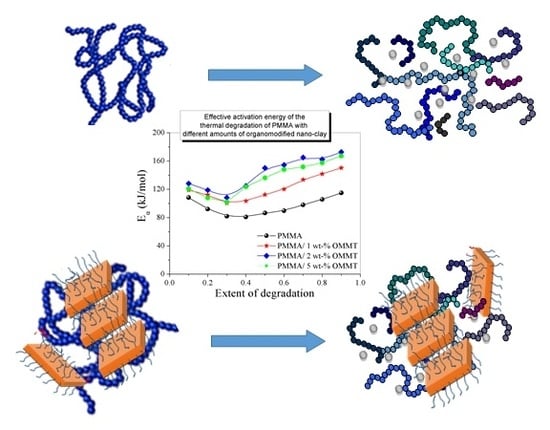
Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Initial Monomer-Clay Mixture
2.3. Synthesis of PMMA/Clay Nanocomposites
2.4. Measurements
3. Background Theory for the Kinetic Analysis of TG Data According to Isoconversional Methods
4. Results and Discussion
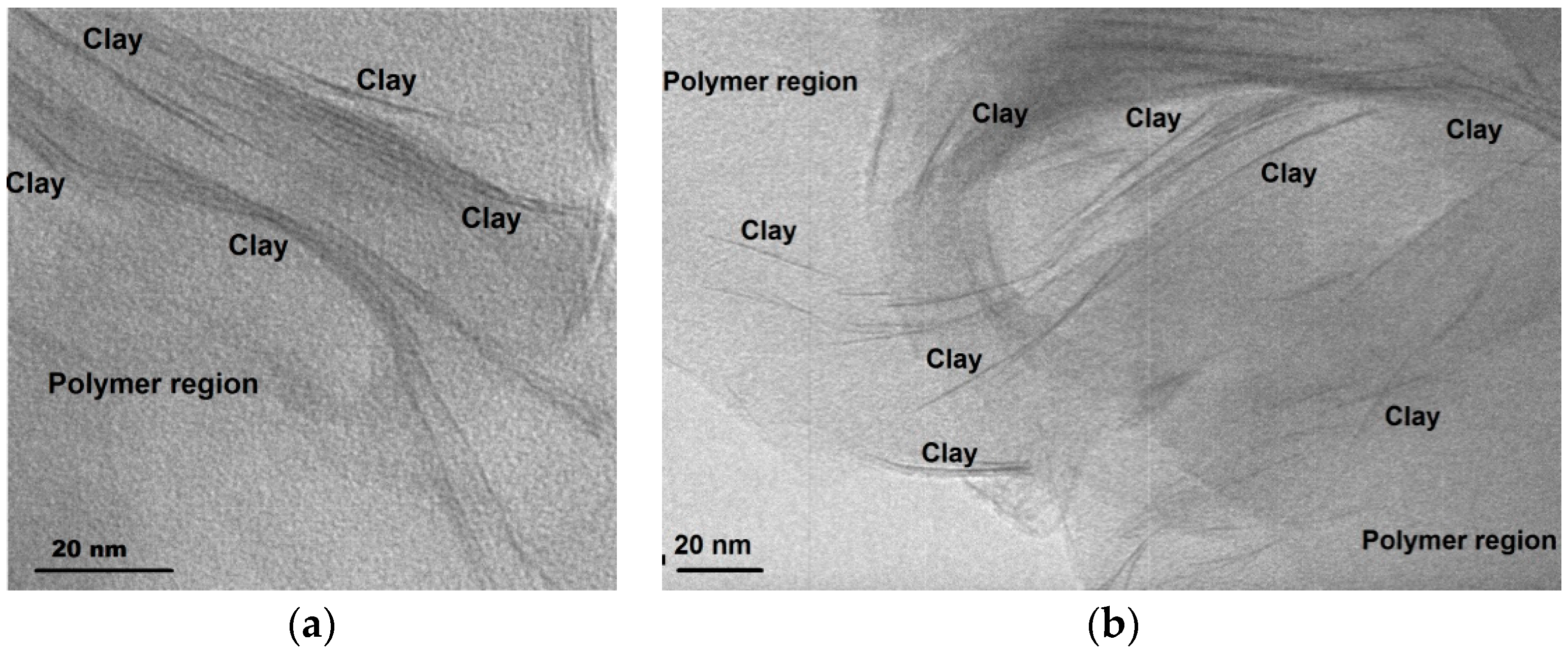
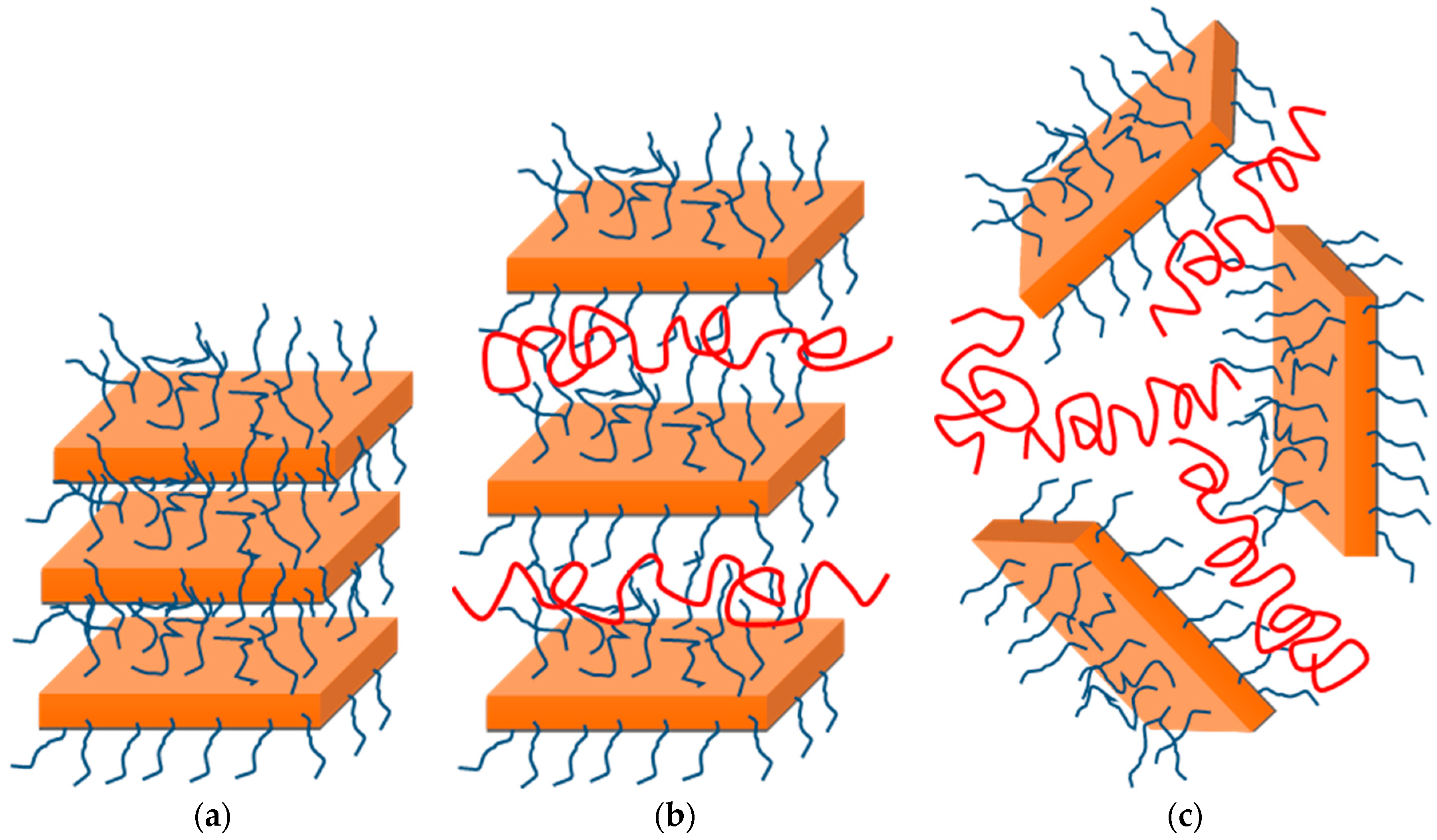
4.1. Structure and Morphology of the Nanocomposites
4.2. Kinetics of Thermal Degradation
4.2.1. Effect of Heating Rate
4.2.2. Isoconversional Kinetic Analysis
4.3. Dynamic Mechanical Thermal Analysis (DMTA)
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and used of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Giannelis, E.P.; Krishnamoorti, R.; Manias, E. Polymer-silicate nanocomposites: Model systems for confined polymers and polymer brushes. Adv. Polym. Sci. 1999, 138, 107–147. [Google Scholar]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Vaia, R.A.; Jandt, K.D.; Kramer, E.J.; Giannelis, E.P. Microstructural evolution of melt intercalated polymer-organically modified layered silicates nanocomposites. Chem. Mater. 1996, 8, 2628–2635. [Google Scholar] [CrossRef]
- Brune, D.A.; Bicerano, J. Micromechanics of nanocomposites: Comparison of tensile and compressive elastic moduli and prediction of effects of incomplete exfoliation and imperfect alignment on modulus. Polymer 2002, 42, 369–387. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the barrier properties of polymer-layered silicate nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Blumstein, A. Polymerization of adsorbed monolayers: II. Thermal degradation of the inserted polymers. J. Polym. Sci. A 1965, 3, 2665–2672. [Google Scholar] [CrossRef]
