The Application of Small-Angle Light Scattering for Rheo-Optical Characterization of Chitosan Colloidal Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Solution Preparation
2.2. Rheological Experiments
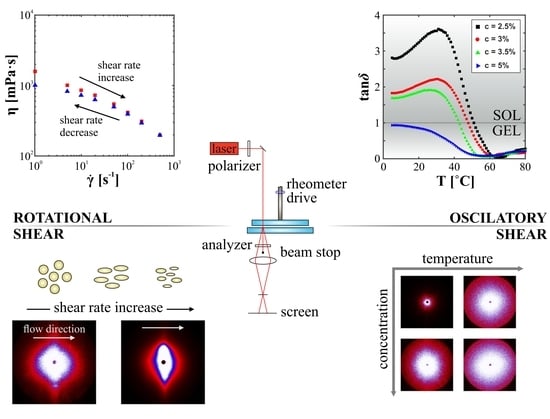
2.3. Small Angle Light Scattering Experiments
3. Results and Discussion
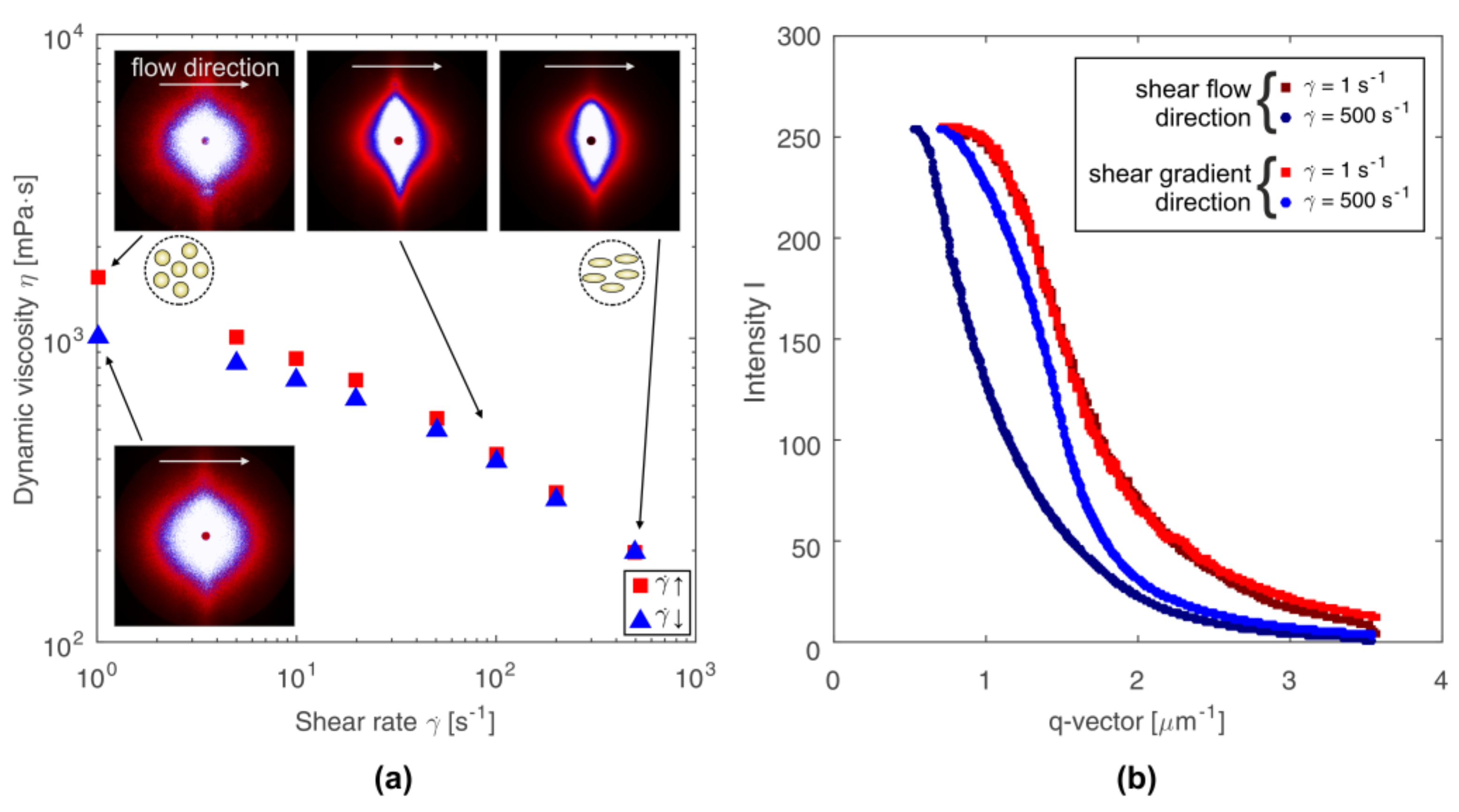
3.1. The Effect of Shear Rate on Structural and Viscous Properties
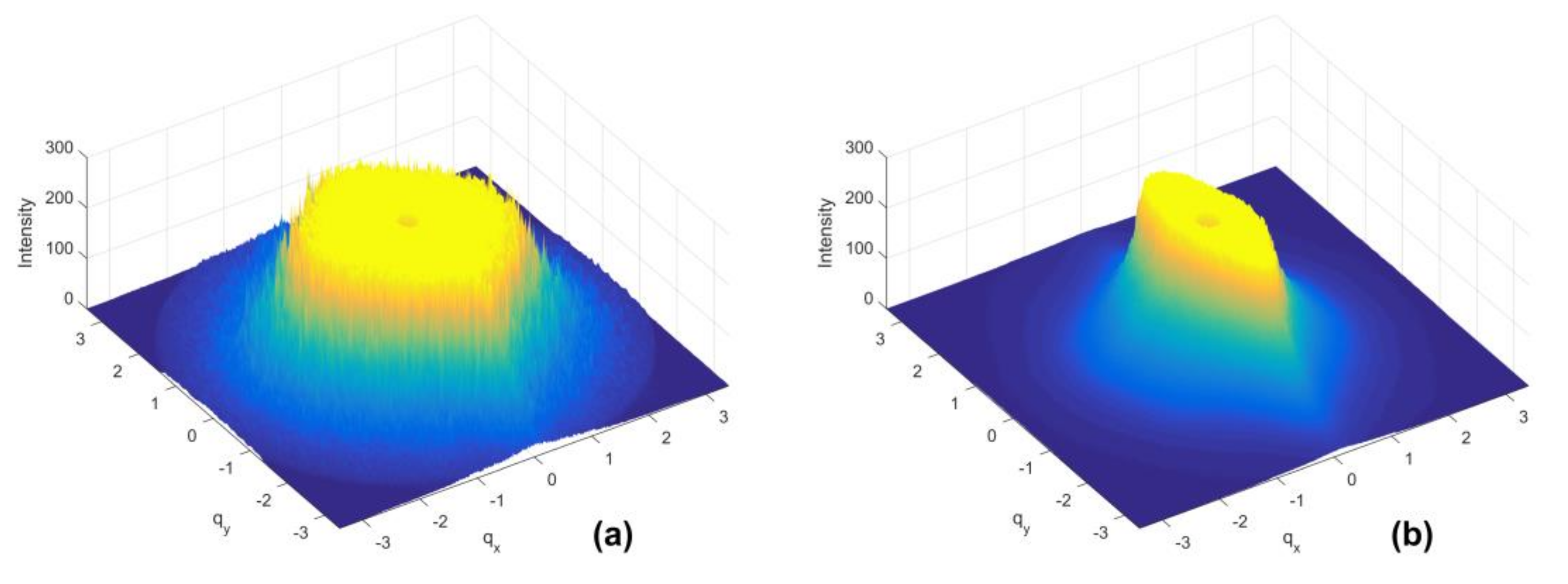
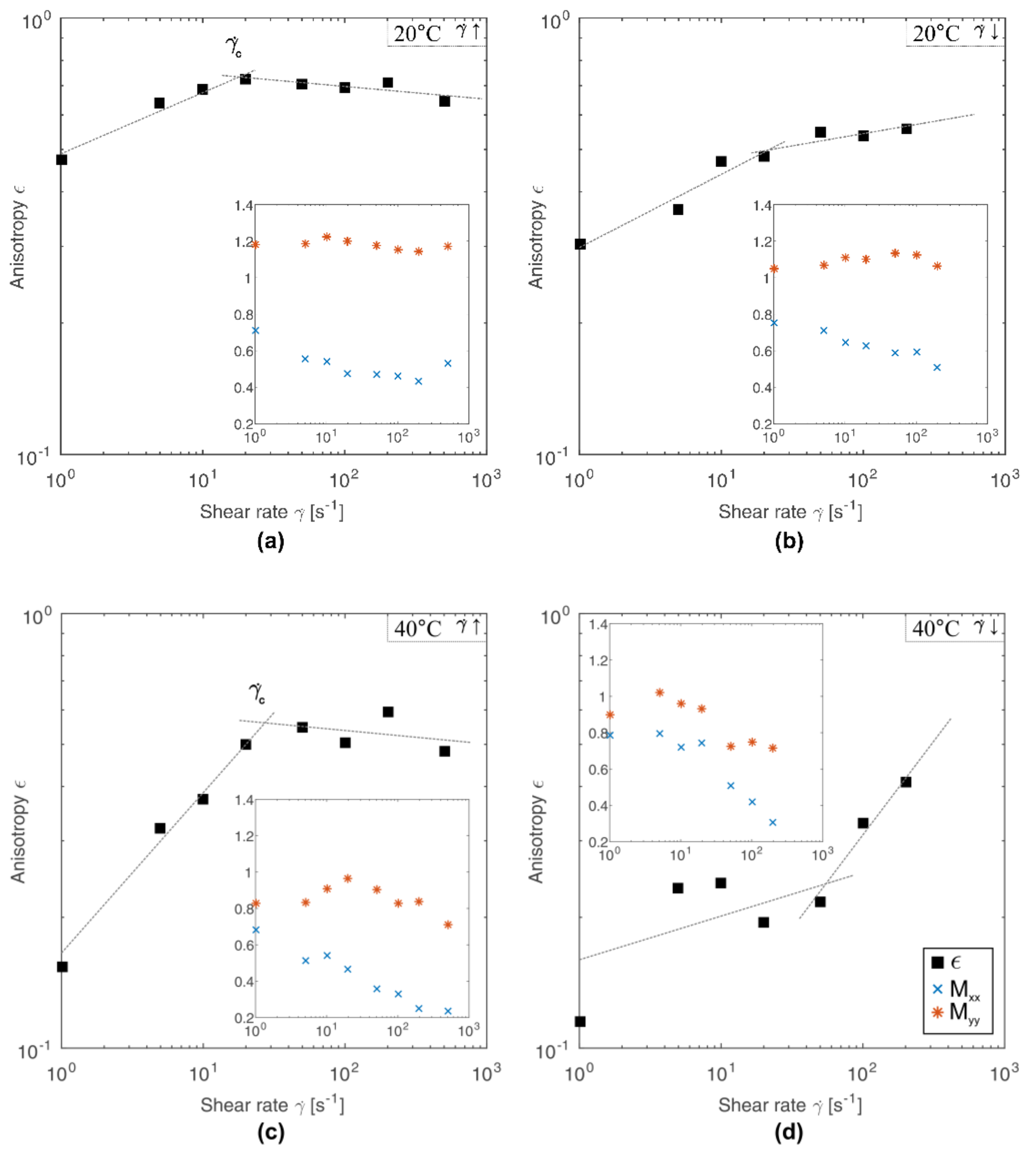
3.2. Quantification of Scattering Pattern Anisotropy
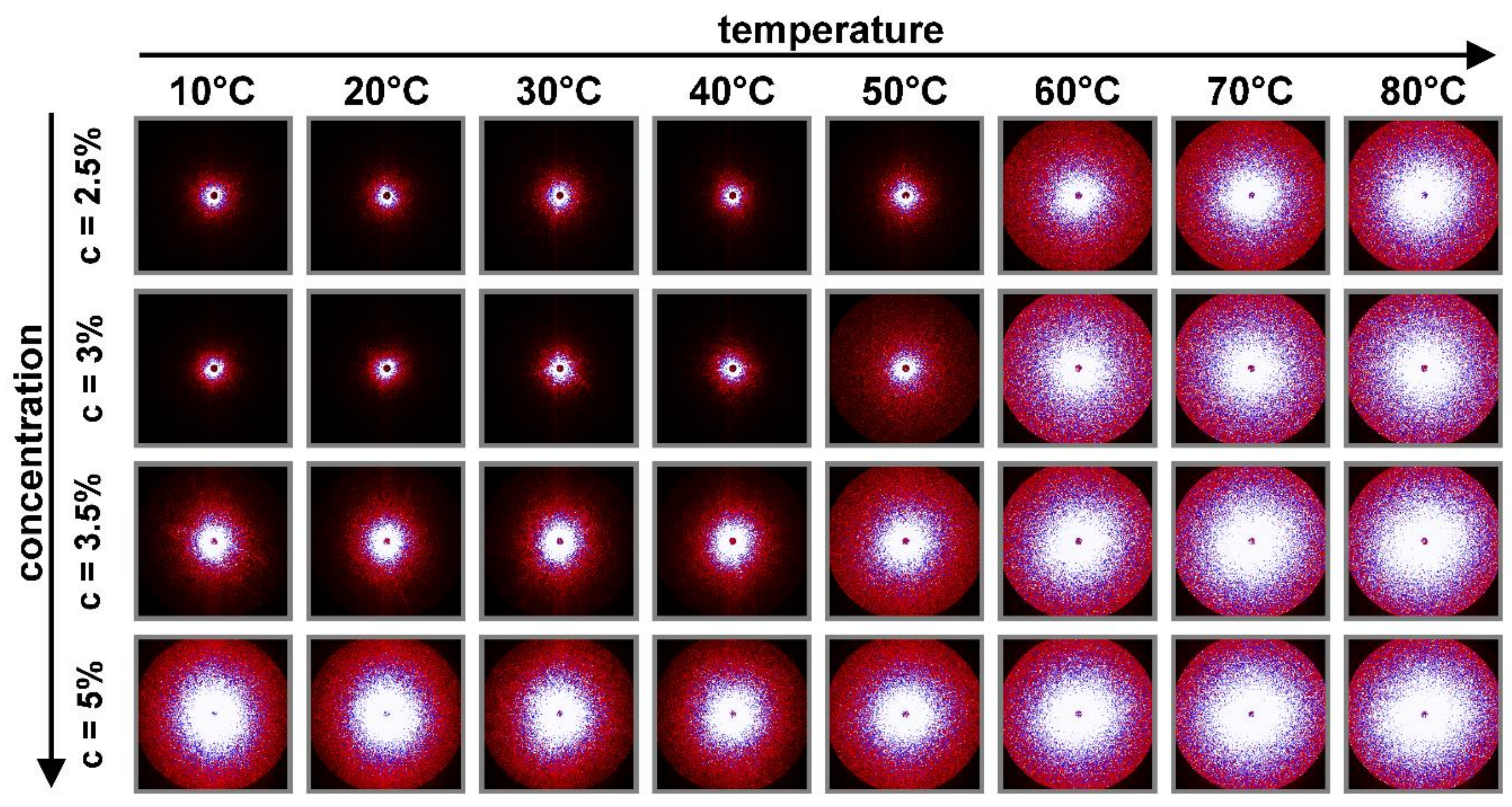
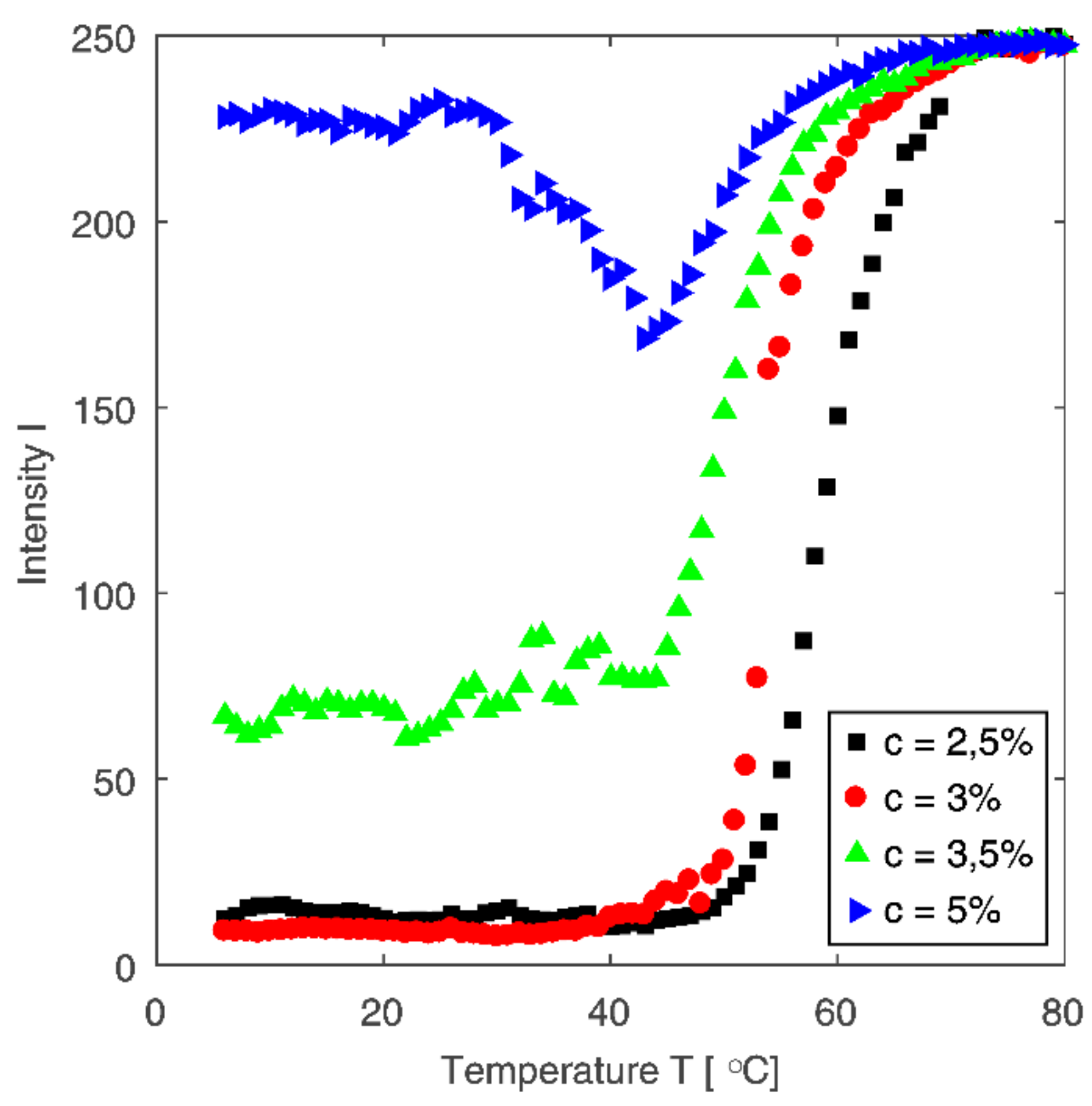
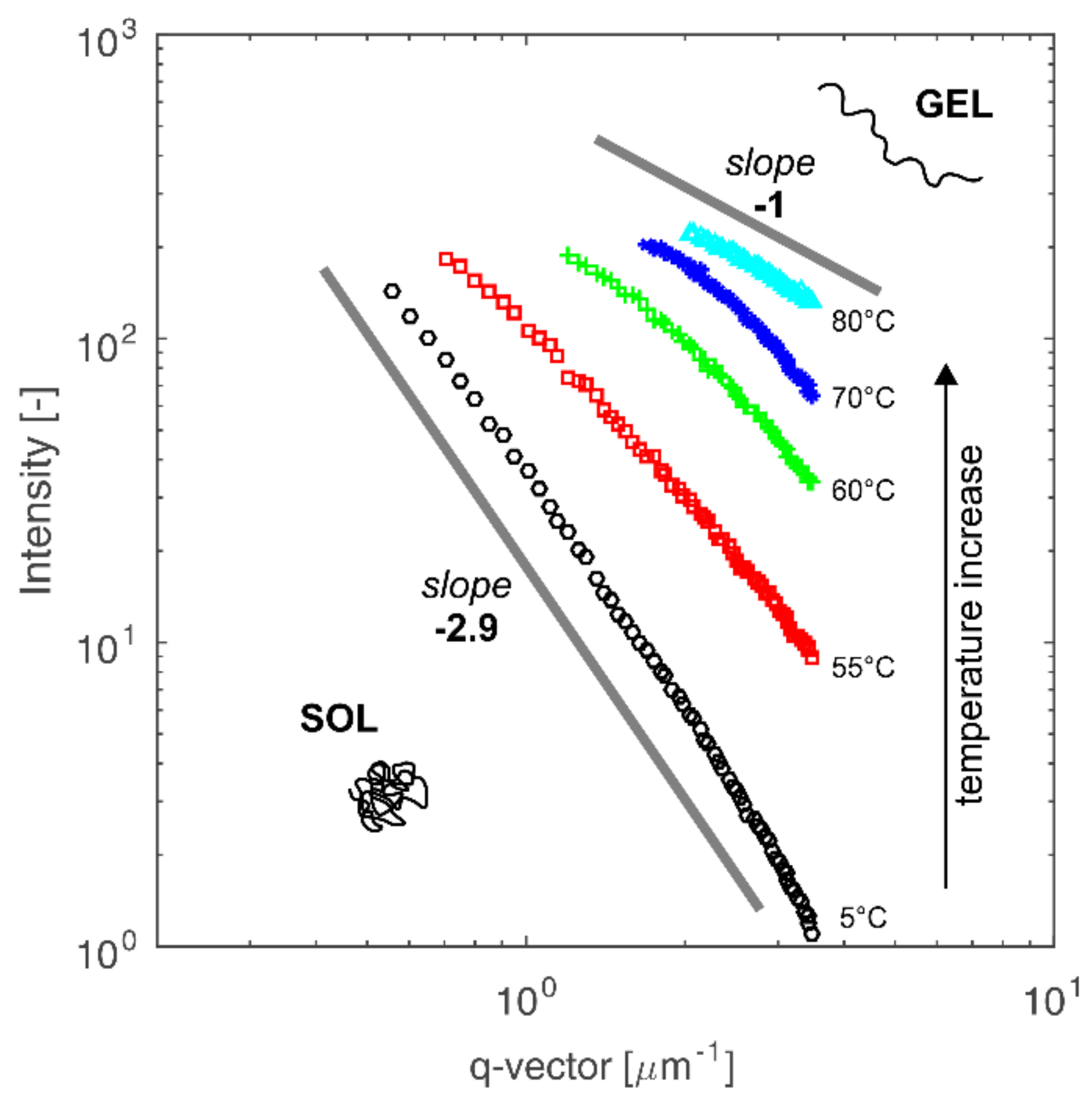
3.3. The Sol-Gel Phase Transition and Temperature of the Gel Point
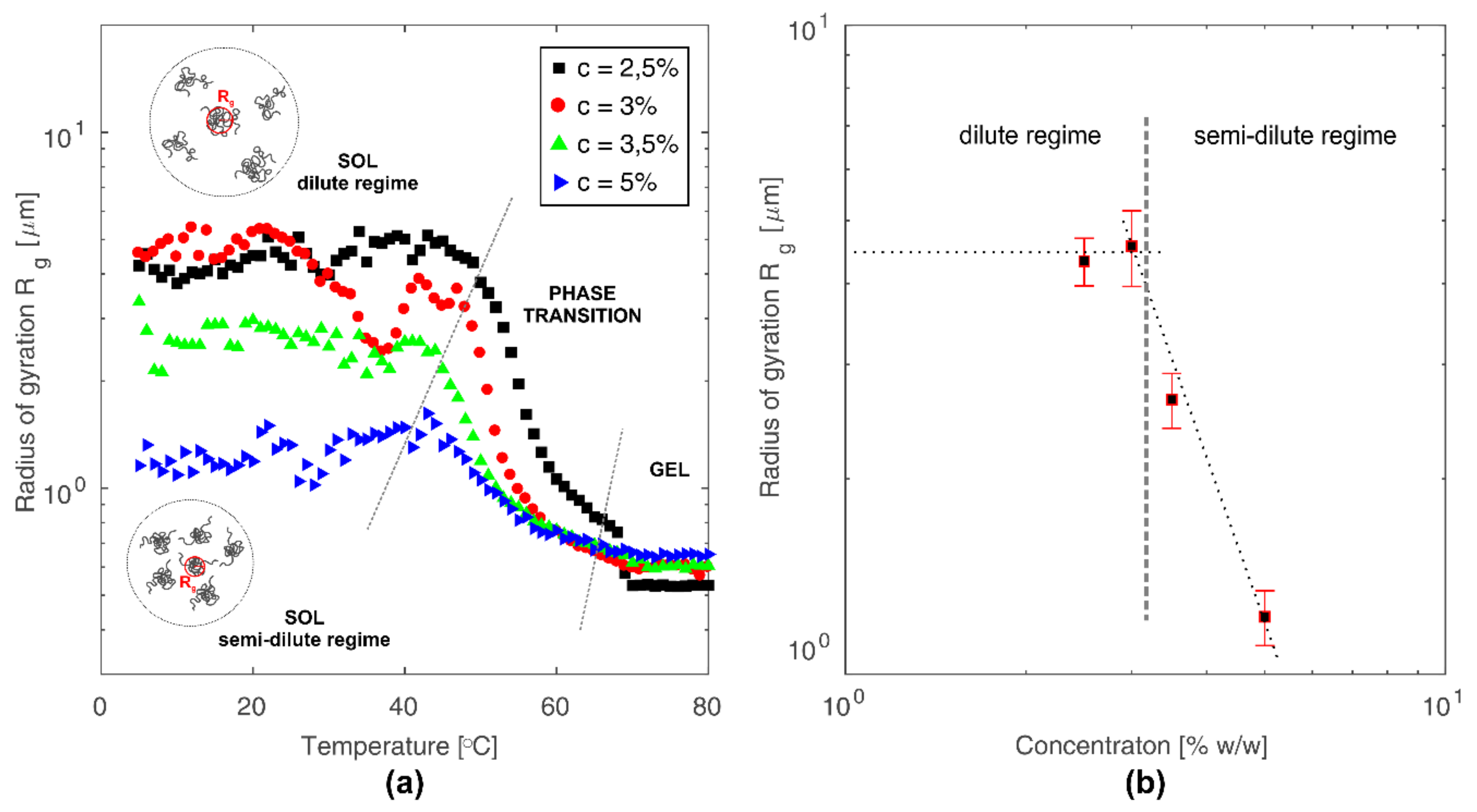
3.4. Radius of Gyration and Chitosan Chains Conformation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crommelin, D.J.A.; Storm, G.; Jiskoot, W.; Stenekes, R.; Mastrobattista, E.; Hennink, W.E. Nanotechnological approaches for the delivery of macromolecules. J. Control. Release Off. J. Control. Release Soc. 2003, 87, 81–88. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical Gelation of Chitosan in the Presence of β-Glycerophosphate: The Effect of Temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, M.; Filion, D.; Buschmann, M.D. Heat-induced transfer of protons from chitosan to glycerol phosphate produces chitosan precipitation and gelation. Biomacromolecules 2008, 9, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Elhefian, E. Rheological study of chitosan and its blends: An overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Cho, J.; Heuzey, M.C.; Begin, A.; Carreau, P.J. Viscoelastic properties of chitosan solutions: Effect of concentration and ionic strength. J. Food Eng. 2006, 74, 500–515. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Chitosan and glycerophosphate concentration dependence of solution behaviour and gel point using small amplitude oscillatory rheometry. Food Hydrocoll. 2006, 20, 936–945. [Google Scholar] [CrossRef]
- Chen, R.H.; Tsaih, M.L. Effect of temperature on the intrinsic viscosity and conformation of chitosans in dilute HCl solution. Int. J. Biol. Macromol. 1998, 23, 135–141. [Google Scholar] [CrossRef]
- Kasaai, M.R. Calculation of Mark-Houwink-Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Christensen, B.E.; Vold, I.M.N.; Vårum, K.M. Chain stiffness and extension of chitosans and periodate oxidised chitosans studied by size-exclusion chromatography combined with light scattering and viscosity detectors. Carbohydr. Polym. 2008, 74, 559–565. [Google Scholar] [CrossRef]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Alvarado-Tenorio, B.; Eugenia Romero-Guzmán, M. A small-angle light scattering instrument to study soft condensed matter. Rev. Latinoam. Metal. Mater. 2010, 30, 190–200. [Google Scholar]
- Nishida, K.; Ogawa, H.; Matsuba, G.; Konishi, T.; Kanaya, T. A high-resolution small-angle light scattering instrument for soft matter studies. J. Appl. Crystallogr. 2008, 41, 723–728. [Google Scholar] [CrossRef]
- Herle, V.; Fischer, P.; Windhab, E.J. Stress Driven Shear Bands and the Effect of Confinement on Their StructuresA Rheological, Flow Visualization, and Rheo-SALS Study. Langmuir 2005, 21, 9051–9057. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Takebe, T.; Suehiro, S. Apparatus to Measure Small-Angle Light Scattering Profiles of Polymers under Shear Flow. Polym. J. 1986, 18, 123–130. [Google Scholar] [CrossRef][Green Version]
