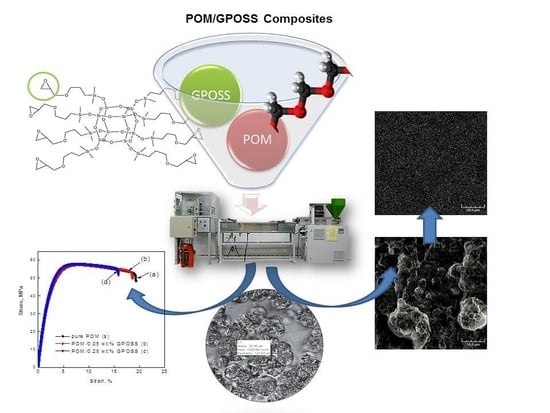
Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the POM/GPOSS Nanocomposites
2.3. Characterization
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Optical Polarized Light Microscopy (PLM)
2.3.3. Wide-Angle X-ray Diffraction (WAXD)
2.3.4. Mechanical Properties
2.3.5. Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. Effect of GPOSS on Melting and Crystallinity
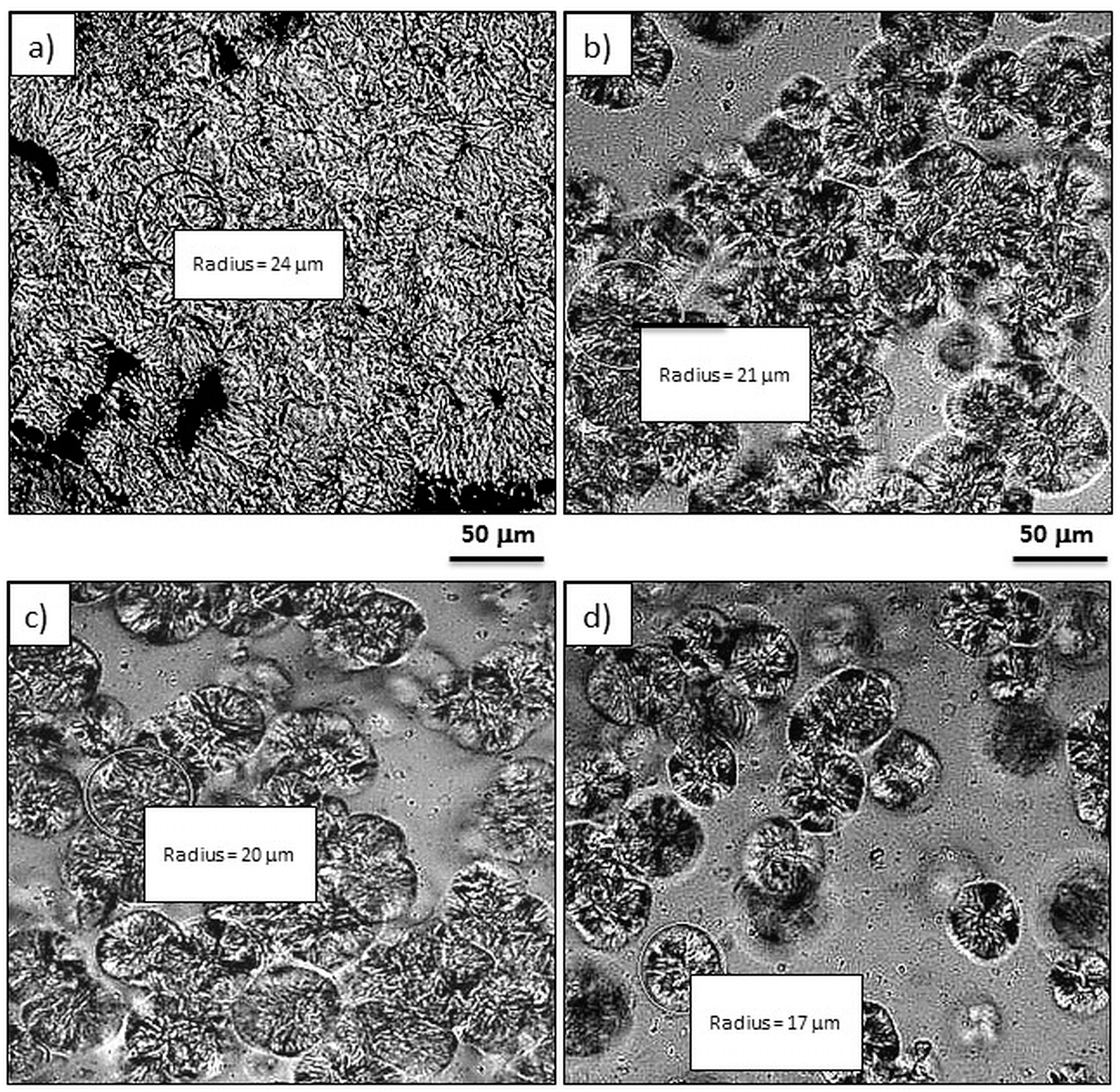
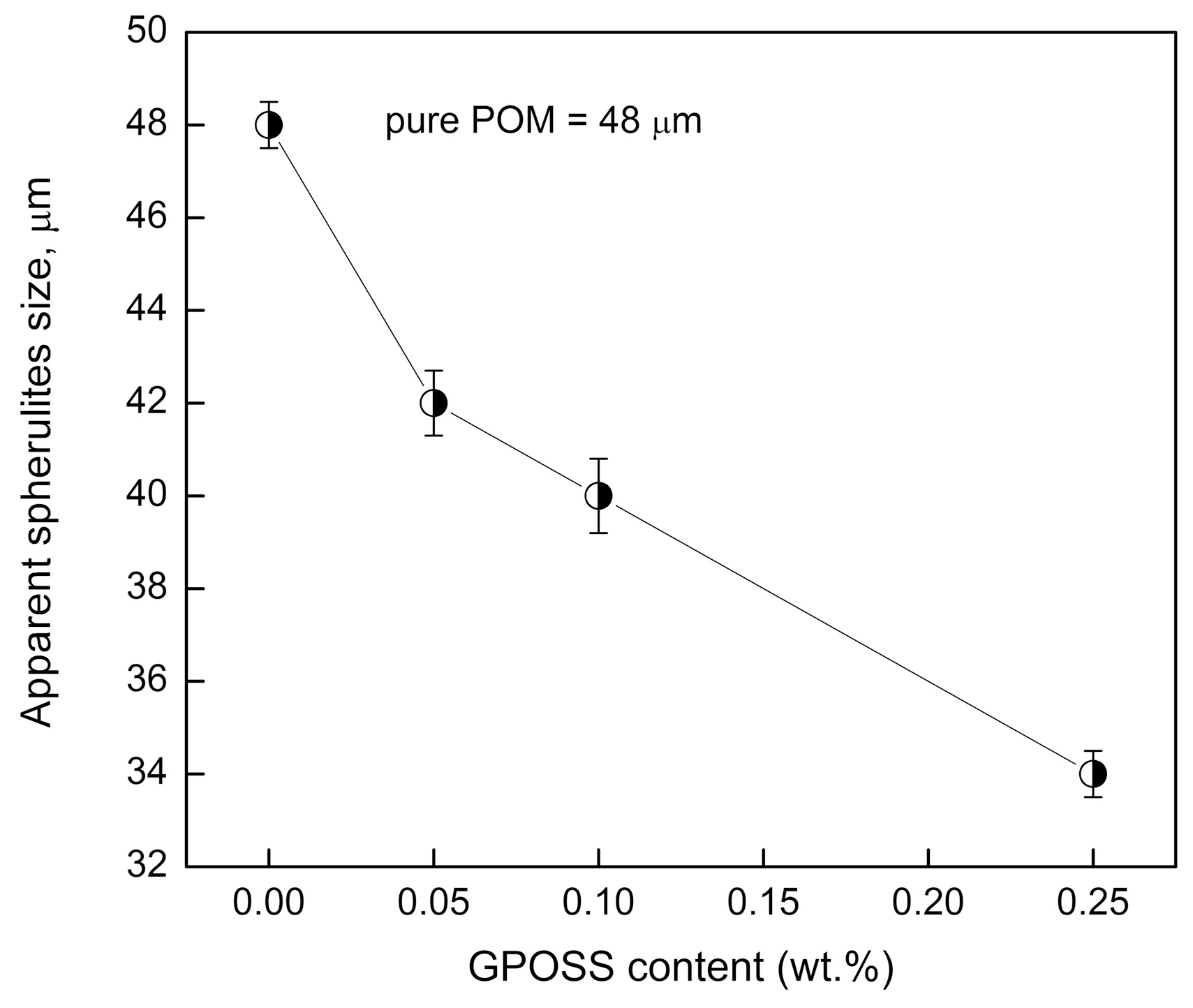
3.2. Effect of GPOSS on the Spherulitic Morphology
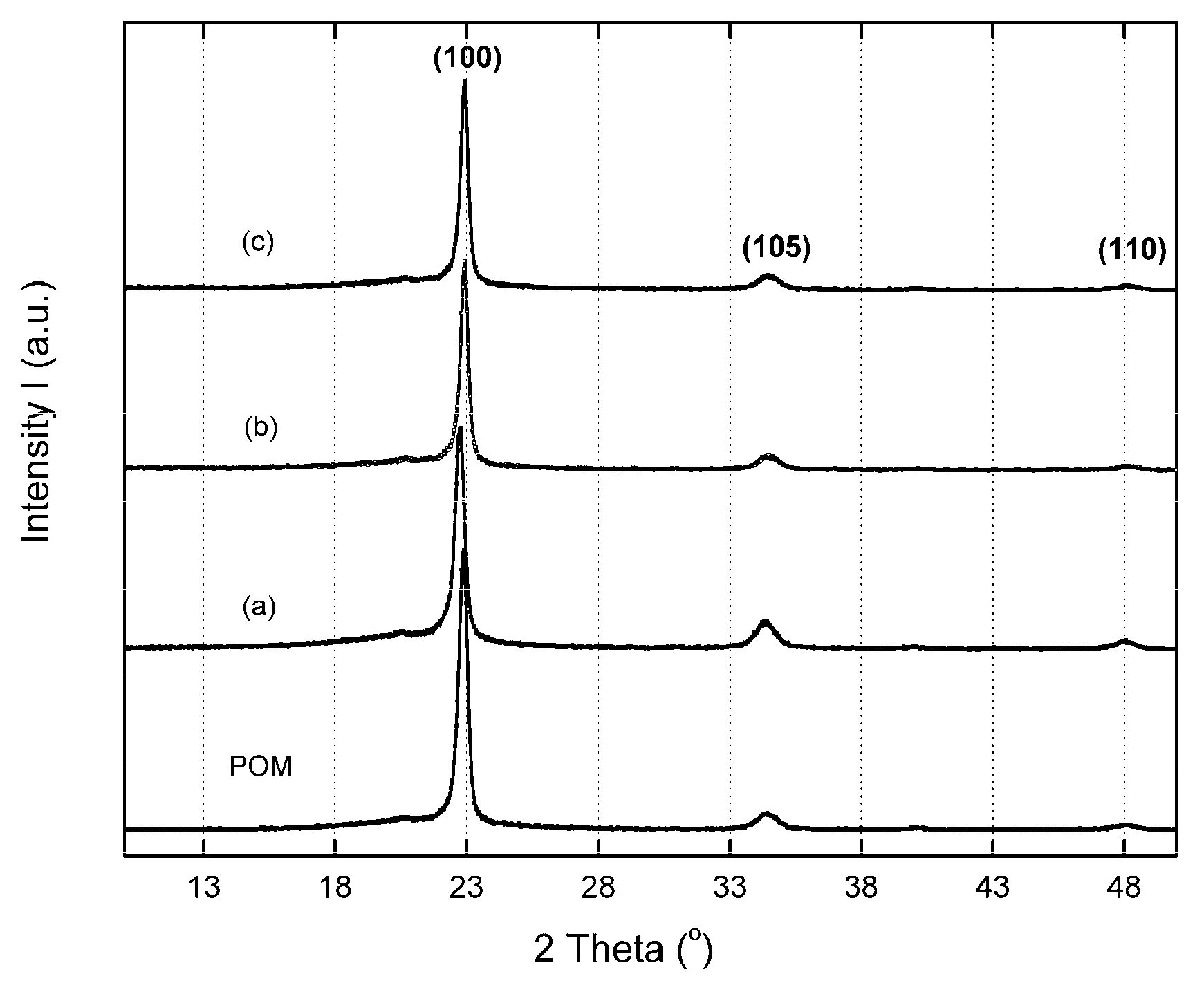
3.3. Effect of GPOSS on the Crystal Structure
3.4. Effect of GPOSS on Mechanical Behaviour
3.5. Effect of GPOSS on Morphology
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sigrid, L.; Visakhn, P.M.; Chandran, S. Polyoxymethylene Handbook; Scrivener Publishing: Beverly, MA, USA, 2014. [Google Scholar]
- Paula, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Piesowicz, E.; Irska, I.; Bryll, K.; Gawdzińska, K.; Bratychak, M. Poly(butylene terephthalate/carbon nanotubes nanocomposites. Part II. Structure and properties. Polimery 2016, 24, 61. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Barczewski, M.; Czarnecka-Komorowska, D.; Andrzejewski, J.; Sterzyński, T.; Dutkiewicz, M.; Dudziec, B. Właściwości przetwórcze termoplastycznych tworzyw polimerowych modyfikowanych silseskwioksanami (POSS). Polimery 2013, 58, 805–815. [Google Scholar] [CrossRef]
