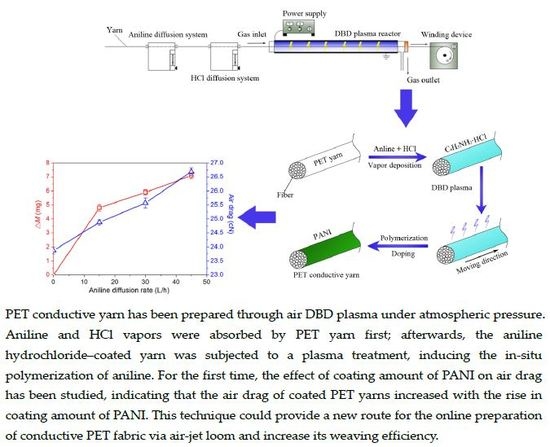
The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn
Abstract
:1. Introduction
2. Materials and Methods
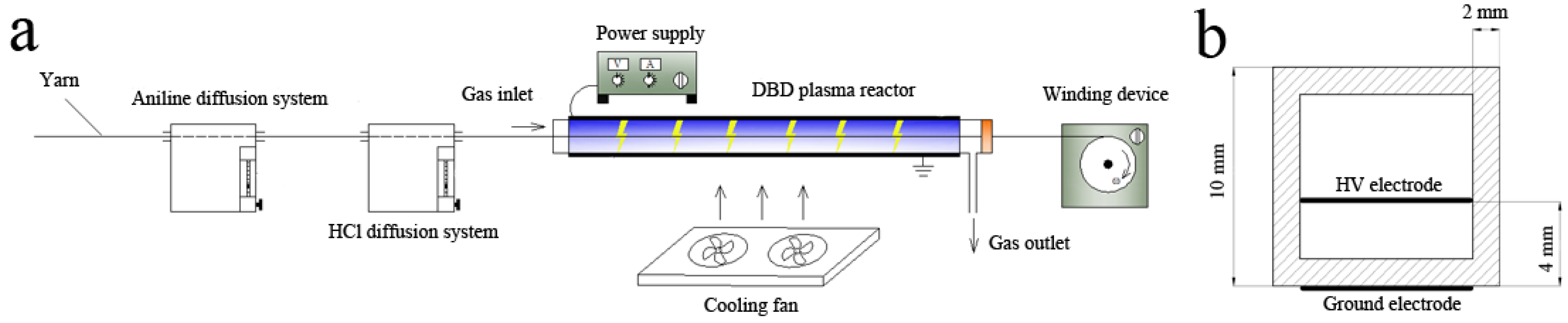
2.1. Experimental Setup and DBD Plasma Treatment Details
2.2. Materials and Reagents
2.3. Yarn Conductivity Test
2.4. Air Drag Measurement
2.5. Yarn Diameter Measurement
2.6. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.7. Fourier Transform-Infrared (FT-IR) Spectroscopy Analyses
2.8. X-Ray Photoelectron Spectroscopy (XPS) Analyses
3. Results and Discussion
3.1. The Polymerization of PANI via DBD Plasma and the Effect of the Coating Amount of PANI on Volume Resistivity
3.2. The Effect of Coating Amount of PANI on Air Drag of PET Yarn
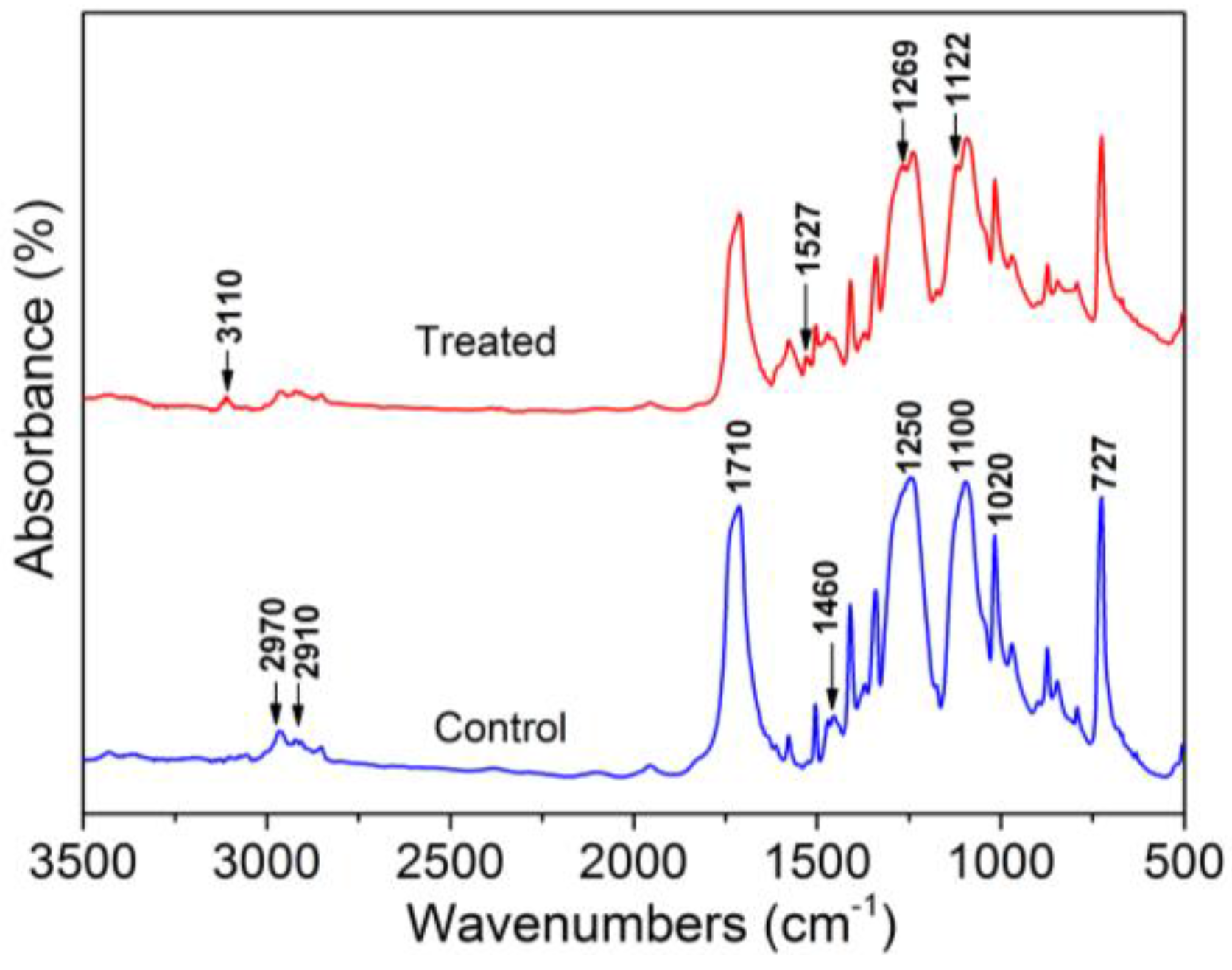
3.3. Chemical Characterization of Treated PET Yarn Surfaces
3.4. Surface Morphology Characterization of Treated PET Yarns
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Yang, L.; Chen, J.; Guo, Y.; Zhang, L. Surface modification of a biomedical polyethylene terephthalate (PET) by air plasma. Appl. Surf. Sci. 2009, 255, 4446–4451. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, S.; Huang, G. Anti-static charge character of the plasma treated PET filter fabric. J. Electrost. 2010, 68, 111–115. [Google Scholar]
- Lawal, A.T.; Wallace, G.G. Vapor phase polymerization of conducting and non-conducting polymers: A review. Talanta 2014, 119, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANI doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. 2011, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.Z.; Shi, M.M.; Deng, M.; Wang, M.; Chen, H.Z. Morphology and conductivity of polyaniline sub-micron fibers prepared by electrospinning. Mater. Sci. Eng. B 2008, 150, 70–76. [Google Scholar] [CrossRef]