- Koenderink, G.H.; Aarts, D.G.A.L.; de Villeneuve, V.W.A.; Philipse, A.P.; Tuinier, R.; Lekkerkerker, H.N.W. Morphology and Kinetics of Phase Separating Transparent Xanthan−Colloid Mixtures. Biomacromolecules 2003, 4, 129–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Läuger, J.; Heyer, P.; Pfeifer, G. A new integrated Rheo Small Angle Light Scattering (Rheo-SALS) device. Annu. Trans. Nord. Rheol. Soc. 2004, Vol. 12, 137–140. [Google Scholar]
- Läuger, J.; Heyer, P. Rheo small angle light scattering (Rheo-SALS) and rheo-microscopy as tools for investigations of structure- property relations in complex fluids. Annu. Trans. Nord. Rheol. Soc. 2006, Vol. 14, 193–196. [Google Scholar]
- DeGroot, J.V.; Macosko, C.W.; Kume, T.; Hashimoto, T. Flow-Induced Anisotropic SALS in Silica-Filled PDMS Liquids. J. Colloid Interface Sci. 1994, 166, 404–413. [Google Scholar] [CrossRef]
- Kume, T.; Hashimoto, T.; Takahashi, T.; Fuller, G.G. Rheo-Optical Studies of Shear-Induced Structures in Semidilute Polystyrene Solutions. Macromolecules 1997, 30, 7232–7236. [Google Scholar] [CrossRef]