- Maciejewski, H.; Dutkiewicz, M.; Byczyński, Ł.; Marciniec, B. Silseskwioksany jako nanonapełniacze. Cz. I. Nanokompozyty z osnową silikonową. Polimery 2012, 57, 535–544. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Marciniec, B. Synteza i charakterystyka silseskwioksanów o strukturze niecałkowicie skondensowanych klatek. Polimery 2013, 10, 741–747. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Mohamed, G.M.; Kuo, S.W. Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, H.; Yu, D.; Feng, L.; Dai, L.; Wang, X.; Lin, T. Superhydrophobic electrospun POSS-PMMA copolymer fibres with highly ordered nanofibrillar and surface structures. Chem. Commun. 2009, 42, 6418–6420. [Google Scholar] [CrossRef] [PubMed]
- Furuheim, K.M.; Axelson, D.E.; Antonsen, H.W.; Helle, T. Phase structural analyses of polyethylene extrusion coatings on high-density papers. I. Monoclinic crystallinity. J. Appl. Polym. Sci. 2004, 91, 218–225. [Google Scholar] [CrossRef]
- Barczewski, M.; Dudziec, B.; Dobrzyńska-Mizera, M.; Sterzyński, T. Synthesis and Influence of Sodium Benzoate Silsesquioxane Based Nucleating Agent on Thermal and Mechanical Properties of Isotactic Polypropylene. J. Macromol. Sci. A 2014, 51, 907–913. [Google Scholar] [CrossRef]