- Burnside, S.D.; Giannelis, E.P. Synthesis and properties of new poly (dimethylsiloxane) nanocomposites. Chem. Mater. 1995, 7, 1597–1600. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: The effect of matrix molecular weight. Polymer 2001, 42, 9929–9940. [Google Scholar] [CrossRef]
- Beyer, G. Nanocomposites: A new class of flame retardants for polymers. Plast. Addit. Compd. 2002, 4, 22–27. [Google Scholar] [CrossRef]
- Kim, C.-M.; Lee, D.-H.; Hoffmann, B.; Kressler, J.; Stoppelmann, G. Influence of nanofillers on the deformation process in layeredsilicate/polyamide 12 nanocomposites. Polymer 2001, 42, 1095–1100. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Kim, S.W.; Jo, W.H.; Lee, M.S.; Ko, M.B.; Jho, J.Y. Preparation of clay–dispersed poly(styrene-co-acrylonitrile) nanocomposites using poly(ε-caprolactone) as a compatibilizer. Polymer 2001, 42, 9837–9842. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Synthesis and characterization of PMMA/organomodified montmorillonite nanocomposites prepared by in situ bulk polymerization. Ind. Eng. Chem. Res. 2011, 50, 571–579. [Google Scholar] [CrossRef]
- Su, S.; Jiang, D.D.; Wilkie, C.A. Methacrylate modified clays and their polystyrene and poly(methyl methacrylate) nanocomposites. Polym. Adv. Technol. 2004, 15, 225–231. [Google Scholar] [CrossRef]
- Chen, K.; Wilkie, C.A.; Vyazovkin, S. Nanoconfinement revealed in degradation and relaxation studies of two structurally different polystyrene-clay systems. J. Phys. Chem. B 2007, 111, 12685–12692. [Google Scholar] [CrossRef] [PubMed]
