Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Membranes
2.2. Permeability Tests
3. Results and Discussion
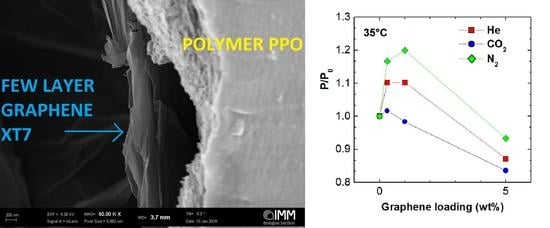
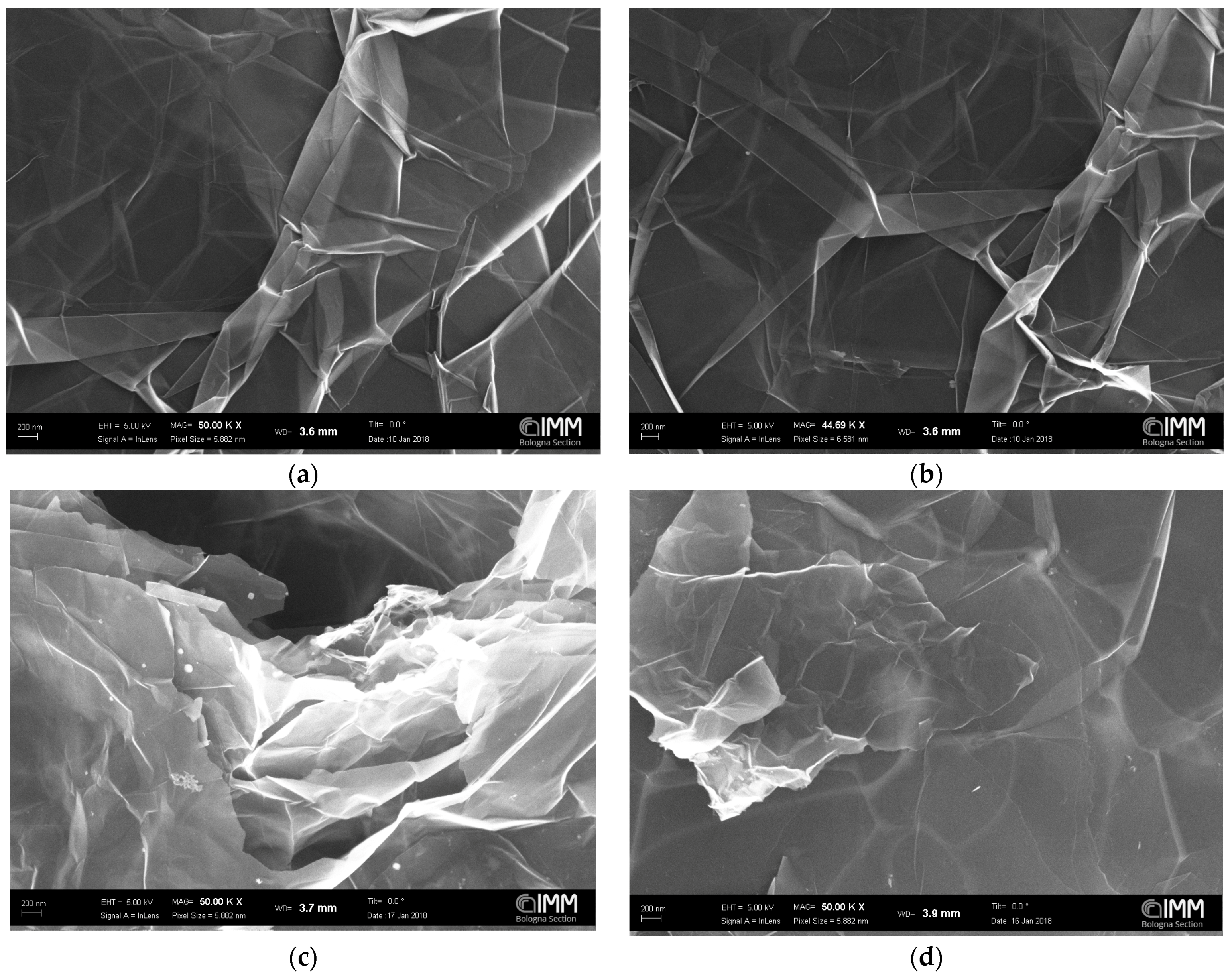
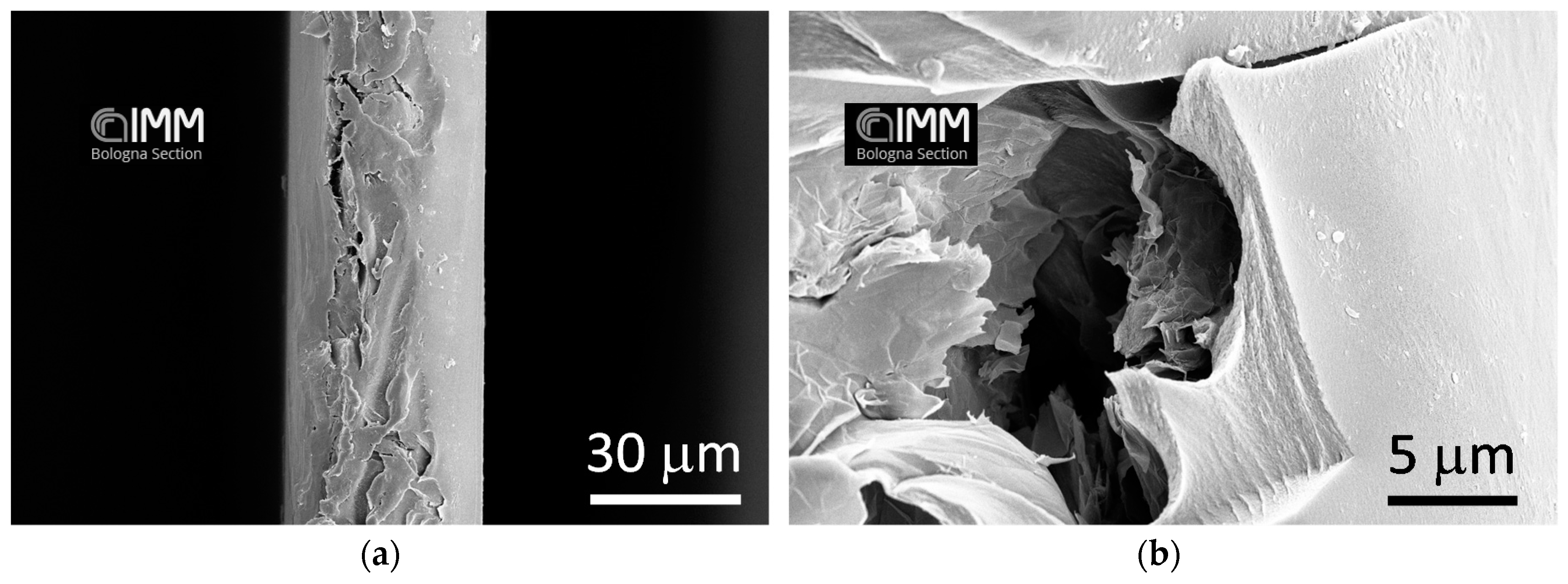
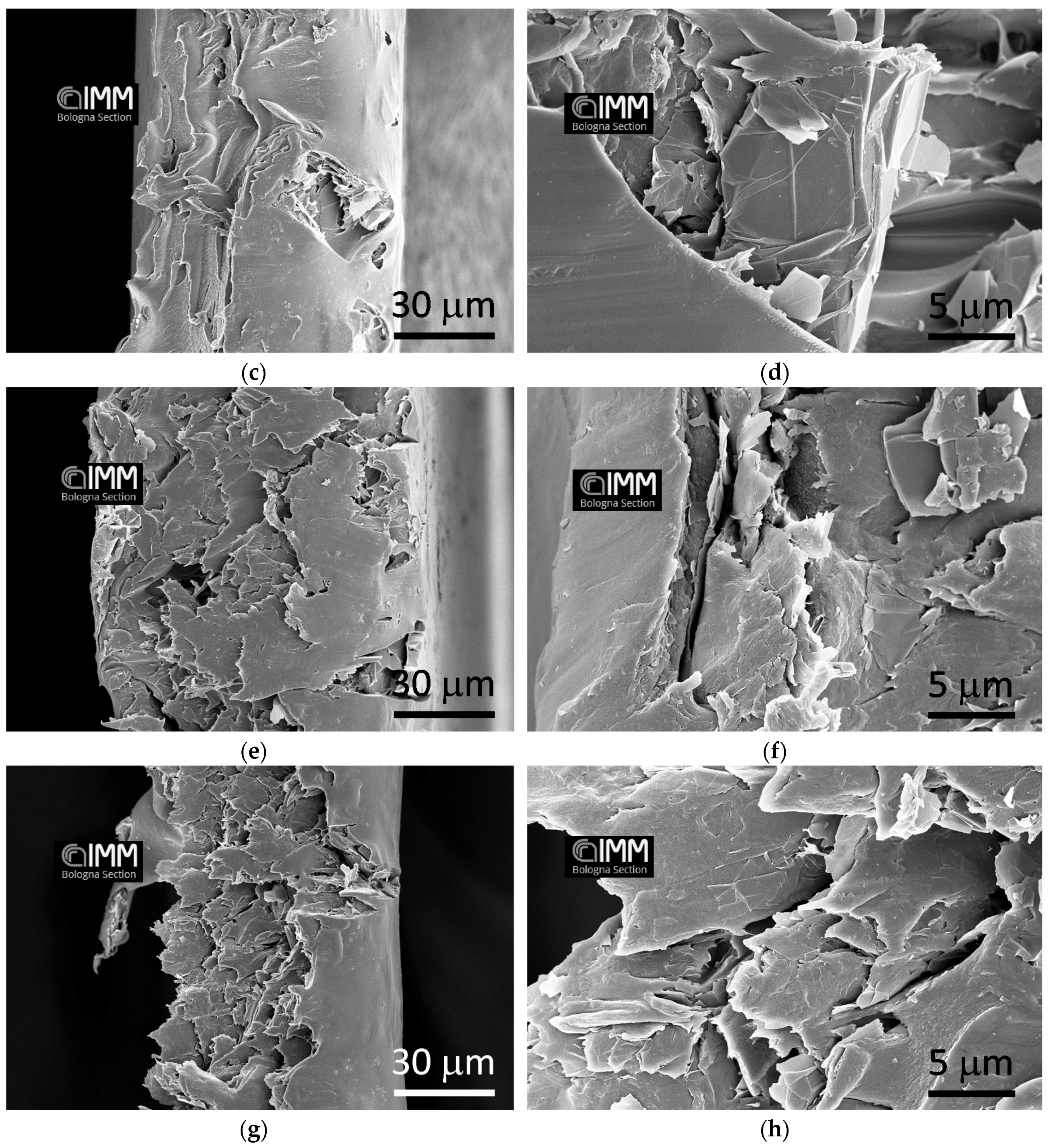
3.1. SEM Analysis
3.2. Permeability
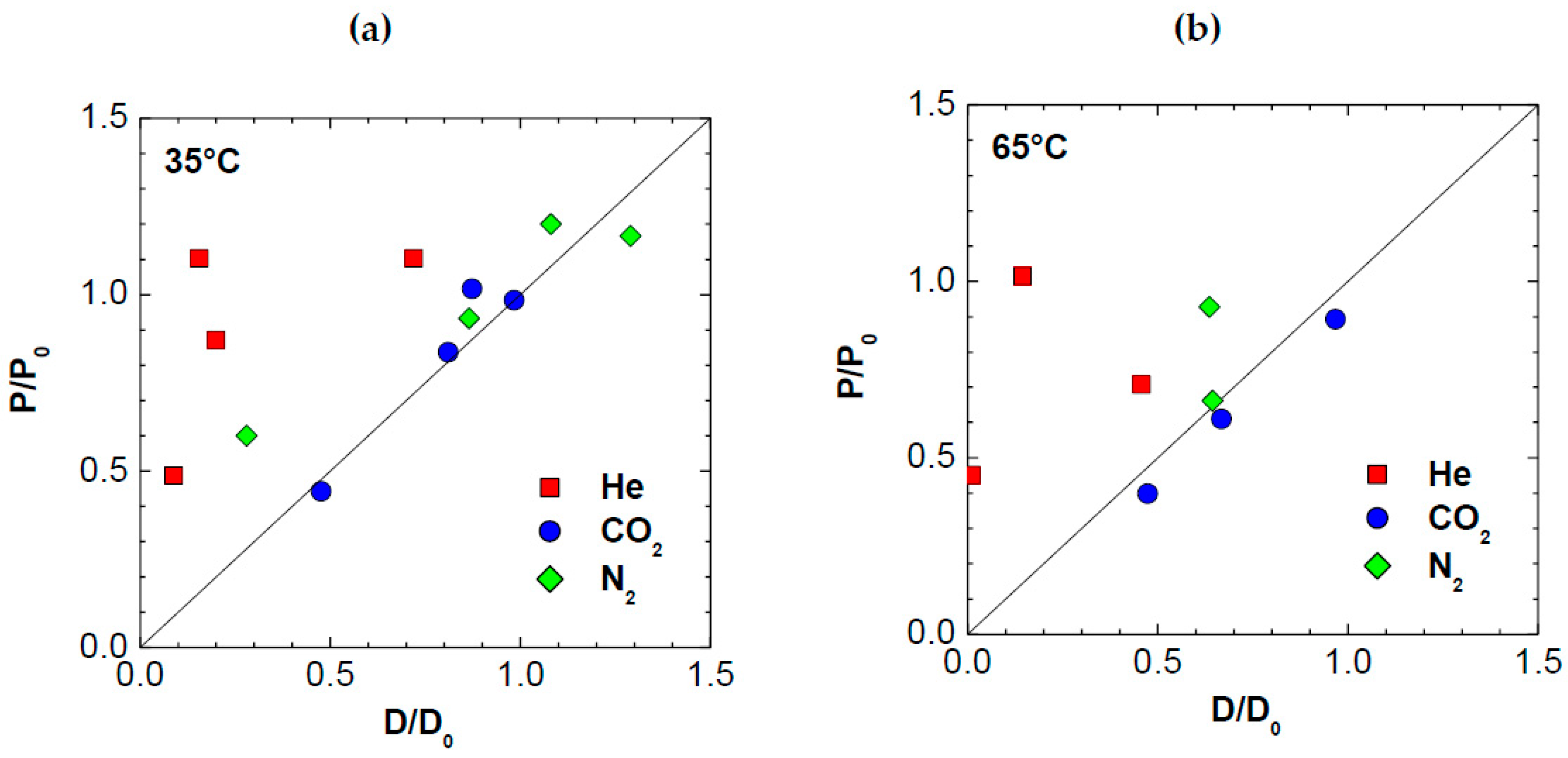
3.3. Selectivity
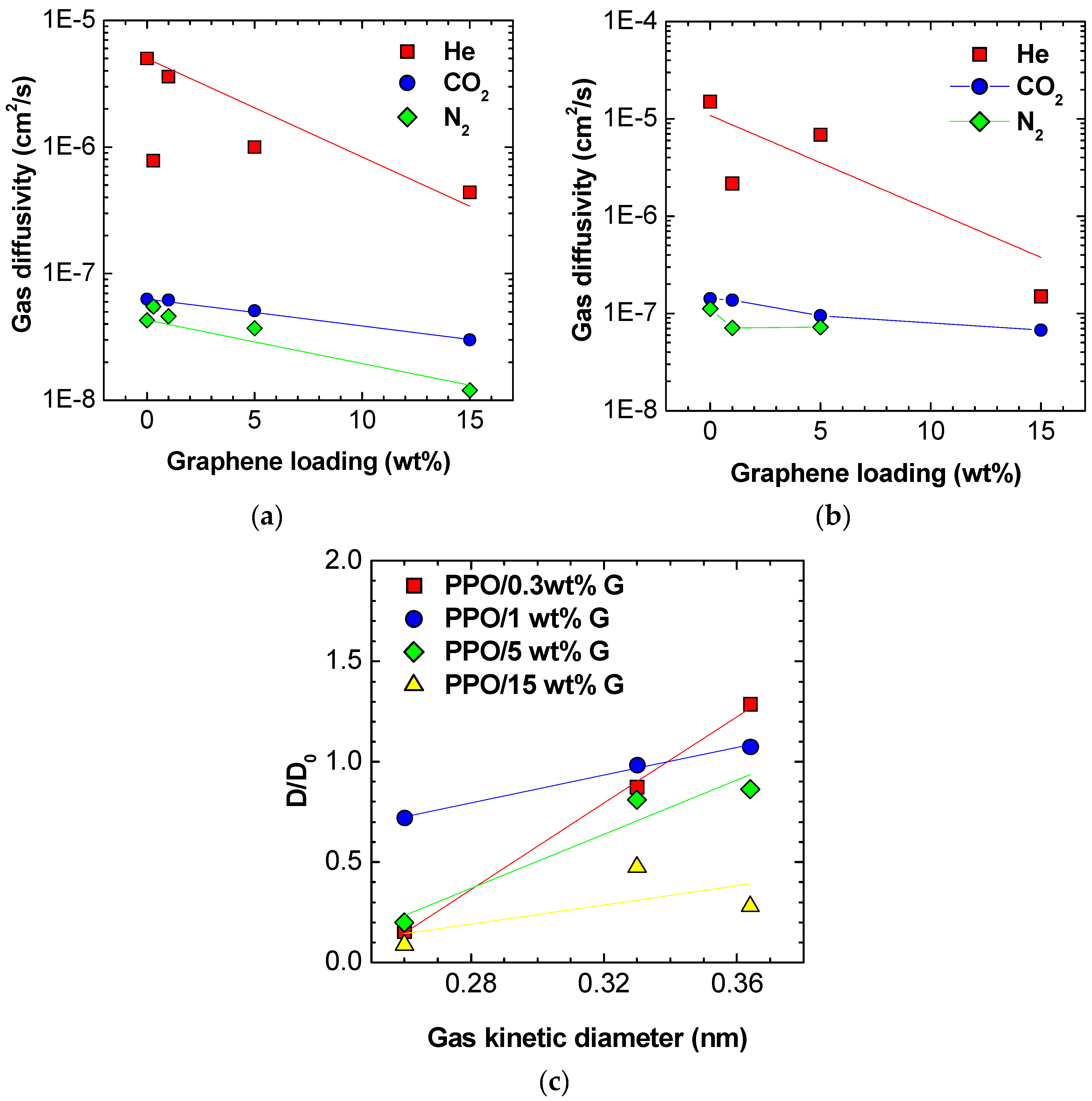
3.4. Diffusivity
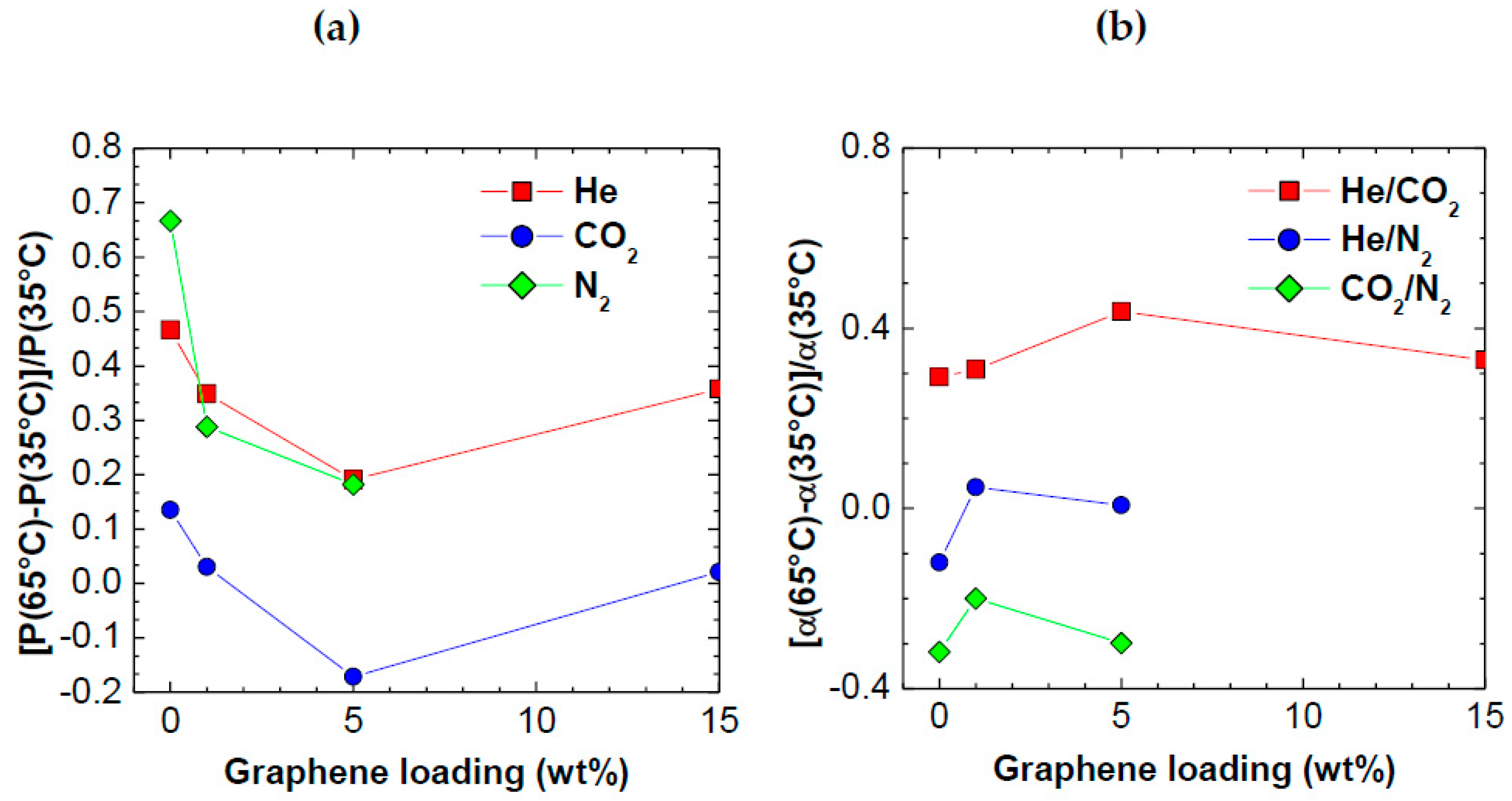
3.5. Analysis of Temperature Effect
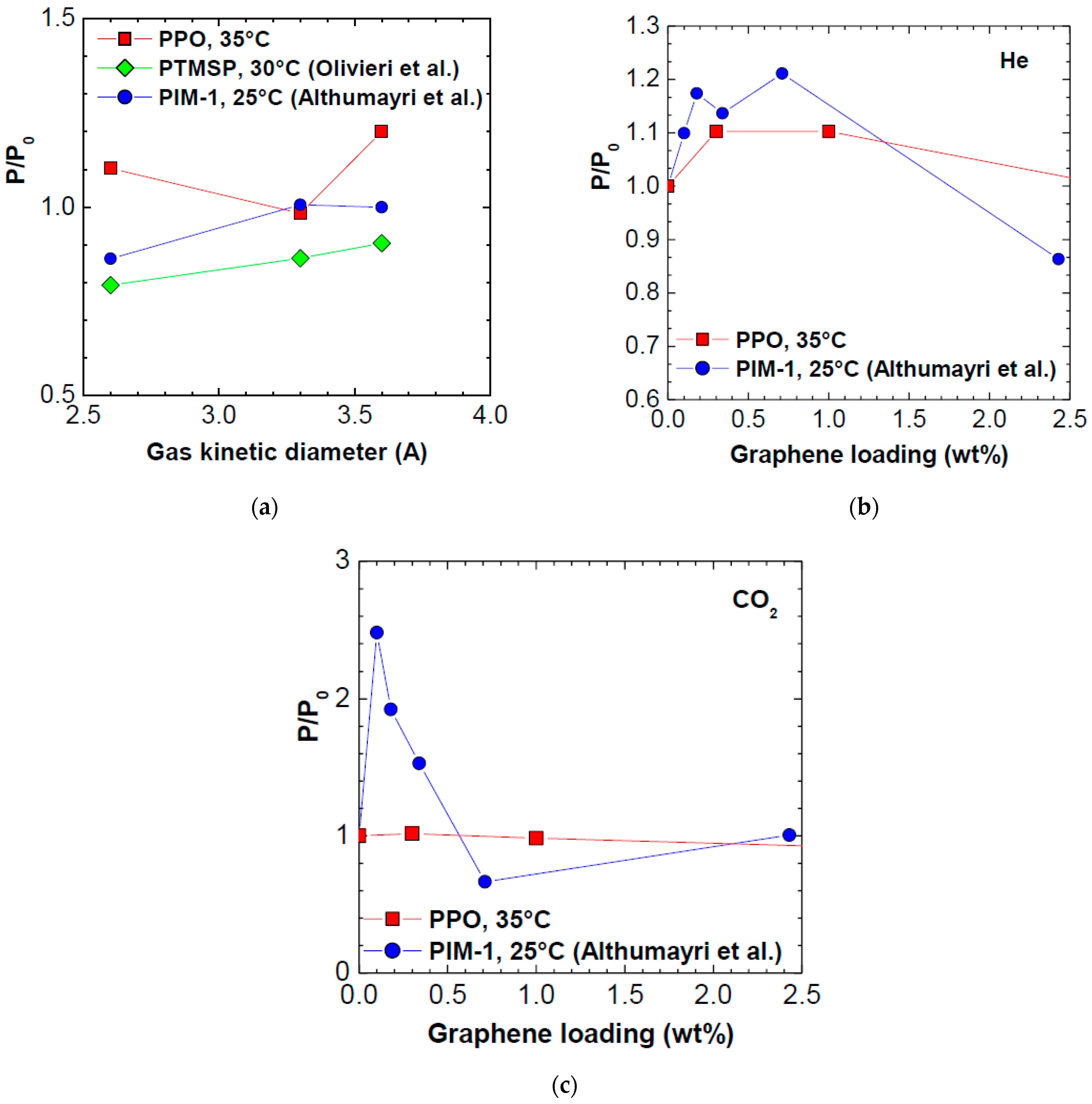
3.6. Comparison with Other Polymers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Bains, P.; Psarras, P.; Wilcox, J. CO2 capture from the industry sector. Prog. Energy Combust. Sci. 2017, 63, 146–172. [Google Scholar] [CrossRef]
- George, G.; Bhoria, N.; AlHallaq, S.; Abdala, A.; Mittal, V. Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 2016, 158, 333–356. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon dioxide capture with membranes at an IGCC power plant. J. Membr. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Marano, J.J.; Ciferino, J.P. Integration of Gas Separation Membranes with IGCC: Identifying the right membrane for the right job. Energy Procedia 2009, 1, 361–368. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of molecular weight and temperature on physical aging of thin glassy poly(2,6-dimethyl-1,4-phenylene oxide) films. J. Polym. Sci. B 2007, 45, 1390–1398. [Google Scholar] [CrossRef]