- Barczewski, M.; Dudziec, B.; Dobrzyńska-Mizera, M.; Sterzyński, T. Influence of the cooling rate on the non-isothermal crystallization of isotactic polypropylene modified with sorbitol derivative and silsesquioxane. Polimery 2013, 58, 920–923. [Google Scholar] [CrossRef]
- Didane, N.; Giraud, S.; Devaux, É.; Lemort, G. Development of fire resistant PET fibrous structures based on phosphinate-POSS blends. Polym. Degrad. Stab. 2012, 97, 879–885. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, Y.G. Preparation and crystallization behavior of polylactide nanocomposites reinforced with POSS-modified montmorillonite. Fibers Polym. 2011, 12, 180–189. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Szołyga, M.; Maciejewski, H.J. Thiirane functional spherosilicate as epoxy resin modifier. J. Therm. Anal. Calorim. 2014, 117, 259–264. [Google Scholar] [CrossRef]
- Pielichowski, K.; Jancia, M.; Hebda, E.; Pagacz, J.; Pielichowski, J.; Marciniec, B.; Franczyk, A. Poliuretany modyfikowane funkcjonalizowanym silseskwioksanem-synteza i właściwości. Polimery 2013, 58, 783–793. [Google Scholar] [CrossRef]
- Illescas, S.; Arostegui, A.; Schiraldi, D.A.; Sánchez-Soto, M.; Velasco, J.I. The Role of Polyhedral Oligomeric Silsesquioxane on the Thermo-Mechanical Properties of Polyoxymethylene Copolymer Based Nanocomposites. J. Nanosci. Nanotechnol. 2010, 10, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Komorowska, D.; Sterzynski, T.; Dutkiewicz, M. Polyhedral oligomeric silsesquioxanes as modifiers of polyoxymethylene structure. AIP Conf. Proc. 2015, 1695, 020013. [Google Scholar] [CrossRef]
- Farmahini-Farahani, M.; Xiao, H.; Zhao, Y. Poly lactic acid nanocomposites containing modified nanoclay with synergistic barrier to water vapor for coated paper. J. Appl. Polym. Sci. 2014, 131, 40952. [Google Scholar] [CrossRef]
- Colomines, G.; Ducruet, V.; Courgneau, C.; Guinault, A.; Domenek, S. Barrier properties of poly(lactic acid) and its morphological changes induced by aroma compound sorption. Polym. Int. 2010, 59, 818–826. [Google Scholar] [CrossRef]
- Philips, S.H.; Haddad, T.S.; Tomczak, S.J. Developments in nanoscience: Polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr. Opin. Solid State Mater. Sci. 2004, 8, 21–29. [Google Scholar] [CrossRef]
- Hossain, M.D.; Yoo, Y.; Lim, K.T. Synthesis of poly(ε-caprolactone)/clay nanocomposites using polyhedral oligomeric silsesquioxane surfactants as organic modifier and initiator. J. Appl. Polym. Sci. 2011, 119, 936–943. [Google Scholar] [CrossRef]