- Saito, S.; Hashimoto, T.; Morfin, I.; Lindner, P.; Boué, F. Structures in a Semidilute Polymer Solution Induced under Steady Shear Flow As Studied by Small-Angle Light and Neutron Scattering. Macromolecules 2002, 35, 445–459. [Google Scholar] [CrossRef]
- Tirado-Miranda, M.; Schmitt, A.; Callejas-Fernández, J.; Fernández-Barbero, A. Dynamic scaling and fractal structure of small colloidal clusters. Colloids Surf. Physicochem. Eng. Asp. 2000, 162, 67–73. [Google Scholar] [CrossRef]
- Maleki, A.; Lafitte, G.; Kjøniksen, A.-L.; Thuresson, K.; Nyström, B. Effect of pH on the association behavior in aqueous solutions of pig gastric mucin. Carbohydr. Res. 2008, 343, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J.; Salem, A.J.; Fuller, G.G. Dynamics of colloidal particles in sheared, non-Newtonian fluids. J. Non-Newton. Fluid Mech. 1990, 34, 89–121. [Google Scholar] [CrossRef]
- Vermant, J.; Van Puyvelde, P.; Moldenaers, P.; Mewis, J.; Fuller, G.G. Anisotropy and Orientation of the Microstructure in Viscous Emulsions during Shear Flow. Langmuir 1998, 14, 1612–1617. [Google Scholar] [CrossRef]
- van Egmond, J.W.; Werner, D.E.; Fuller, G.G. Time-dependent small-angle light scattering of shear-induced concentration fluctuations in polymer solutions. J. Chem. Phys. 1992, 96, 7742–7757. [Google Scholar] [CrossRef]