- Dranca, I.; Ungur, N.; Lupascu, T.; Petuhov, O. Comparative kinetics study of the thermal and thermo-oxidative degradation of a polystyrene-clay nanocomposite by TGA and DSC. Chem. J. Mold. 2010, 5, 68–72. [Google Scholar]
- Erceg, M.; Makrić, J.; Kovačić, T. Kinetic Analysis of the Non-Isothermal Degradation of PEO Nanocomposites. In Proceedings of the 14th European Conference on Composite Materials, Budapest, Hungary, 7–10 June 2010. [Google Scholar]
- Kandare, E.; Deng, H.; Wang, D. Thermal stability and degradation kinetics of poly(methyl methacrylate)/layered copper hydroxyl methacrylate composites. Polym. Adv. Technol. 2006, 17, 312–319. [Google Scholar] [CrossRef]
- Li, H.; Kim, H. Thermal degradation and kinetic analysis of PVDF/modified MMT nanocomposite membranes. Desalination 2008, 234, 9–15. [Google Scholar] [CrossRef]
- Majoni, S.; Su, S.; Hossenlopp, J.M. The effect of boron-containing layered hydroxysalt (LHS) on the thermal stability and degradation kinetics of poly(methyl methacrylate). Polym. Degrad. Stab. 2010, 95, 1593–1604. [Google Scholar] [CrossRef][Green Version]
- Qiu, L.; Chen, W.; Qu, B. Morphology and thermal stabilization mechanism of LLDPE/MMT and LLDPE/LDH nanocomposites. Polymer 2006, 47, 922–930. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Nanoclay nucleation effect in the thermal stabilization of a polymer nanocomposite: A kinetic mechanism change. J. Phys. Chem. C 2012, 116, 11797–11807. [Google Scholar] [CrossRef]