- Zhang, F.; Halverson, P.A.; Lunt, B.; Linford, M.R. Wet spinning of pre-doped polyaniline into an aqueous solution of a polyelectrolyte. Synth. Met. 2006, 156, 932–937. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Jin, H.F.; Wang, X.H.; Jing, X.B. Morphology of conductive blend fibers of polyaniline and polyamide-11. Synth. Met. 2001, 123, 481–485. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Sun, Z.C.; Li, J. Conductive polyaniline/poly-ω-aminoundecanoyle blending fiber. Synth. Met. 1999, 102, 1198–1199. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, H.J.; Lee, H.A.; Ryu, K.S. Fabrication and physical properties of conductive polyacrylonitrile-polyaniline derivative fibers. Synth. Met. 2003, 135, 399–400. [Google Scholar] [CrossRef]
- Gregory, R.V.; Kimbrell, W.C.; Kuhn, H.H. Conductive textiles. Synth. Met. 1989, 28, 823–835. [Google Scholar] [CrossRef]
- Kim, B.; Koncar, V.; Devaux, E.; Dufour, C.; Viallier, P. Electrical and morphological properties of PP and PET conductive polymer fibers. Synth. Met. 2004, 146, 167–174. [Google Scholar] [CrossRef]
- Oh, K.W.; Kim, S.H.; Kim, E.A. Improved surface characteristics and the conductivity of polyaniline-nylon 6 fabrics by plasma treatment. J. Appl. Polym. Sci. 2010, 81, 684–694. [Google Scholar] [CrossRef]
- Jin, X.; Xiao, C.; Wang, W. Electrical and mechanical properties of novel polyaniline coated polycaprolactam fibers. Synth. Met. 2010, 160, 368–372. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Singh, N.; Venkatachalam, S. Shielding behaviour of conducting polymer-coated fabrics in X-band, W-band and radio frequency range. Synth. Met. 2002, 129, 261–267. [Google Scholar] [CrossRef]
- Kim, B.; Koncar, V.; Dufour, C. Polyaniline-coated PET conductive yarns: Study of electrical, mechanical, and electro-mechanical properties. J. Appl. Polym. Sci. 2006, 101, 1252–1256. [Google Scholar] [CrossRef]
- Hirase, R.; Shikata, T.; Shirai, M. Selective formation of polyaniline on wool by chemical polymerization, using potassium iodate. Synth. Met. 2004, 146, 73–77. [Google Scholar] [CrossRef]
- Goswami, S.; Mitra, M.K.; Chattopadhyay, K.K. Enhanced field emission from polyaniline nano-porous thin films on PET substrate. Synth. Met. 2009, 159, 2430–2436. [Google Scholar] [CrossRef]