- Mehio, N.; Dai, S.; Jiang, D. Quantum Mechanical Basis for Kinetic Diameters of Small Gaseous Molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; He, Z.; Pinnau, I.; Freeman, B.D.; Meakin, P.; Hill, A.J. Effect of Nanoparticles on Gas Sorption and Transport in Poly(1-trimethylsilyl-1-propyne). Macromolecules 2003, 36, 6844–6855. [Google Scholar] [CrossRef]
- Merkel, T.C.; He, Z.; Pinnau, I.; Freeman, B.D.; Meakin, P.; Hill, A.J. Sorption and Transport in Poly(2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole-co-tetrafluoroethylene) Containing Nanoscale Fumed Silica. Macromolecules 2003, 36, 8406–8414. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.; Galizia, M.; De Angelis, M.G.; Sarti, G.C. Gas and Vapor Transport in Mixed Matrix Membranes Based on Amorphous Teflon AF1600 and AF2400 and Fumed Silica. Ind. Eng. Chem. Res. 2010, 49, 11920–11935. [Google Scholar] [CrossRef]
- Galizia, M.; De Angelis, M.G.; Messori, M.; Sarti, G.C. Mass transport in hybrid PTMSP/Silica membranes. Ind. Eng. Chem. Res. 2014, 53, 9243–9255. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Gaddoni, R.; Sarti, G.C. Gas Solubility, Diffusivity, Permeability, and Selectivity in Mixed Matrix Membranes Based on PIM-1 and Fumed Silica. Ind. Eng. Chem. Res. 2013, 52, 10506–10520. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C. Gas sorption and permeation in mixed matrix membranes based on glassy polymers and silica nanoparticles. Curr. Opin. Chem. Eng. 2012, 1, 148–155. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable Fabrication of Carbon Nanotube/Polymer Nanocomposite Membranes for High Flux Gas Transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Khan Muntazim, M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic, microporosity for gas separation. Nanoscale Res. Lett. 2012, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Pechar, T.W.; Marand, E. Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 192, 330–339. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C. Solubility and Diffusivity of Gases in Mixed Matrix Membranes Containing Hydrophobic Fumed Silica: Correlations and Predictions Based on the NELF Model. Ind. Eng. Chem. Res. 2008, 47, 5214–5226. [Google Scholar] [CrossRef]
- Zhu, J.; Lim, J.; Lee, C.-H.; Joh, H.-I.; Kim, H.C.; Park, B.; You, N.-H.; Lee, S. Multifunctional polyimide/graphene oxide composites via in situ polymerization. J. Appl. Polym. Sci. 2014, 131, 40177. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Sun, C.; Wen, B.; Bai, B. Recent advances in nanoporous graphene membrane for gas separation and water purification. Sci. Bull. 2015, 60, 1807–1823. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, M.; Li, C.; Shi, G. Graphene-Based Membranes for Molecular Separation. J. Phys. Chem. Lett. 2015, 6, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.W.; Cho, Y.H.; Park, H.B. Graphene-based membranes: Status and prospects. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150024. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2013, 131, 39628. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, H.; Yoo, B.; Park, J.; Gleason, K.; Freeman, B.D.; Park, H.B. High performance CO2-phylic graphene oxide membranes under wet conditions. Chem. Commun. 2014, 50, 13563–13566. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.-R.; Xu, R. Membranes with Fast and Selective Gas-Transport Channels of Laminar Graphene Oxide for Efficient CO2 Capture. Angew. Chem. 2015, 127, 588–592. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, C.; Chen, Y.-F.; Wang, T.; Du, C.-H.; Wu, L.-G. Enhancement on the permeation performance of polyimide mixed matrix membranes by incorporation of graphene oxide with different oxidation degrees. Polym. Adv. Technol. 2015, 26, 330–337. [Google Scholar] [CrossRef]
- Gonciaruk, A.; Althumayri, K.; Harrison, W.J.; Budd, P.M.; Siperstein, F.R. PIM-1/graphene composite: A combined experimental and molecular simulation study. Microporous Mesoporous Mater. 2015, 209, 126–134. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A 2016, 374, 20150031. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Prestat, E.; Zhou, K.-G.; Gorgojo, P.; Althumayri, K.; Harrison, W.; Budd, P.M.; Haigh, S.J.; Casiraghi, C. Synthesis and characterization of composite membranes made of graphene and polymers of intrinsic microporosity. Carbon 2016, 102, 357–366. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of Graphene and Graphene Oxide Nanoplatelets on the Gas Permselectivity and Aging Behavior of Poly(trimethylsilyl propyne) (PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Aguilar-Vega, M.; Paul, D.R. Gas transport properties of phenylene ethers. J. Polym. Sci. B 1993, 31, 1577–1589. [Google Scholar] [CrossRef]
- Le Roux, J.D.; Paul, D.R.; Kampaby, J.; Lagow, R.J. Surface fluorination of poly(phenylene oxide) composite membranes; Part I. Transport properties. J. Membr. Sci. 1994, 90, 1–35. [Google Scholar] [CrossRef]