- Bourbigot, S.; Gilman, J.W.; Wilkie, C.A. Kinetic analysis of the thermal degradation of polystyrene-montmorillonite nanocomposite. Polym. Degrad. Stab. 2004, 84, 483–492. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I.; Fan, X.; Advincula, R. Degradation and relaxation kinetics of polystyrene-clay nanocomposite prepared by surface initiated polymerization. J. Phys. Chem. B 2004, 108, 11672–11679. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I.; Fan, X.; Advincula, R. Kinetics of the thermal and thermo-oxidative degradation of a polystyrene–clay nanocomposite, Macromol. Rapid Commun. 2004, 25, 498–503. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Natarajan, U. Thermal and mechanical properties of melt processed intercalated poly(methylmethacrylate)–organoclay nanocomposites over a wide range of filler loading. Polym. Int. 2008, 57, 738–743. [Google Scholar] [CrossRef]
- Kumar, S.; Jog, J.P.; Natarajan, U. Preparation and characterization of poly(methylmethacrylate)–clay nanocomposites via melt intercalation: The effect of organoclay on the structure and thermal properties. J. Appl. Polym. Sci. 2003, 89, 1186–1194. [Google Scholar] [CrossRef]
- Dhibar, A.K.; Mallick, S.; Rath, T.; Khatua, B.B. Effect of clay platelet dispersion as affected by the manufacturing techniques on thermal and mechanical properties of PMMA-clay nanocomposites. J. Appl. Polym. Sci. 2009, 113, 3012–3018. [Google Scholar] [CrossRef]
- Kim, S.; Wilkie, C.A. Transparent and flame retardant PMMA nanocomposites. Polym. Adv. Technol. 2008, 19, 496–506. [Google Scholar] [CrossRef]