- Shao, Y.; Aizhao, P.; Lin, H. POSS end-capped diblock copolymers: Synthesis, micelle self-assembly and properties. J. Colloid Interface Sci. 2014, 425, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Durmus, A.; Kasgoz, A.; Erca, N.; Akn, D.; Şanl, S. Effect of Polyhedral Oligomeric Silsesquioxane (POSS) Reinforced Polypropylene (PP) Nanocomposite on the Microstructure and Isothermal Crystallization Kinetics of Polyoxymethylene (POM). Polymer 2012, 53, 5347–5357. [Google Scholar] [CrossRef]
- Illescas, S.; Sanchez-Soto, M.; Milliman, H.; Schiraldi, D.A.; Arostegui, A. The morphology and properties of melt-mixed polyoxymethylene/monosilanolisobutyl-POSS composites. High Perform. Polym. 2011, 23, 457–467. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, F. Some comments on the melting and recrystallization of polyoxymethylene by high-speed and StepScan differential scanning calorimetry. Polimery 2004, 49, 558–560. [Google Scholar]
- Szubert, K.B.; Marciniec, M.; Dutkiewicz, M.; Potrzebowski, M.; Maciejewski, H. Functionalization of spherosilicates via hydrosilylation catalyzed by well-defined rhodium siloxide complexes immobilized on silica. J. Mol. Catal. A: Chem. 2014, 391, 150–157. [Google Scholar] [CrossRef]
- Szwarc-Rzepka, K.; Marciniec, B.; Jesionowski, T. Immobilization of multifunctional silsesquioxane cage on precipitated silica supports. Adsorption 2013, 19, 483. [Google Scholar] [CrossRef]