- Minelli, M.; De Angelis, M.G.; Doghieri, F.; Marini, M.; Toselli, M.; Pilati, F. Oxygen permeability of novel organic–inorganic coatings: I. Effects of organic–inorganic ratio and molecular weight of the organic component. Eur. Polym. J. 2008, 44, 2581–2588. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford Press: London, UK, 1956. [Google Scholar]
- Luo, S.; Stevens, K.A.; Park, J.S.; Moon, J.D.; Liu, Q.; Freeman, B.D.; Guo, R. Highly CO2-selective gas separation membranes based on segmented copolymers of poly(ethylene oxide) reinforced with pentiptycene-containing polyimide hard segments. ACS Appl. Mater. Inter. 2016, 8, 2306–2317. [Google Scholar] [CrossRef] [PubMed]

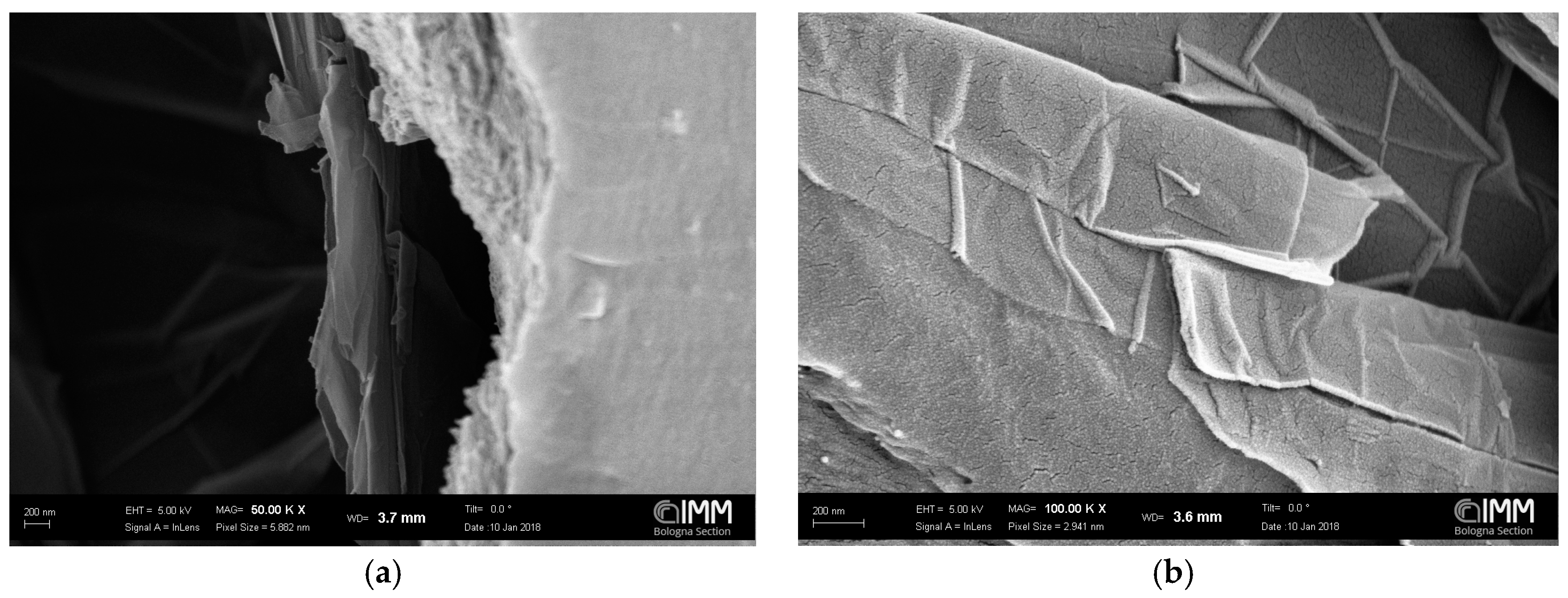
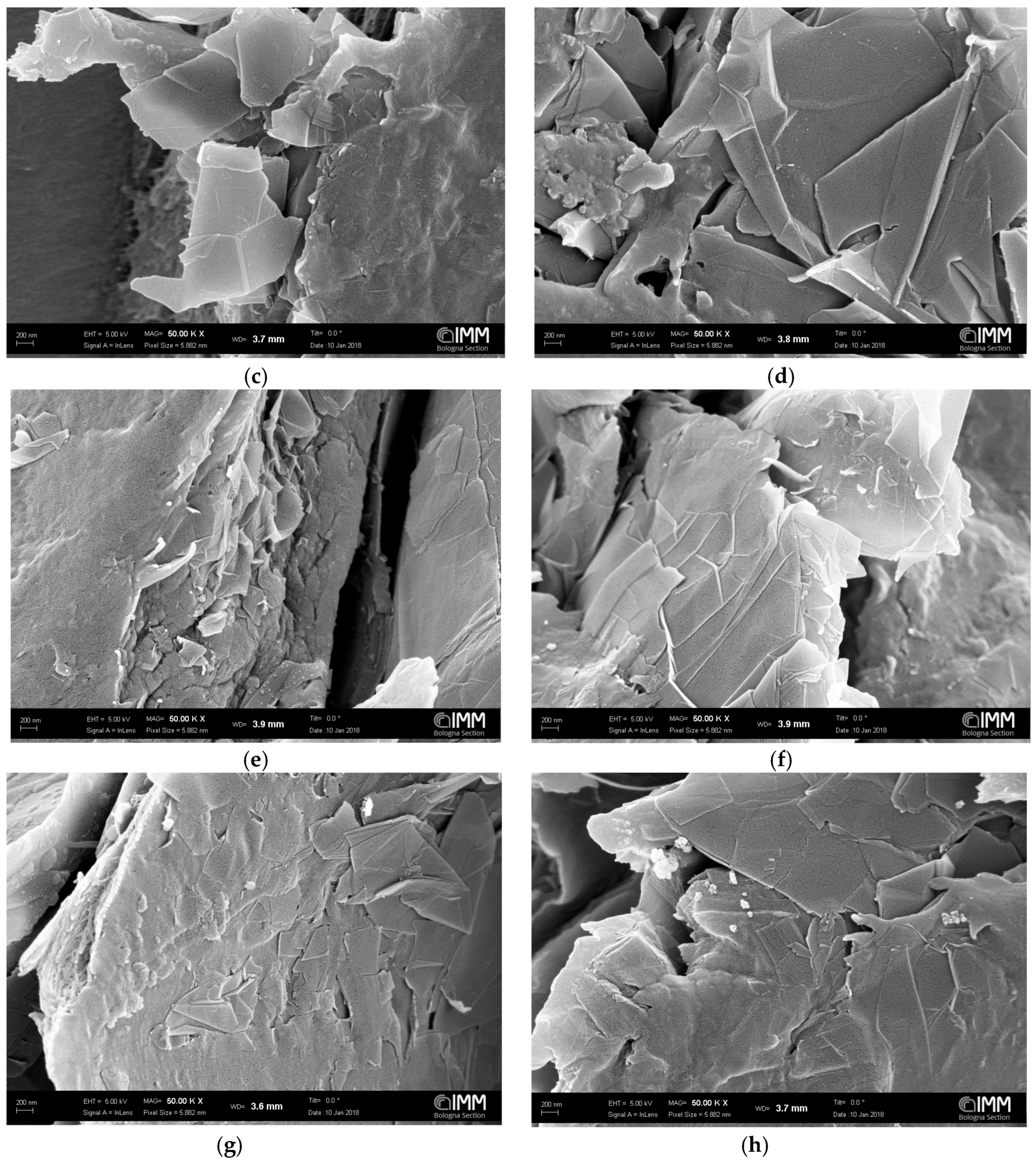
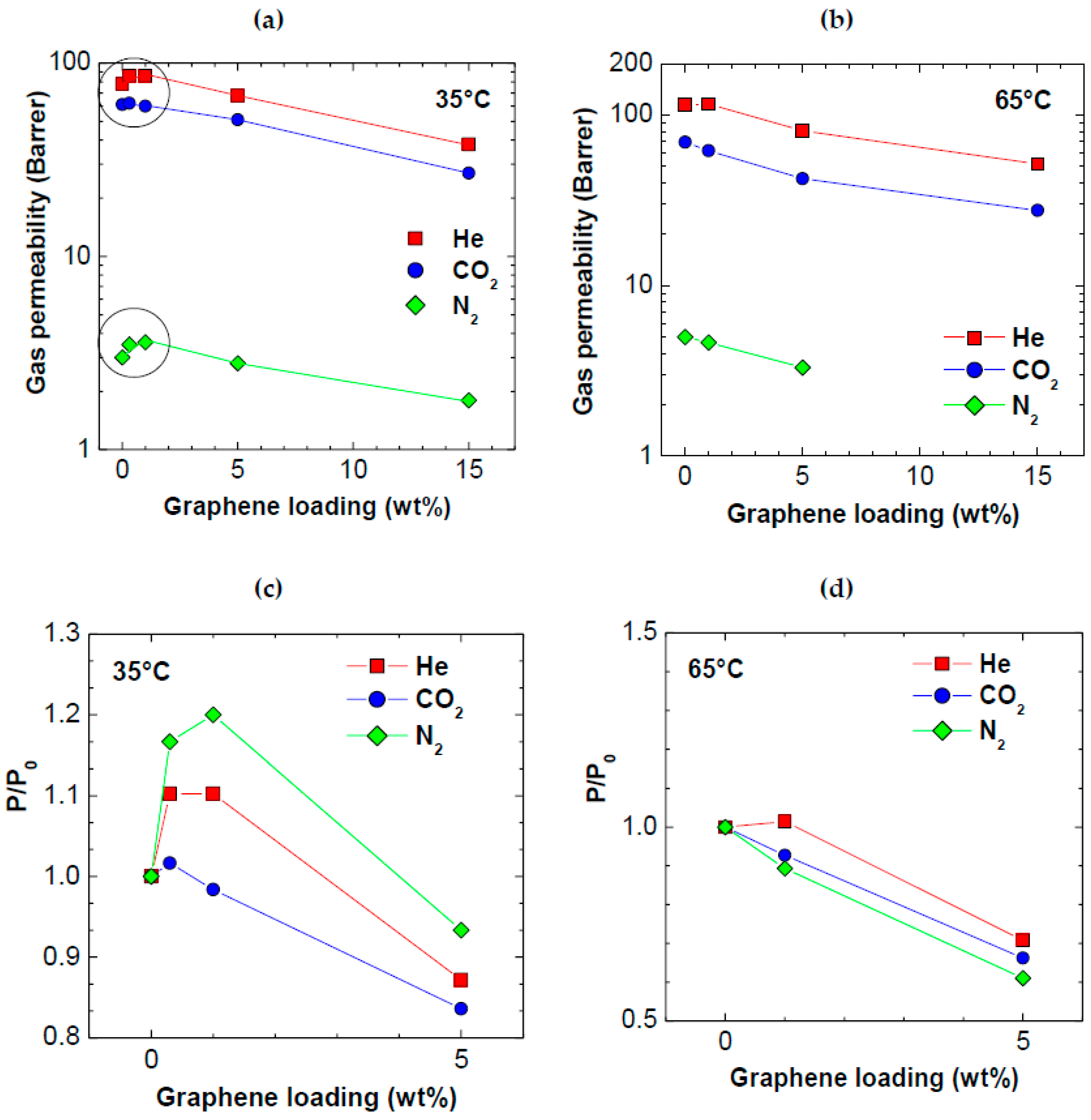
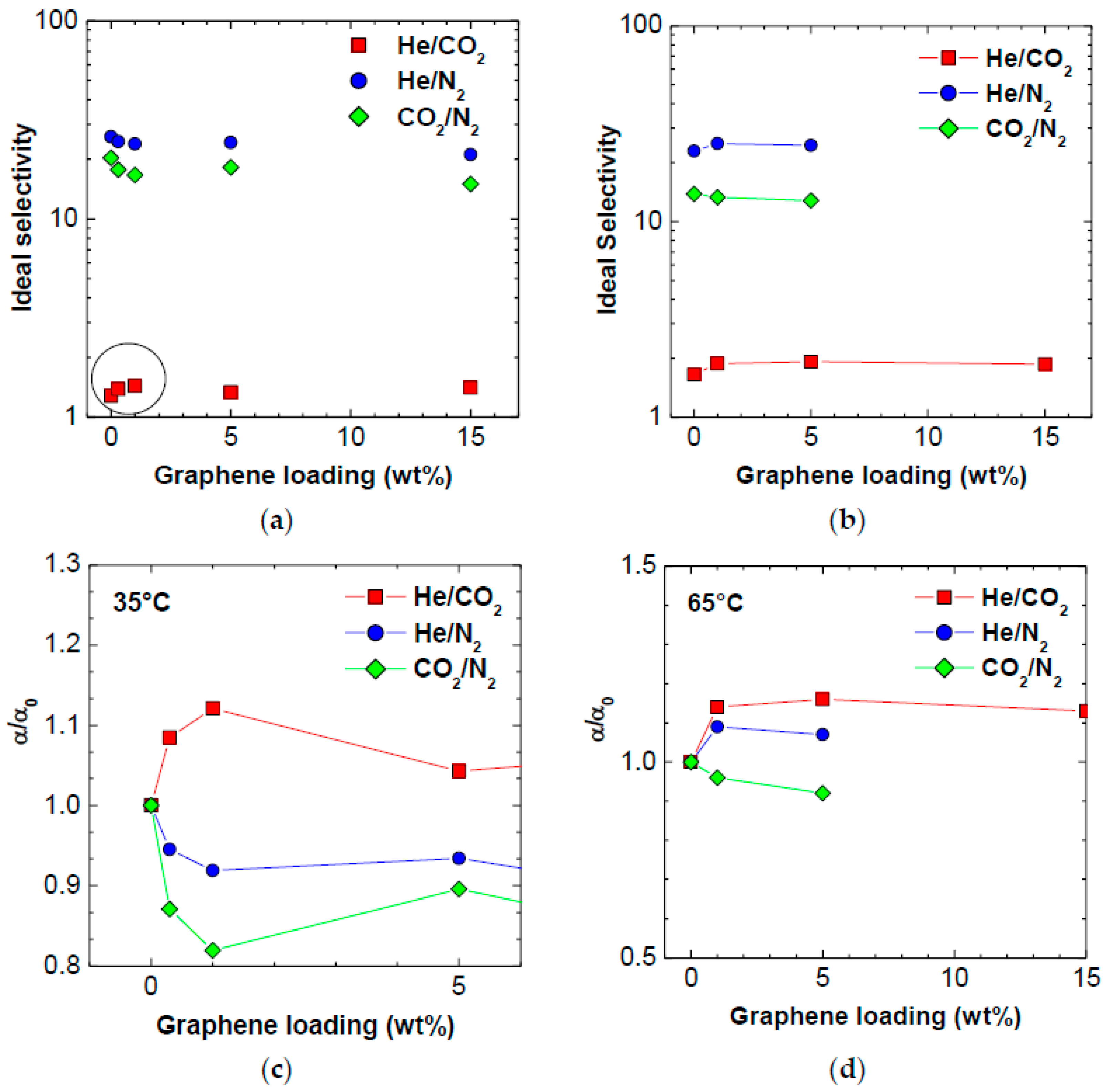
| Graphene Type | Graphene wt % in Polymer (g/100 g of PPO) | Treatment | Sonication Time | Stirring Time | Thickness (m) |
|---|---|---|---|---|---|
| XT7 | 0.3 | Heating at 200 °C under vacuum for 1 d | 60 min | 1 d | 41 ± 2.1 |
| XT6 a | 1 | 15 min | 82 ± 3.9 72 ± 4.2 | ||
| XT6 | 5 | 88 ± 2.6 | |||
| XT6 | 15 | 55 ± 4.6 |
| Permeability at 35 °C, Barrer | PPO | PPO/0.3 wt % Graphene XT7 | PPO/1 wt % Graphene XT6 | PPO/5 wt % Graphene XT6 | PPO/15 wt % Graphene XT6 |
|---|---|---|---|---|---|
| He | 78 ± 3.8 | 86 ± 4.2 | 86 ± 4.1 | 68 ± 2.0 | 38 ± 3.2 |
