration rate and aqueous re-dispersion. J. Polym. Res. 2017, 24, 191–201. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Pierleoni, D.; Xia, Z.Y.; Christian, M.; Ligi, S.; Minelli, M.; Morandi, V.; Doghieri, F.; Palermo, V. Graphene-based coatings on polymer films for gas barrier applications. Carbon 2016, 96, 503–512. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Lin, S.-Y.; Tsai, H.-C. Butyl rubber nanocomposites with monolayer MoS2 additives: Structural characteristics, enhanced mechanical, and gas barrier properties. Polymers 2018, 10, 238–250. [Google Scholar] [CrossRef]
- Biscarat, J.; Bechelany, M.; Pochat-Bohatier, C.; Miele, P. Graphene-like BN/gelatin nanobiocomposites for gas barrier applications. Nanoscale 2015, 7, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jeong, J.-M.; Huh, Y.S.; Choi, B.G. High-performance supercapacitor based on three-dimensional MoS2/graphene aerogel composites. Compos. Sci. Technol. 2015, 121, 123–128. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Lee, K.G.; Chang, S.-J.; Kim, J.W.; Han, Y.-K.; Lee, S.J.; Choi, B.G. Ultrathin sandwich-like MoS2@N-doped carbon nanosheets for anodes of lithium ion batteries. Nanoscale 2015, 7, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-wall Carbon Nanotube in Neural Phaeochiromocytoma-drived PC 12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Chen, Y.; Behan, G.; Zhang, H.; Petravic, M.; Glushenkov, A.M. Large-scale mechanical peeling of boron nitride nanosheets by low-energy ball milling. J. Mater. Chem. 2011, 21, 11862–11866. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Istrate, O.M.; May, P.; Barwich, S.; Bell, A.P.; Khan, U.; Coleman, J.N. Boron nitride nanosheets as barrier enhancing fillers in melt processed composites. Nanoscale 2015, 7, 4443–4450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.; Fang, Z.; Xu, J.; Cao, F.; Wan, J.; Preston, C.; Yang, B.; Hu, L. Highly thermally conductive papers with percolative layered boron nitride nanosheets. ACS Nano 2014, 8, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Song, W.L.; Wang, P.; Cao, L.; Anderson, A.; Meziani, M.J.; Farr, A.J.; Sun, Y.P. Polymer/boron nitride nanocomposite materials for superior thermal transport performance. Angew. Chem. Int. Ed. 2012, 51, 6498–6501. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754–1604762. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.-B.; Wong, C.-P. A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Jeong, J.-M.; Kang, H.G.; Kim, H.-J.; Park, J.; Kim, D.H.; Jung, Y.M.; Hwang, S.Y.; Han, Y.K.; Choi, B.G. Scalable Water-based Production of Highly Conductive Two-dimensional Nanosheets with Ultrahigh Volumetric Capacitance and Rate Capability. Adv. Energy Mater. 2018, in press. [Google Scholar] [CrossRef]
- Oh, D.X.; Kim, S.; Lee, D.; Hwang, D.S. Tunicate-mimetic nanofibrous hydrogel adhesive with improved wet adhesion. Acta Biomater. 2015, 20, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.X.; Shin, S.; Lim, C.; Hwang, D.S. Dopamine-mediated sclerotization of regenerated chitin in ionic liquid. Materials 2013, 6, 3826–3839. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Oh, D.X.; Heo, J.; Lee, H.-K.; Choy, S.; Hawker, C.J.; Hwang, D.S. Formation, removal, and reformation of surface coatings on various metal oxide surfaces inspired by mussel adhesives. ACS Appl. Mater. Interfaces 2015, 7, 24656–24662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A Review of the Mechanical Properties of Carbon Nanotube–Polymer Composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Lee, S.A.; Jeon, H.; Choi, S.-S.; Park, J.; Hwang, S.Y.; Jegal, J.; Oh, D.X.; Kim, B.C.; Hwang, S.S. Crystallization Derivation of Amine Functionalized T12 Polyhedral Oligomeric Silsesquioxane-conjugated Poly(ethylene Terephthalate). Compos. Sci. Technol. 2017, 146, 42–48. [Google Scholar] [CrossRef]
- Kim, T.; Koo, J.M.; Ryu, M.H.; Jeon, H.; Kim, S.-M.; Park, S.-A.; Oh, D.X.; Park, J.; Hwang, S.Y. Sustainable Terpolyester of High Tg Based on Bio Heterocyclic Monomer of Dimethyl Furan-2, 5-Dicarboxylate and Isosorbide. Polymer 2017, 132, 122–132. [Google Scholar] [CrossRef]
- Park, S.-A.; Choi, J.; Ju, S.; Jegal, J.; Lee, K.M.; Hwang, S.Y.; Oh, D.X.; Park, J. Copolycarbonates of Bio-based Rigid Isosorbide and Flexible 1,4-Cyclohexanedimethanol: Merits over Bisphenol-A Based Polycarbonates. Polymer 2017, 116, 153–159. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-L.; Hanif, Z.; Park, S.-A.; Choi, B.G.; Tran, T.H.; Hwang, D.S.; Park, J.; Hwang, S.Y.; Oh, D.X. Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity. Polymers 2018, 10, 501. https://doi.org/10.3390/polym10050501
Nguyen H-L, Hanif Z, Park S-A, Choi BG, Tran TH, Hwang DS, Park J, Hwang SY, Oh DX. Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity. Polymers. 2018; 10(5):501. https://doi.org/10.3390/polym10050501
Chicago/Turabian StyleNguyen, Hoang-Linh, Zahid Hanif, Seul-A Park, Bong Gill Choi, Thang Hong Tran, Dong Soo Hwang, Jeyoung Park, Sung Yeon Hwang, and Dongyeop X. Oh. 2018. "Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity" Polymers 10, no. 5: 501. https://doi.org/10.3390/polym10050501
APA StyleNguyen, H.-L., Hanif, Z., Park, S.-A., Choi, B. G., Tran, T. H., Hwang, D. S., Park, J., Hwang, S. Y., & Oh, D. X. (2018). Sustainable Boron Nitride Nanosheet-Reinforced Cellulose Nanofiber Composite Film with Oxygen Barrier without the Cost of Color and Cytotoxicity. Polymers, 10(5), 501. https://doi.org/10.3390/polym10050501