- Jeziórska, R.; Swierz-Motysia, B.; Szadkowska, A.; Marciniec, B.; Maciejewski, H.; Dutkiewicz, M.; Leszczyńska, I. Effect of POSS on Morphology, Thermal and Mechanical Properties of Polyamide 6. Polimery 2011, 56, 809–816. [Google Scholar]
- Siengchin, S.; Psarras, G.C.; Karger-Kocsis, J. POM/PU/carbon nanofiber composites produced by water-mediated melt compounding: Structure, thermo-mechanical and dielectrical properties. J. Appl. Polym. Sci. 2010, 117, 1804–1812. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics: Crystal Nucleation, Growth, Annealing; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Plummer, C.J.G.; Menu, P.; Cudré-Mauroux, N.; Kausch, H.-H. The effect of crystallization conditions on the properties of polyoxymethylene. J. Appl. Polym. Sci. 1995, 55, 489–500. [Google Scholar] [CrossRef]
- Mück, K.-F. Physical Constants of Poly(oxymethylene). In Wiley Database of Polymer Properties; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Pelzbauer, Z.; Galeski, A. Growth rate and morphology of polyoxymethylene supermolecular structures. J. Polym. Sci. Polym. Symp. 1972, 8, 23–32. [Google Scholar] [CrossRef]
- Yousef, S.; Visco, A.; Galtieri, G.; Njuguna, J. Flexural, impact, rheological and physical characterizations of POM reinforced by carbon nanotubes and paraffin oil. Polym. Adv. Technol. 2016, 27, 1338–1344. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, L. Nucleation effect of polyamide on polyoxymethylene. Polym. Eng. Sci. 2005, 45, 1174–1179. [Google Scholar] [CrossRef]