- Achilias, D.S.; Siafaka, P.; Nikolaidis, A.K. Polymerization Kinetics and Thermal Properties of Poly(alkyl methacrylate)/Organomodified Montmorillonite Nanocomposites. Polym. Int. 2012, 61, 1510–1518. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Xie, S.; Zhang, S. Synthesis and properties of poly(methylmethacrylate)/montmorillonite (PMMA/MMT) nanocomposites. Polym. Int. 2003, 52, 892–898. [Google Scholar] [CrossRef]
- Qu, X.; Guan, T.; Liu, G.; She, Q.; Zhang, L. Preparation, structural characterization, and properties of poly(methyl methacrylate)/montmorillonite nanocomposites by bulk polymerization. J. Appl. Polym. Sci. 2005, 97, 348–357. [Google Scholar] [CrossRef]
- Su, S.; Wilkie, C.A. Exfoliated poly(methyl methacrylate) and polystyrene nanocomposites occur when the clay cation contains a vinyl monomer. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1124–1135. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Liou, S.-J.; Lin, C.-Y.; Cheng, C.-Y.; Chang, Y.-W. Anticorrosively enhanced PMMA-clay nanocomposite materials with quaternary alkylphosphonium salt as an intercalating agent. Chem. Mater. 2002, 14, 154–161. [Google Scholar] [CrossRef]
- Zhao, Q.; Samulski, E.T. A comparative study of poly(methyl methacrylate) and polystyrene/clay nanocomposites prepared in supercritical carbon dioxide. Polymer 2006, 47, 663–671. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S.; Karayannidis, G.P. Effect of the type of the organic modifier on the polymerization kinetics and the properties of poly(methyl methacrylate)/organomodified montmorillonite nanocomposites. Eur. Polym. J. 2012, 48, 240–251. [Google Scholar] [CrossRef]