| N2 | 3.0 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.2 | 2.8 ± 0.1 | 1.8 ± 0.2 |
| CO2 | 61 ± 2.0 | 62 ± 2.9 | 60 ± 2.9 | 51 ± 1.5 | 27 ± 2.3 |
| Permeability at 65 °C, Barrer | |||||
| He | 114 ± 5.0 | - | 116 ± 6.7 | 81.0 ± 2.4 | 51.6 ± 4.4 |
| N2 | 5.00 ± 0.4 | - | 4.64 ± 0.3 | 3.31 ± 0.1 | - |
| CO2 | 69.3 ± 2 | - | 61.9 ± 3.6 | 42.3 ± 1.2 | 27.6 ± 2.4 |
| Ideal Selectivity at 35 °C | PPO | PPO/0.3 wt % Graphene XT7 | PPO/1 wt % Graphene XT6 | PPO/5 wt % Graphene XT6 | PPO/15 wt % Graphene XT6 |
|---|---|---|---|---|---|
| He/CO2 | 1.28 | 1.39 | 1.43 | 1.33 | 1.41 |
| He/N2 | 26.0 | 24.6 | 23.9 | 24.3 | 21.1 |
| CO2/N2 | 20.3 | 17.7 | 16.7 | 18.2 | 15.0 |
| Ideal Selectivity at 65 °C | PPO | ||||
| He/CO2 | 1.65 | - | 1.88 | 1.92 | 1.87 |
| He/N2 | 22.9 | - | 25.0 | 24.5 | - |
| CO2/N2 | 13.9 | - | 13.3 | 12.8 | - |
| Diffusivity at 35 °C, cm2/s | PPO | PPO/0.3 wt % Graphene XT7 | PPO/1 wt % Graphene XT6 | PPO/5 wt % Graphene XT6 | PPO/15 wt % Graphene XT6 |
|---|---|---|---|---|---|
| He | (5.0 ± 0.5) × 10−6 | (7.8 ± 0.8) × 10−7 | (3.6 ± 0.3) × 10−6 | (10 ± 0.6) × 10−7 | (4.4 ± 0.8) × 10−7 |
| N2 | (4.3 ± 0.4) × 10−8 | (5.5 ± 0.5) × 10−8 | (4.6 ± 0.4) × 10−8 | (3.7 ± 0.2) × 10−8 | (1.2 ± 0.2) × 10−8 |
| CO2 | (6.3 ± 0.6) × 10−8 | (5.5 ± 0.5) × 10−8 | (6.2 ± 0.6) × 10−8 | (5.1 ± 0.3) × 10−8 | (3.0 ± 0.5) × 10−8 |