- Liu, Z.; Maleki, A.; Zhu, K.; Kjøniksen, A.-L.; Nyström, B. Intramolecular and intermolecular association during chemical cross-linking of dilute solutions of different polysaccharides under the influence of shear flow. J. Phys. Chem. B 2008, 112, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, G.H.; Larson, R.G. Viscoelasticity of homogeneous polymer melts near a critical point. J. Chem. Phys. 1987, 86, 1553–1560. [Google Scholar] [CrossRef]
- Aliaghaie, M.; Mirzadeh, H.; Dashtimoghadam, E.; Taranejoo, S. Investigation of gelation mechanism of an injectable hydrogel based on chitosan by rheological measurements for a drug delivery application. Soft Matter 2012, 8, 7128–7137. [Google Scholar] [CrossRef]
- Rivas-Araiza, R.; Alcouffe, P.; Rochas, C.; Montembault, A.; David, L. Micron Range Morphology of Physical Chitosan Hydrogels. Langmuir 2010, 26, 17495–17504. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, T.; Ohkura, M.; Kaji, K.; Furusaka, M.; Misawa, M. Structure of Poly(vinyl alcohol) Gels Studied by Wide- and Small-Angle Neutron Scattering. Macromolecules 1994, 27, 5609–5615. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Mirzadeh, H.; Afshar Taromi, F.; Nyström, B. Thermoresponsive biopolymer hydrogels with tunable gel characteristics. RSC Adv. 2014, 4, 39386–39393. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Bahlakeh, G.; Salimi-Kenari, H.; Hasani-Sadrabadi, M.M.; Mirzadeh, H.; Nyström, B. Rheological Study and Molecular Dynamics Simulation of Biopolymer Blend Thermogels of Tunable Strength. Biomacromolecules 2016, 17, 3474–3484. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Debye, P. Molecular-weight Determination by Light Scattering. J. Phys. Colloid Chem. 1947, 51, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Ermi, B.D.; Amis, E.J. Influence of Backbone Solvation on Small Angle Neutron Scattering from Polyelectrolyte Solutions. Macromolecules 1997, 30, 6937–6942. [Google Scholar] [CrossRef]
- Tirado-Miranda, M.; Schmitt, A.; Callejas-Fernández, J.; Fernández-Barbero, A. Colloidal Clusters with Finite Binding Energies: Fractal Structure and Growth Mechanism. Langmuir 1999, 15, 3437–3444. [Google Scholar] [CrossRef]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; John Wiley & Sons, Inc.: New York, NY, USA, 2002; ISBN 0-471-22451-0. [Google Scholar]