- Chrissafis, K. Kinetics of Thermal Degradation of Polymers. J. Therm. Anal. Calorim. 2009, 95, 273–283. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and polypropylene. Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to phenolic plastic. J. Polym. Sci. C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of thermogravimetry of polymers. J. Res. Natl. Bur. Stand. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dollimore, D. Linear and nonlinear procedures in isoconversional computations of the activation energy of non-isothermal reactions in solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Vyazovkin, S. Evaluation of activation energy of thermally stimulated solid state reactions under arbitrary variation of temperature. J. Comput. Chem. 1997, 18, 393–402. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetic study of stabilizing effect of oxygen on thermal degradation of poly(methyl methacrylate). J. Phys. Chem. B 1999, 103, 8087–8092. [Google Scholar] [CrossRef]
- Kang, B.-S.; Kim, S.G.; Kim, J.-S.J. Thermal degradation of poly (methyl methacrylate) polymers: Kinetics and recovery of monomers using a fluidized bed reactor. Anal. Appl. Pyrolysis 2008, 81, 7–13. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-Y. The effect of end groups on the thermal degradation of poly (methyl methacrylate). Polym. Degrad. Stab. 2003, 82, 81–88. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly (methyl methacrylate)(PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Staggs, J.E.J. Population balance models for the thermal degradation of PMMA. Polymer 2007, 48, 3868–3876. [Google Scholar]