| Diffusivity at 65 °C, cm2/s | |||||
| He | (1.5 ± 0.2) × 10−5 | - | (2.2 ± 0.3) × 10−6 | (6.9 ± 0.4) × 10−6 | (1.5 ± 0.7) × 10−7 |
| N2 | (1.1 ± 0.1) × 10−7 | - | (7.1 ± 0.8) × 10−8 | (7.2 ± 0.4) × 10−8 | - |
| CO2 | (1.4 ± 0.1) × 10−7 | - | (1.4 ± 0.2) × 10−7 | (9.5 ± 0.6) × 10−8 | (6.7 ± 1.1) × 10−8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rea, R.; Ligi, S.; Christian, M.; Morandi, V.; Giacinti Baschetti, M.; De Angelis, M.G. Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation. Polymers 2018, 10, 129. https://doi.org/10.3390/polym10020129
Rea R, Ligi S, Christian M, Morandi V, Giacinti Baschetti M, De Angelis MG. Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation. Polymers. 2018; 10(2):129. https://doi.org/10.3390/polym10020129
Chicago/Turabian StyleRea, Riccardo, Simone Ligi, Meganne Christian, Vittorio Morandi, Marco Giacinti Baschetti, and Maria Grazia De Angelis. 2018. "Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation" Polymers 10, no. 2: 129. https://doi.org/10.3390/polym10020129
APA StyleRea, R., Ligi, S., Christian, M., Morandi, V., Giacinti Baschetti, M., & De Angelis, M. G. (2018). Permeability and Selectivity of PPO/Graphene Composites as Mixed Matrix Membranes for CO2 Capture and Gas Separation. Polymers, 10(2), 129. https://doi.org/10.3390/polym10020129