- Owczarz, P.; Ziółkowski, P.; Modrzejewska, Z.; Kuberski, S.; Dziubiński, M. Rheo-Kinetic Study of Sol-Gel Phase Transition of Chitosan Colloidal Systems. Polymers 2018, 10, 47. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarz, P.; Ziółkowski, P.; Dziubiński, M. The Application of Small-Angle Light Scattering for Rheo-Optical Characterization of Chitosan Colloidal Solutions. Polymers 2018, 10, 431. https://doi.org/10.3390/polym10040431
Owczarz P, Ziółkowski P, Dziubiński M. The Application of Small-Angle Light Scattering for Rheo-Optical Characterization of Chitosan Colloidal Solutions. Polymers. 2018; 10(4):431. https://doi.org/10.3390/polym10040431
Chicago/Turabian StyleOwczarz, Piotr, Patryk Ziółkowski, and Marek Dziubiński. 2018. "The Application of Small-Angle Light Scattering for Rheo-Optical Characterization of Chitosan Colloidal Solutions" Polymers 10, no. 4: 431. https://doi.org/10.3390/polym10040431
APA StyleOwczarz, P., Ziółkowski, P., & Dziubiński, M. (2018). The Application of Small-Angle Light Scattering for Rheo-Optical Characterization of Chitosan Colloidal Solutions. Polymers, 10(4), 431. https://doi.org/10.3390/polym10040431