- Tsuge, S.; Mukarami, K.; Esaki, M.; Takeuchi, T. Thermal Analysis; Chihara, H., Ed.; Heyden: London, UK, 1977; p. 289. [Google Scholar]
- Grassie, N.; Vance, E. Degradation evidence for the nature of chain termination and transfer with benzene in the polymerization of methyl methacrylate. Trans. Faraday Soc. 1953, 49, 184–189. [Google Scholar] [CrossRef]
- Jellinek, H.H.D. Thermal degradation of polymethyl methacrylate: Energies of activation. Makromol. Chem. 1968, 115, 89–102. [Google Scholar] [CrossRef]
- Gao, Z.; Kaneko, T.; Hou, D.; Nakada, M. Kinetics of thermal degradation of poly(methyl methacrylate) studied with the assistance of the fractional conversion at the maximum reaction rate. Polym. Degrad. Stab. 2004, 84, 399–403. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The kinetics and mechanisms of the thermal degradation of poly(methyl methacrylate) studied by thermal analysis-fourier transform infrared spectroscopy. Polymer 2001, 42, 4825–4835. [Google Scholar] [CrossRef]
- Liaw, J.H.; Hsueh, T.Y.; Tan, T.-S.; Wang, Y.; Chiao, S.-M. Twin-screw compounding of poly(methyl methacrylate)/clay nanocomposites: Effects of compounding temperature and matrix molecular weight. Polym. Int. 2007, 56, 1045–1052. [Google Scholar] [CrossRef]
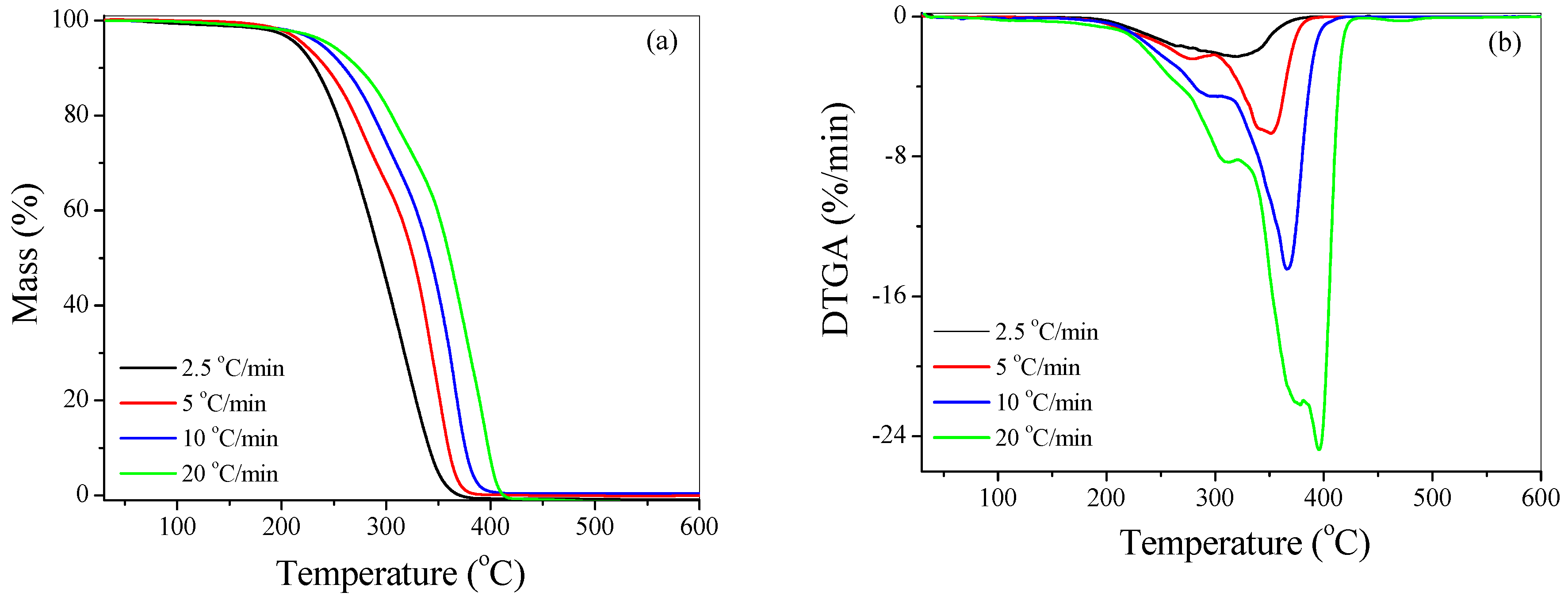
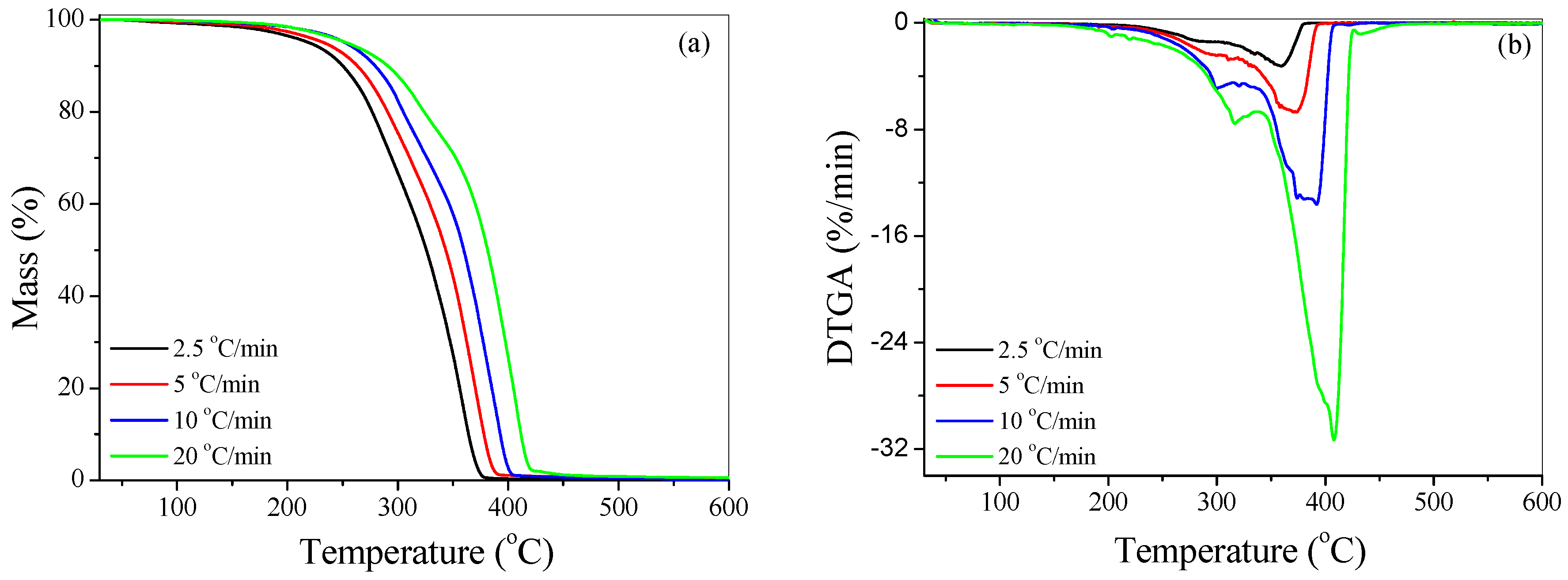

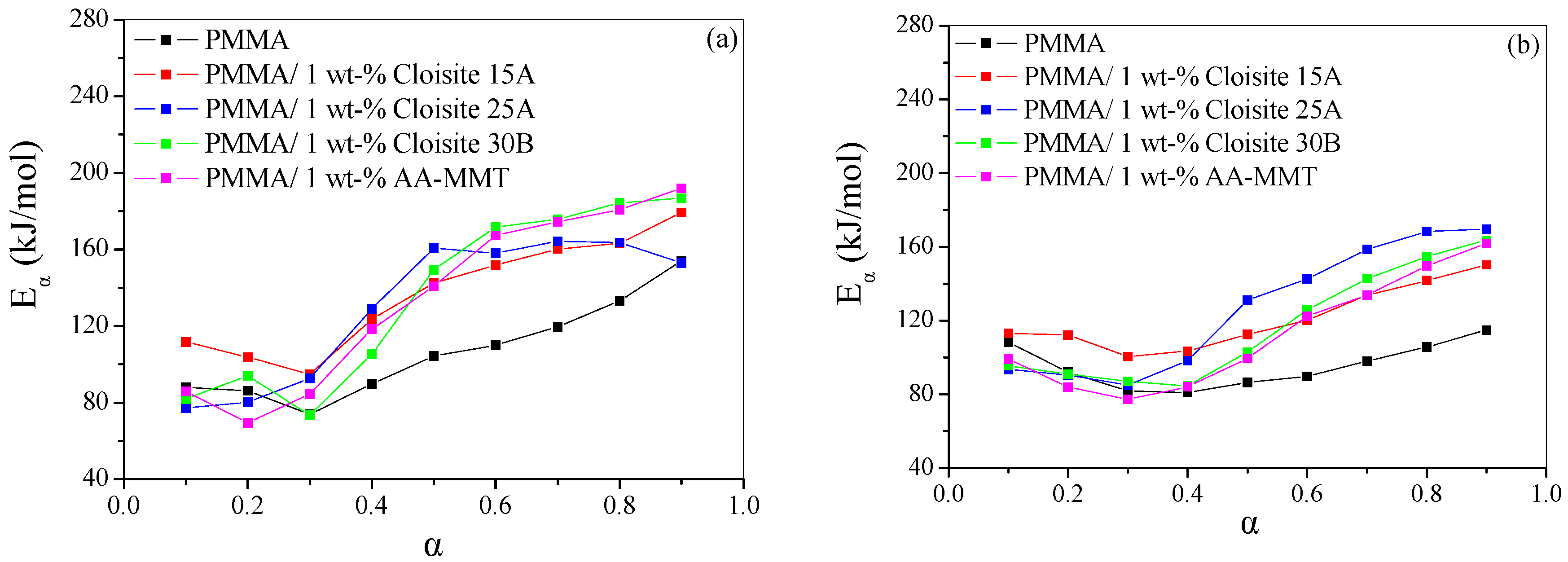

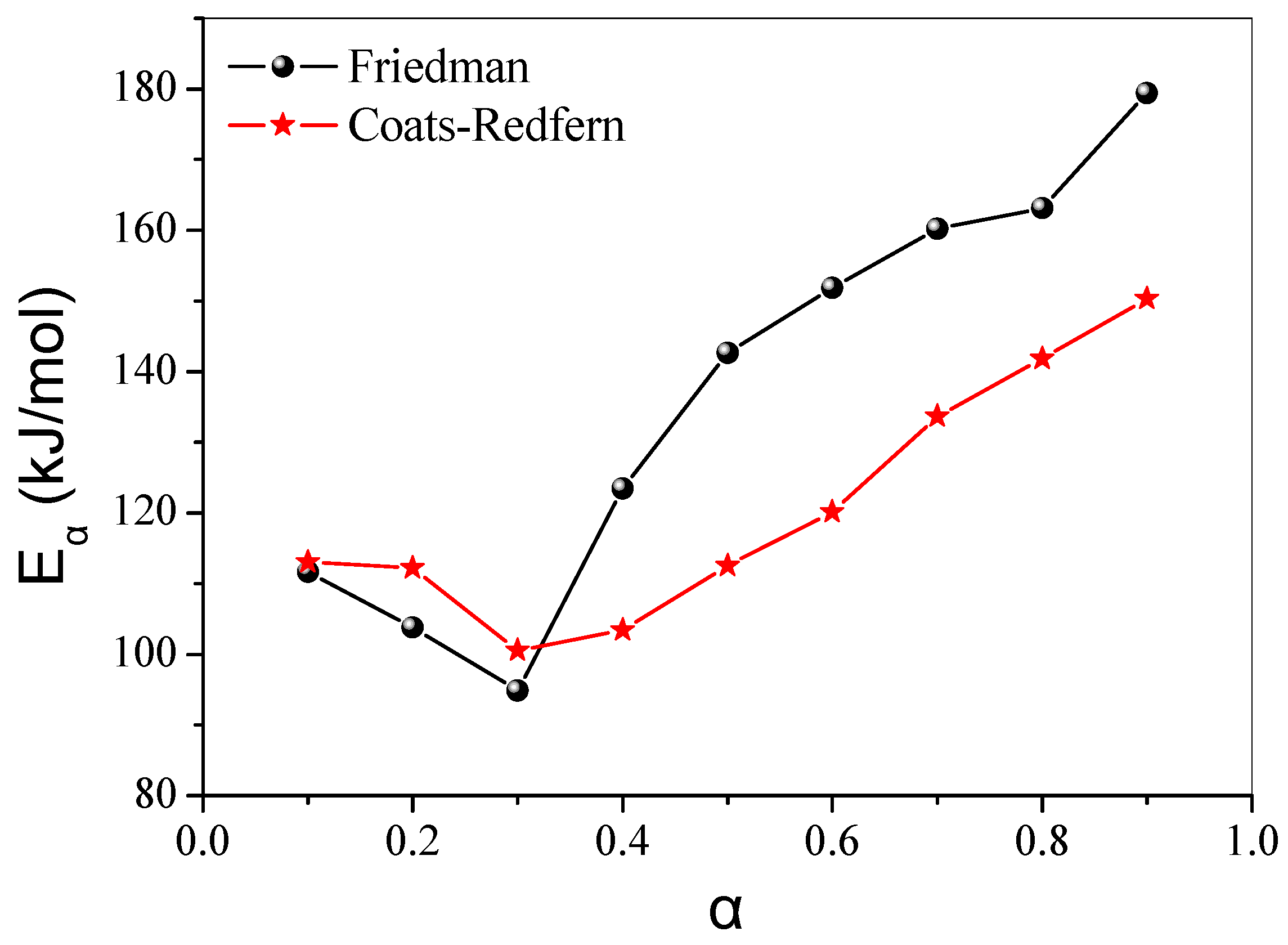
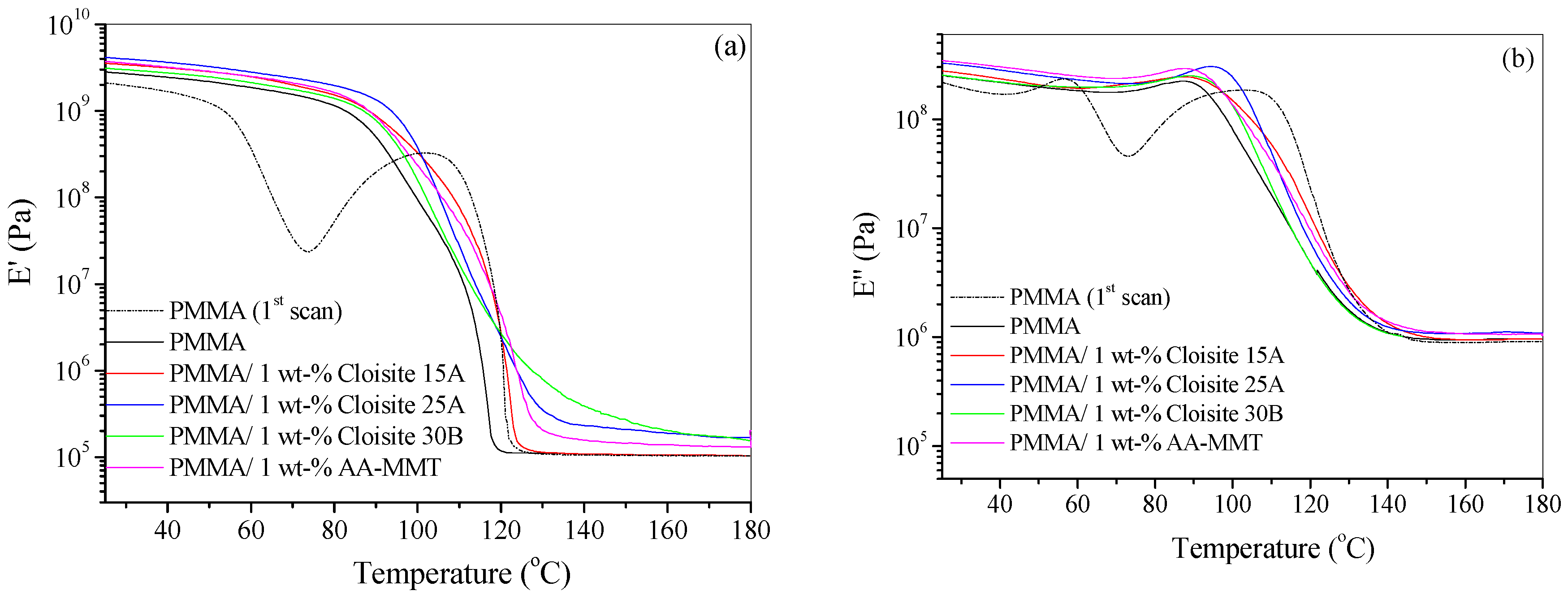