- Nobile, M.R.; Bove, L.; Somma, E.; Kruszelnicka, I.; Sterzynski, T. Rheological and structure investigation of shear-induced crystallization of isotactic polypropylene. Polym. Eng. Sci. 2005, 45, 153–162. [Google Scholar] [CrossRef]
- Everaert, V.; Groeninck, G.; Koch, M.H.J.; Reynaers, H. Influence of fractionated crystallization on the semicrystalline structure of (POM/(PS/PPE)) blends. Static and time-resolved SAXS, WAXD and DSC studies. Polymer 2003, 44, 3491–3508. [Google Scholar] [CrossRef]
- Pielichowska, K.; Szczygielska, A.; Spasówka, E. Preparation and characterization of polyoxymethylene-copolymer/hydroxyapatite nanocomposites for long-term bone implants. Polym. Adv. Technol. 2012, 23, 1141–1150. [Google Scholar] [CrossRef]

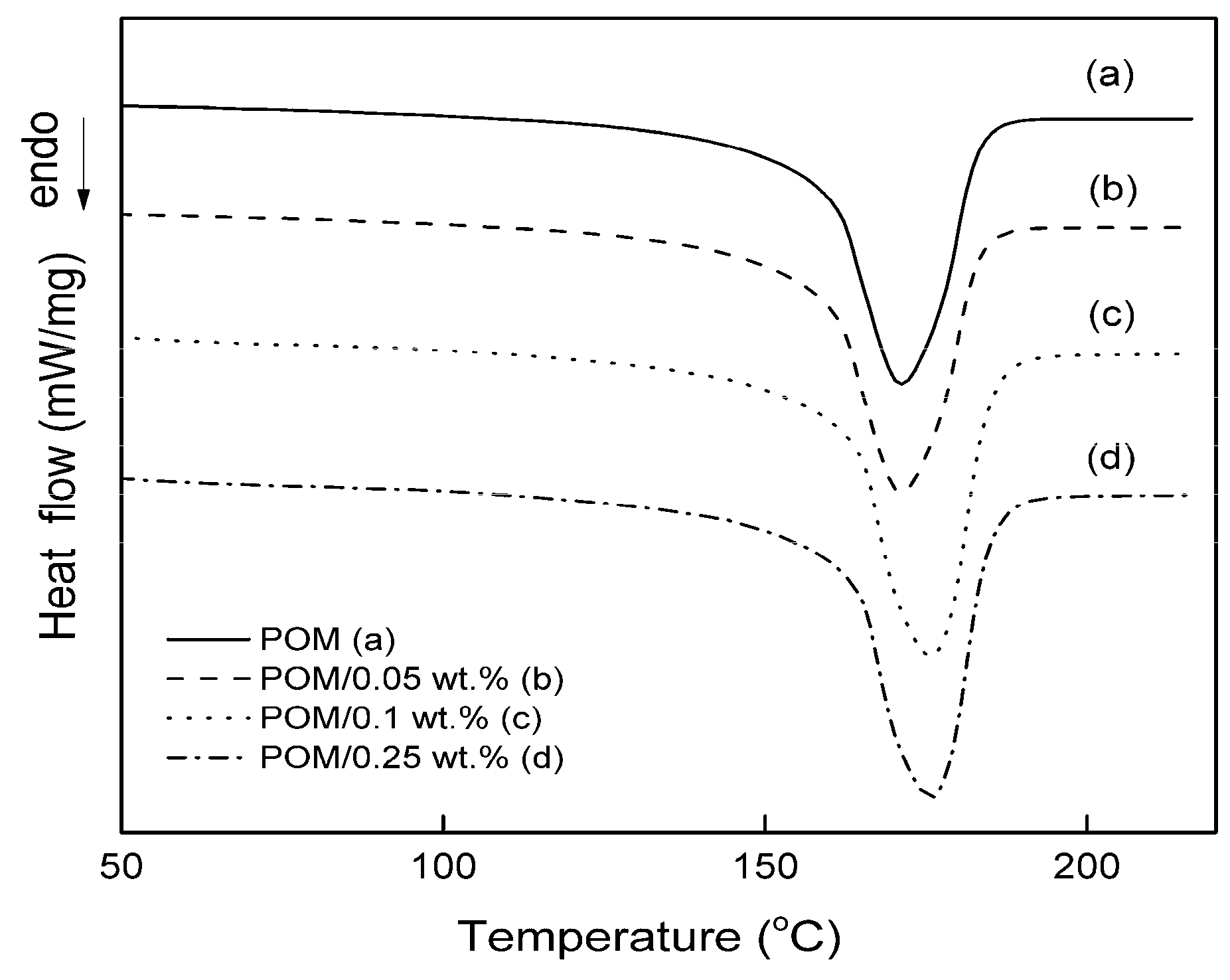
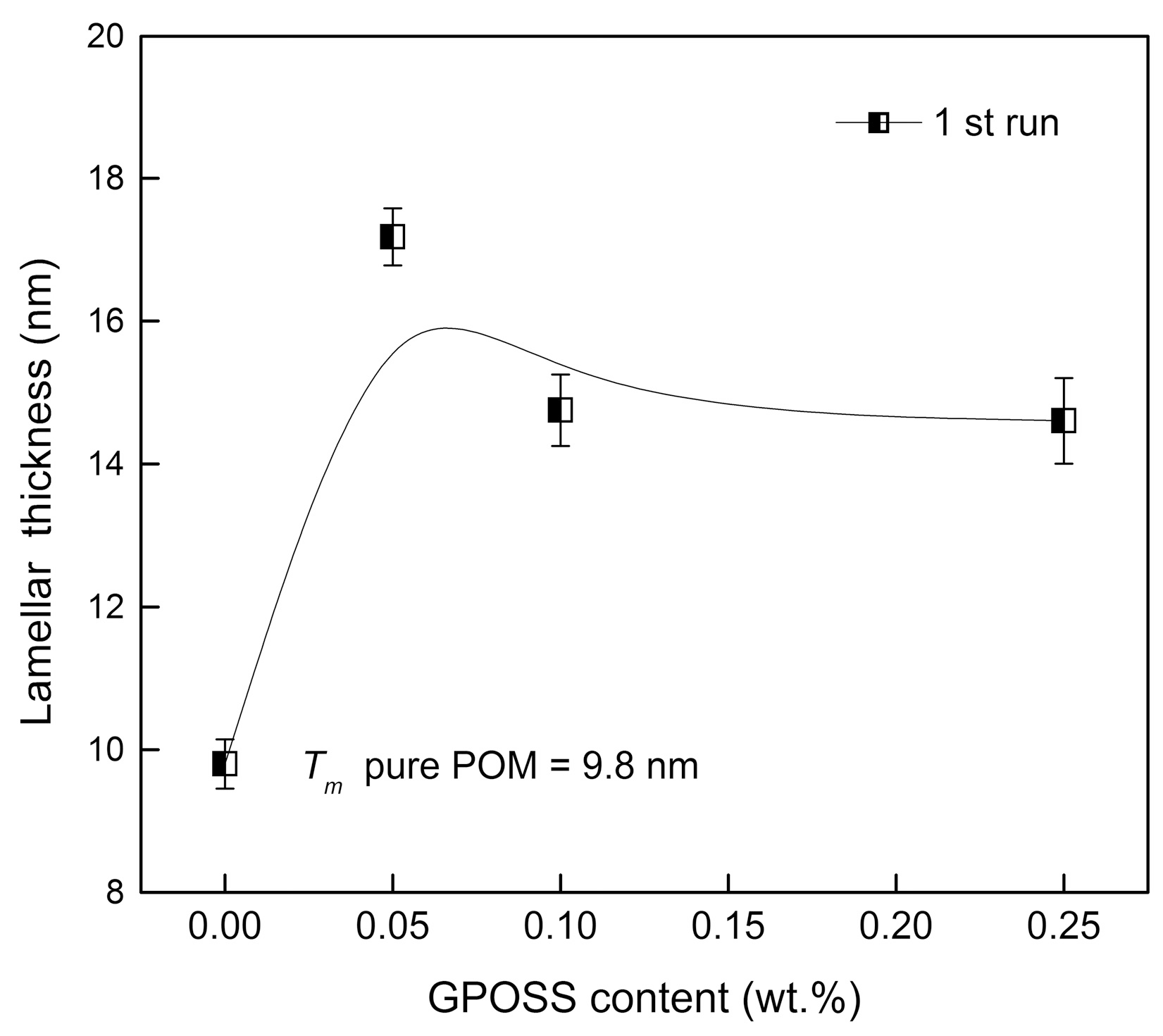


| Samples | Tm1 (°C) | Tm1 onset (°C) | ∆Hm1 (J/g) | lc (nm) | ∆Hm2 (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| pure POM | 169.2 | 158.0 | −138.5 | 9.8 | −134.3 | 72 |
| POM/0.05 wt % GPOSS | 171.2 | 159.0 | −142.0 | 11.4 | −135.8 | 73 |
| POM/0.1 wt % GPOSS | 173.7 | 160.0 | −143.5 | 14.2 | −137.4 | 74 |