- Kutanis, S.; Karakisla, M.; Akbulut, U.; Sacak, M. The conductive polyaniline/poly(ethylene terephthalate) composite fabrics. Compos. Part A 2007, 38, 609–614. [Google Scholar] [CrossRef]
- Neelakandan, R.; Madhusoothanan, M. Electrical resistivity studies on polyaniline coated PET fabrics. J. Eng. Fibers Fabr. 2010, 5, 16–24. [Google Scholar]
- Nouri, M.; Kish, M.H.; Entezami, A.A.; Edrisi, M. Conductivity of textile fibers treated with aniline. Iran. Polym. J. 2000, 9, 49–58. [Google Scholar]
- Bai, S.; Ye, J.; Luo, R.; Chen, A.; Li, D. Hierarchical polyaniline microspheres loading on flexible PET films for NH3 sensing at room temperature. RSC Adv. 2016, 6, 6939–6945. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Cai, Z.S.; Zhou, Z.Y.; Fu, X.L. Fabrication of conductive network formed by polyaniline-ZnO composite on fabric surfaces. Thin Solid Films 2011, 519, 5887–5891. [Google Scholar] [CrossRef]
- Huang, H.; Liu, W. Polyaniline/poly(ethylene terephthalate)conducting composite fabric with improved fastness to washing. J. Appl. Polym. Sci. 2006, 102, 5775–5780. [Google Scholar] [CrossRef]
- Seki, Y.; Sarikanat, M.; Sever, K.; Erden, S.; Gulec, H.A. Effect of the low and radio frequency oxygen plasma treatment of jute fiber on mechanical properties of jute fiber/PET composite. Fibers Polym. 2010, 11, 1159–1164. [Google Scholar] [CrossRef]
- Yuan, X.W.; Jayaraman, K.; Bhattacharyya, D. Mechanical properties of plasma-treated sisal fibre-reinforced polypropylene composites. J. Adhes. Sci. Technol. 2004, 18, 1027–1045. [Google Scholar] [CrossRef]
- Li, R.Z.; Ye, L.; Mai, Y.W. Application of plasma technologies in fibre-reinforced polymer composites: A review of recent developments. Compos. Part A 1997, 28, 73–86. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Verschuren, J.; Clerck, K.D.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Marais, S.; Gouanve, F.; Bonnesoeur, A.; Grenet, J.; Poncin-Epaillard, F.; Morvanm, C.; Métayer, M. Unsaturated PET composites reinforced with flax fibers: Effect of cold plasma and autoclave treatments on mechanical and permeation properties. Compos. Part A 2005, 36, 975–986. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anantharaman, M.R.; Venkitachalam, S.; Jayalekshmi, S. Characterization of low dielectric constant polyaniline thin film synthesized by ac plasma polymerization technique. J. Phys. D Appl. Phys. 2002, 35, 240–245. [Google Scholar] [CrossRef]
- Lakshmi, G.B.V.S.; Dhillon, A.; Siddiqui, A.M.; Zulfequar, M.; Avasthi, D.K. RF-plasma polymerization and characterization of polyaniline. Eur. Polym. J. 2009, 45, 2873–2877. [Google Scholar] [CrossRef]
- Cruz, G.J.; Morales, J.; Castillo-Ortega, M.M.; Olayo, R. Synthesis of polyaniline films by plasma polymerization. Synth. Met. 1997, 88, 213–218. [Google Scholar] [CrossRef]
- Olayo, M.G.; Morales, J.; Cruz, G.J.; Olayo, R.; Ordonez, E.; Barocio, S.R. On the influence of electron energy on iodine-doped polyaniline formation by plasma polymerization. J. Polym. Sci. Polym. Phys. 2001, 39, 175–183. [Google Scholar] [CrossRef]
- Nastase, C.; Nastase, F.; Dumitru, A.; Ionescu, M.; Stamatin, I. Thin film composites of nanocarbons-polyaniline obtained by plasma polymerization technique. Compos. Part A 2005, 36, 481–485. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. ACTA B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Qiu, Y. Surface modification of ultrahigh modulus polyethylene fibers by an atmospheric pressure plasma jet. J. Appl. Polym. Sci. 2008, 108, 25–33. [Google Scholar] [CrossRef]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Kim, D.Y.; Lee, H.-K.; Shin, B.J.; Lee, D.H.; Tae, H.-S. Atmospheric Pressure Plasma Polymerization Synthesis and Characterization of Polyaniline Films Doped with and without Iodine. Materials 2017, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, D.S.; Salunkhe, R.R.; Jamadade, V.S.; Dubal, D.P.; Pawar, S.M.; Lokhande, C.D. Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Z.; Liu, D.; Zhang, X.; Zhang, L. Response of air drag force of the polyethylene terephthalate (PET) yarn treated via dielectric barrier discharge (DBD) plasma to its varying surface characteristics. Text. Res. J. 2015, 86, 2140–2150. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Ma, Y.; Su, Y.; Wu, M.; Feng, Z.; Liu, D. Research on the Effect of Dielectric Barrier Discharge (DBD) Plasma Remote Treatment on Drag Force of Polyethylene Terephthalate (PET) Yarns in Air Flow with Different Humidities. Plasma Chem. Plasma Process. 2017, 37, 1573–1586. [Google Scholar] [CrossRef]
- Detsri, E.; Dubas, S.T. Interfacial Polymerization of Water-Soluble Polyaniline and Its Assembly Using the Layer-By-Layer Technique. JOM 2009, 19, 39–44. [Google Scholar]
- Wang, Y.; Jing, X.; Kong, J. Polyaniline nanofibers prepared with hydrogen peroxide as oxidant. Synth. Met. 2007, 157, 269–275. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Qian, X.; Shen, J.; An, X. Polyaniline/cellulose fiber composite prepared using persulfate as oxidant for Cr(VI)-detoxification. Carbohyd. Polym. 2013, 92, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pei, Y.; Xie, D. Surface modification of ultra-high molecularweight polyethylene by argon plasma. Appl. Surf. Sci. 2010, 256, 3941–3945. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Ebrahimi, I. Atmospheric air-plasma treatment of polyester fiber to improve the performance of nanoemulsion silicone. Appl. Surf. Sci. 2011, 257, 4062–4068. [Google Scholar] [CrossRef]
- Andanson, J.M.; Kazarian, S.G. In situ ATR-FTIR Spectroscopy of Poly(ethylene terephthalate) Subjected to High-Temperature Methanol. Macromol. Symp. 2008, 265, 195–204. [Google Scholar] [CrossRef]
- Tiwari, A.; Sen, V.; Dhakate, S.R.; Mishra, A.P.; Singh, V. Synthesis, characterization, and hoping transport properties of HCl doped conducting biopolymer-co-polyaniline zwitterion hybrids. Polym. Adv. Technol. 2008, 19, 909–914. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V. Microwave-induced synthesis of electrical conducting gum acacia-graft-polyaniline. Carbohydr. Polym. 2008, 74, 427–434. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Iroh, J.O. Characterization of polyaniline-polypyrrole composite coatings on low carbon steel: A XPS and infrared spectroscopy study. Appl. Surf. Sci. 2003, 218, 58–69. [Google Scholar] [CrossRef]