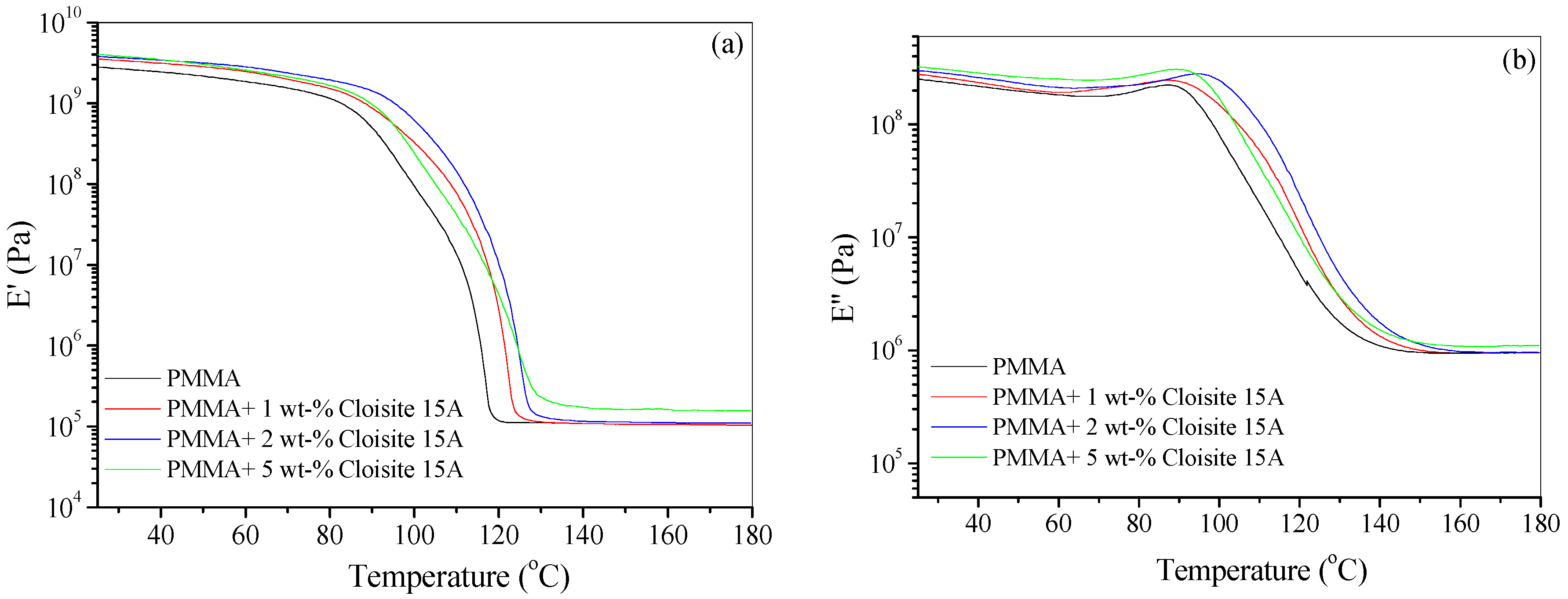
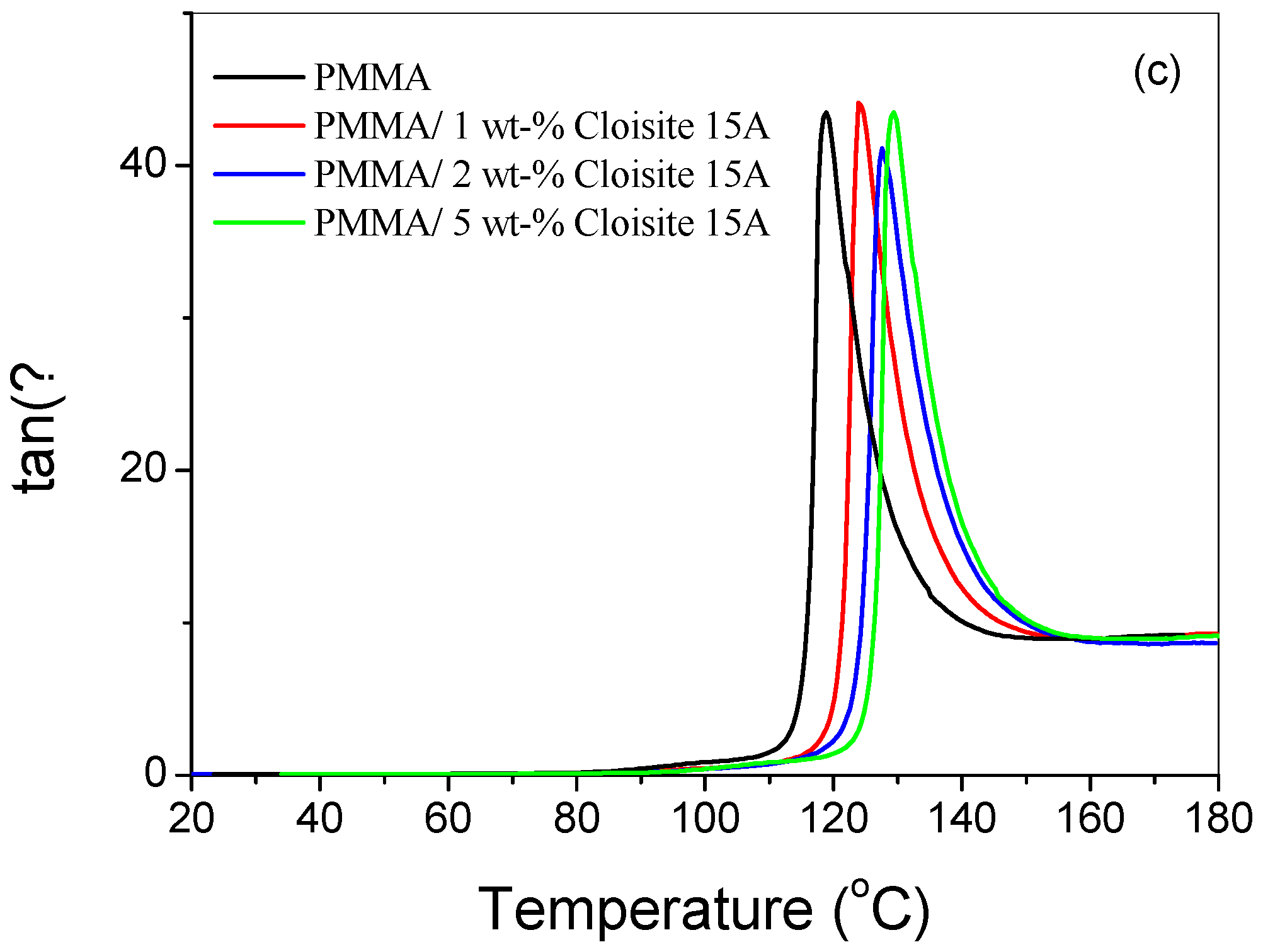
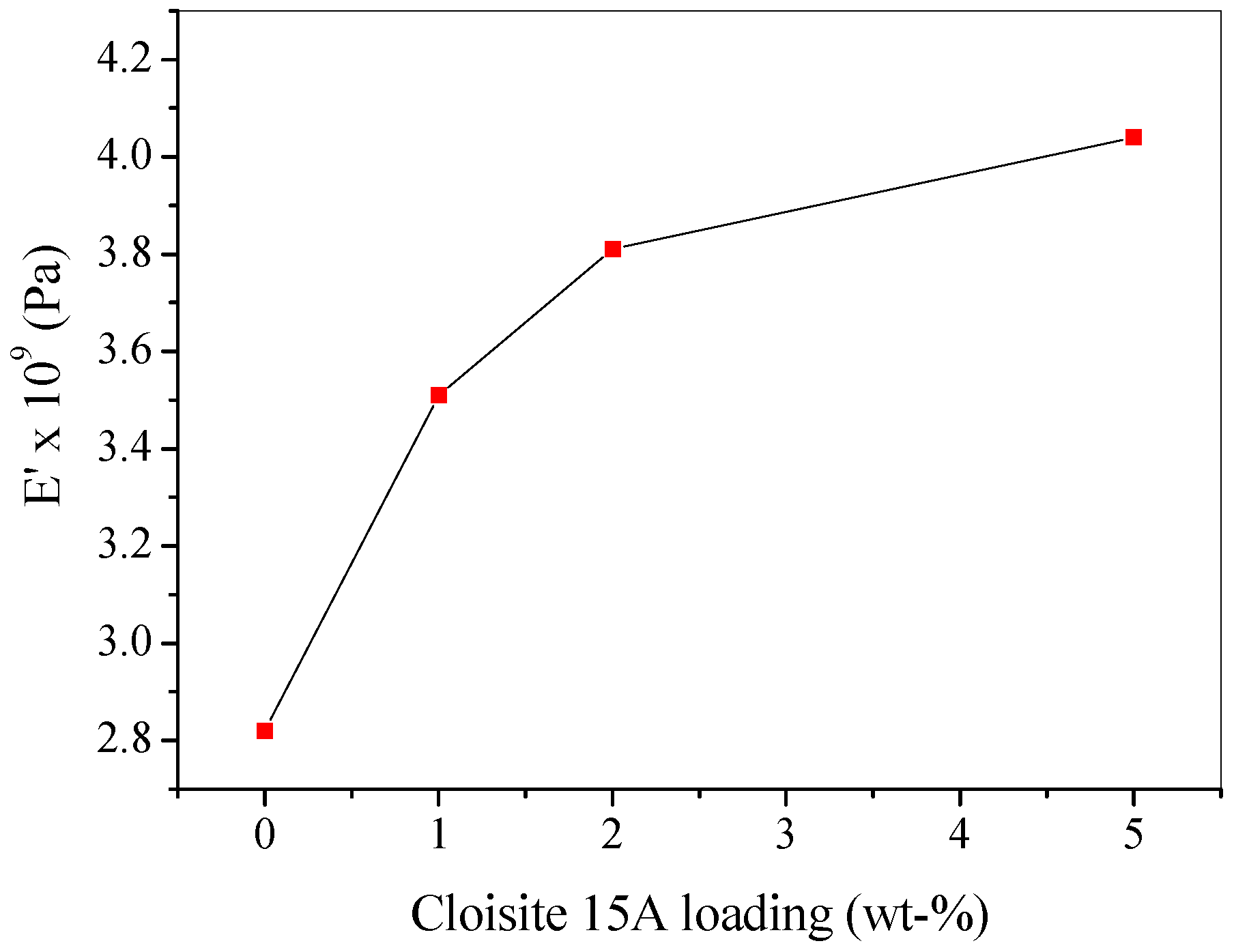
| Sample | Heating Rate (°C/min) | T1% (°C) | T10% (°C) | T50% (°C) | Tp1 (°C) | Tp2 (°C) | Residue (%) |
|---|---|---|---|---|---|---|---|
| PMMA | 2.5 | 136 | 231 | 294 | 271 | 319 | 0 |
| 5 | 143 | 242 | 326 | 277 | 348 | 0 | |
| 10 | 154 | 259 | 342 | 293 | 366 | 0.1 | |
| 20 | 162 | 272 | 360 | 311 | 392 | 0.3 | |
| PMMA + 1 wt % Cloisite 15A | 2.5 | 142 | 248 | 326 | 326 | 326 | 0.1 |
| 5 | 154 | 262 | 344 | 344 | 346 | 0.3 | |
| 10 | 176 | 279 | 360 | 360 | 364 | 0.6 | |
| 20 | 183 | 290 | 381 | 381 | 387 | 0.8 |
| Sample | Storage Modulus, E′ × 109 (Pa) |
|---|---|
| PMMA | 2.82 |
| PMMA + 1 wt % Cloisite 15A | 3.54 |
| PMMA + 1 wt % Cloisite 25A | 4.14 |
| PMMA + 1 wt % Cloisite 30B | 3.11 |
| PMMA + 1 wt % AA-MMT | 3.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaidis, A.K.; Achilias, D.S. Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization. Polymers 2018, 10, 491. https://doi.org/10.3390/polym10050491
Nikolaidis AK, Achilias DS. Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization. Polymers. 2018; 10(5):491. https://doi.org/10.3390/polym10050491
Chicago/Turabian StyleNikolaidis, Alexandros K., and Dimitris S. Achilias. 2018. "Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization" Polymers 10, no. 5: 491. https://doi.org/10.3390/polym10050491
APA StyleNikolaidis, A. K., & Achilias, D. S. (2018). Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization. Polymers, 10(5), 491. https://doi.org/10.3390/polym10050491