| POM/0.25 wt % GPOSS | 175.7 | 163.2 | −154.1 | 17.2 | −149.9 | 81 |
| Samples | L100 (Å) |
|---|---|
| pure POM | 228 |
| POM/0.05 wt % GPOSS | 217 |
| POM/0.1 wt % GPOSS | 186 |
| POM/0.25 wt % GPOSS | 143 |
| Samples | Ultimate tensile strength (MPa) | Young’s modulus (GPa) | Tensile stress at break (MPa) |
|---|---|---|---|
| pure POM | 55.00 ± 0.52 | 1.83 ± 0.13 | 45.97 ± 0.65 |
| POM/0.05 wt % GPOSS | 57.50 ± 0.43 | 2.44 ± 0.23 | 49.78 ± 0.78 |
| POM/0.1 wt % GPOSS | 57.64 ± 0.90 | 2.48 ± 0.15 | 49.90 ± 0.69 |
| POM/0.25 wt % GPOSS | 57.80 ± 0.95 | 2.60 ± 0.25 | 50.25 ± 0.73 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka-Komorowska, D.; Sterzynski, T. Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene. Polymers 2018, 10, 203. https://doi.org/10.3390/polym10020203
Czarnecka-Komorowska D, Sterzynski T. Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene. Polymers. 2018; 10(2):203. https://doi.org/10.3390/polym10020203
Chicago/Turabian StyleCzarnecka-Komorowska, Dorota, and Tomasz Sterzynski. 2018. "Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene" Polymers 10, no. 2: 203. https://doi.org/10.3390/polym10020203
APA StyleCzarnecka-Komorowska, D., & Sterzynski, T. (2018). Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene. Polymers, 10(2), 203. https://doi.org/10.3390/polym10020203