- Sabbatini, L.; Malitesta, C.; De Giglio, E.; Losito, I.; Torsi, L.; Zambonin, P.G. Electrosynthesised thin polymer films: The role of XPS in the design of application oriented innovative materials. J. Electron. Spectrosc. Relat. Phenom. 1999, 100, 35–53. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, P.; MacDiarmid, A.G. Selective Deposition of Conducting Polymers on Hydroxyl-Terminated Surfaces with Printed Monolayers of Alkylsiloxanes as Templates. Lamgmuir 1997, 13, 6480–6484. [Google Scholar] [CrossRef]

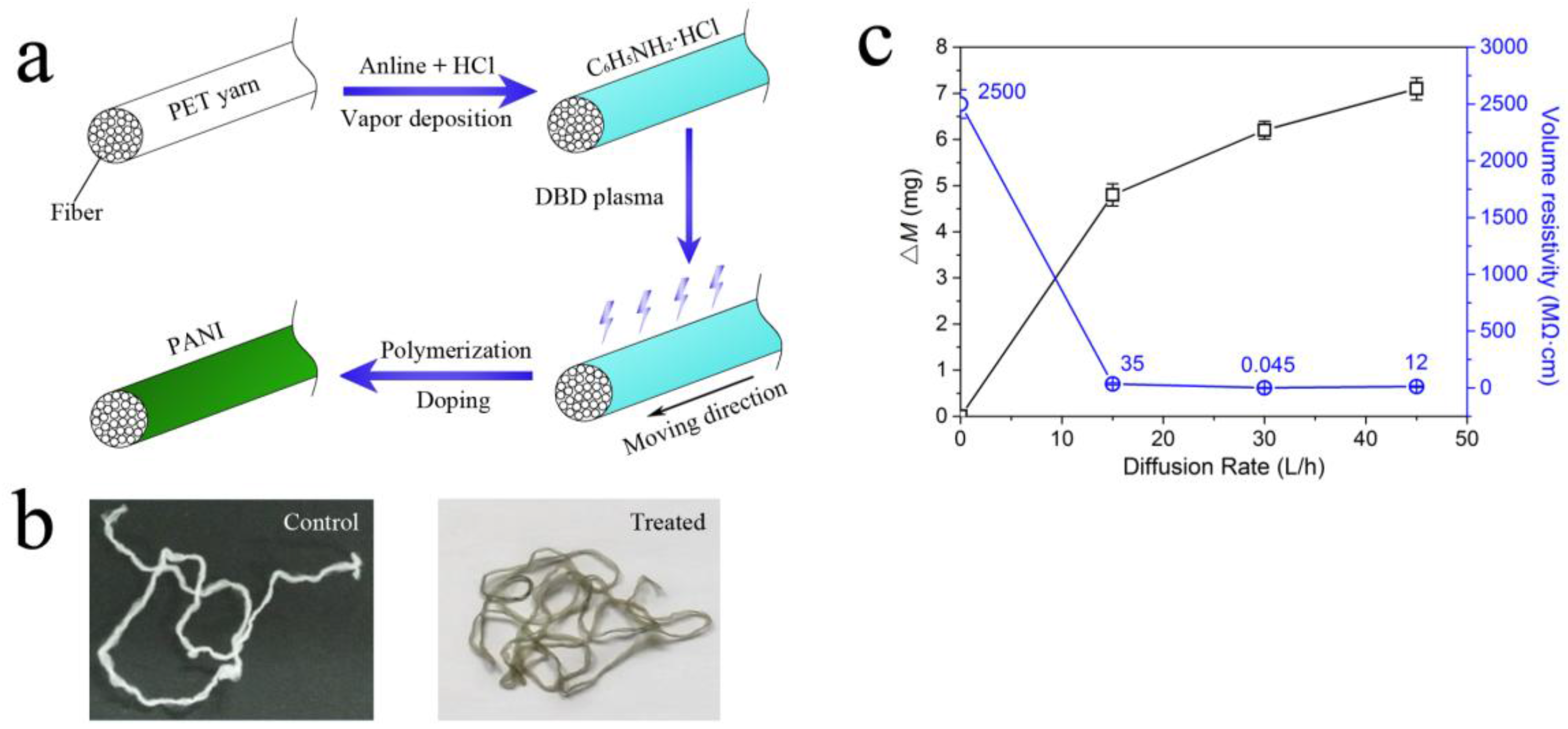


| Diffusion rate (L/h) | ΔM (mg) | d (μm) | Fd (cN) | Fd/d (cN/μm) |
|---|---|---|---|---|
| 0 | 0 | 251.6 | 23.87 | 0.094 |
| 15 | 4.8 | 252.3 | 24.88 | 0.099 |
| 30 | 5.9 | 251.1 | 25.57 | 0.102 |
| 45 | 7.1 | 252.4 | 26.69 | 0.106 |
| Element | Control | Treated |
|---|---|---|
| C1s | 75.8 | 74.9 |
| O1s | 24.2 | 19.2 |
| N1s | 0 | 4.6 |
| Cl2p | 0 | 1.3 |
| C1s and N1s groups | ||
| C–H/C–C | 67.4 | 62.6 |
| C–O/C–N | 22.9 | 25.7 |
| O=C–O | 9.7 | 11.7 |
| =N– | 0 | 34.7 |
| –NH– | 0 | 39.7 |
| N+ | 0 | 25.6 |
| N+/NTotal | - | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, D.; Pan, Z. The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn. Polymers 2018, 10, 351. https://doi.org/10.3390/polym10040351
Liu S, Liu D, Pan Z. The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn. Polymers. 2018; 10(4):351. https://doi.org/10.3390/polym10040351
Chicago/Turabian StyleLiu, Shuai, Deqi Liu, and Zhijuan Pan. 2018. "The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn" Polymers 10, no. 4: 351. https://doi.org/10.3390/polym10040351
APA StyleLiu, S., Liu, D., & Pan, Z. (2018). The Effect of Polyaniline (PANI) Coating via Dielectric-Barrier Discharge (DBD) Plasma on Conductivity and Air Drag of Polyethylene Terephthalate (PET) Yarn. Polymers, 10(4), 351. https://doi.org/10.3390/polym10040351