Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyaniline Nanotube Matrix Synthesis
2.3. Synthesis of Pristine and Oxidized CNOs
2.4. Methods
3. Results and Discussion
3.1. Nanocomposite PANINT/CNOsox Electrode Preparation Procedure
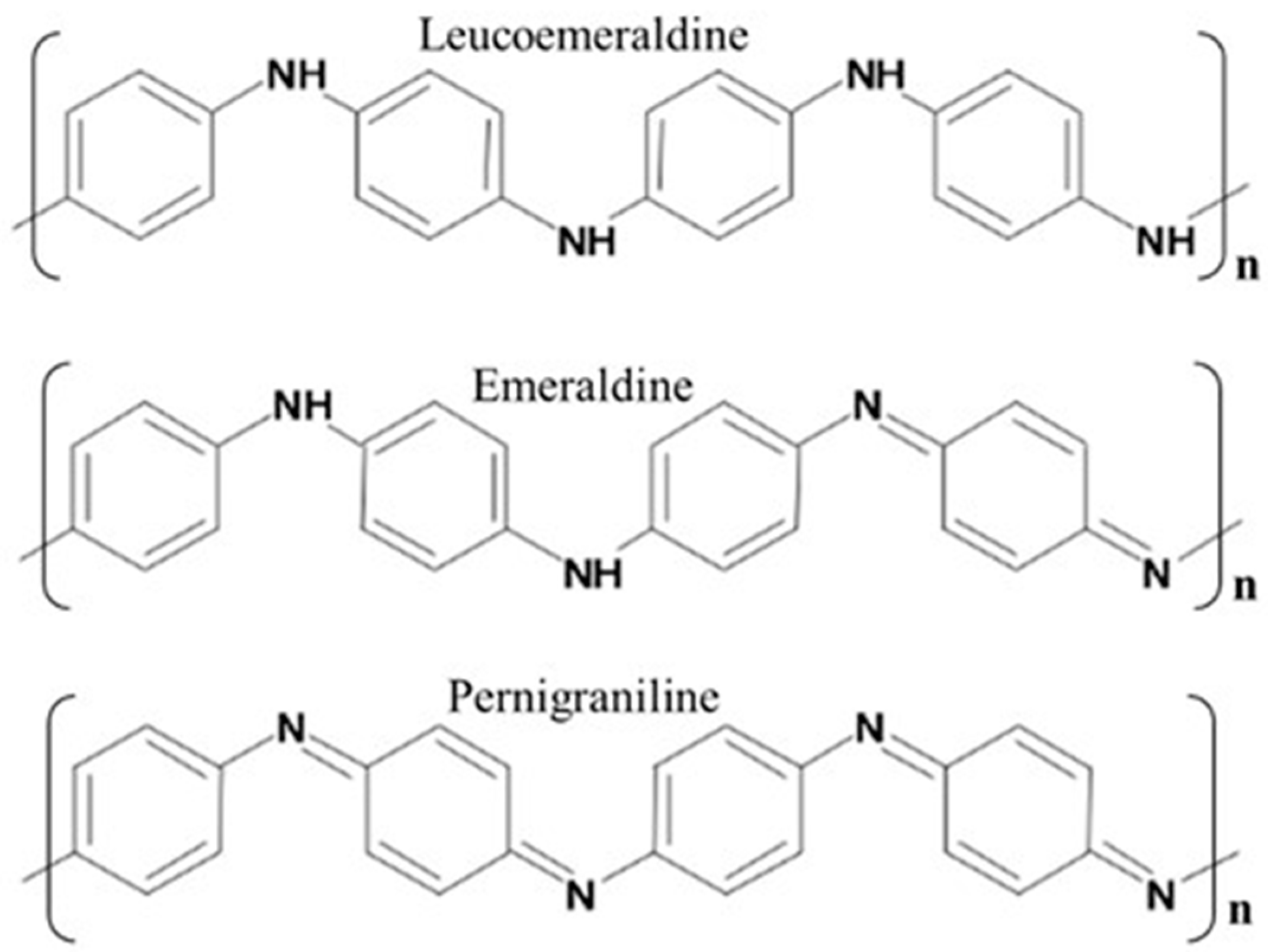
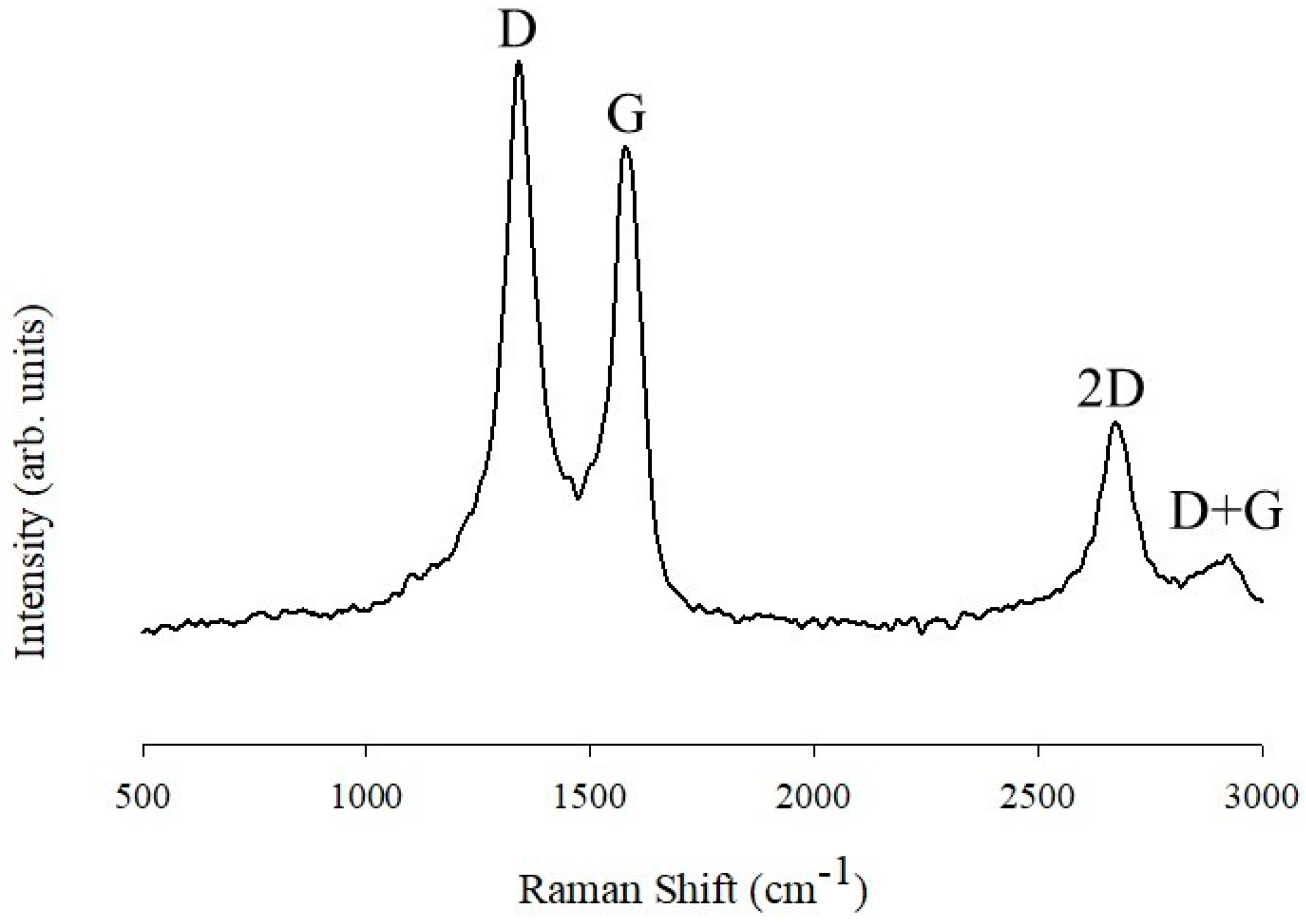
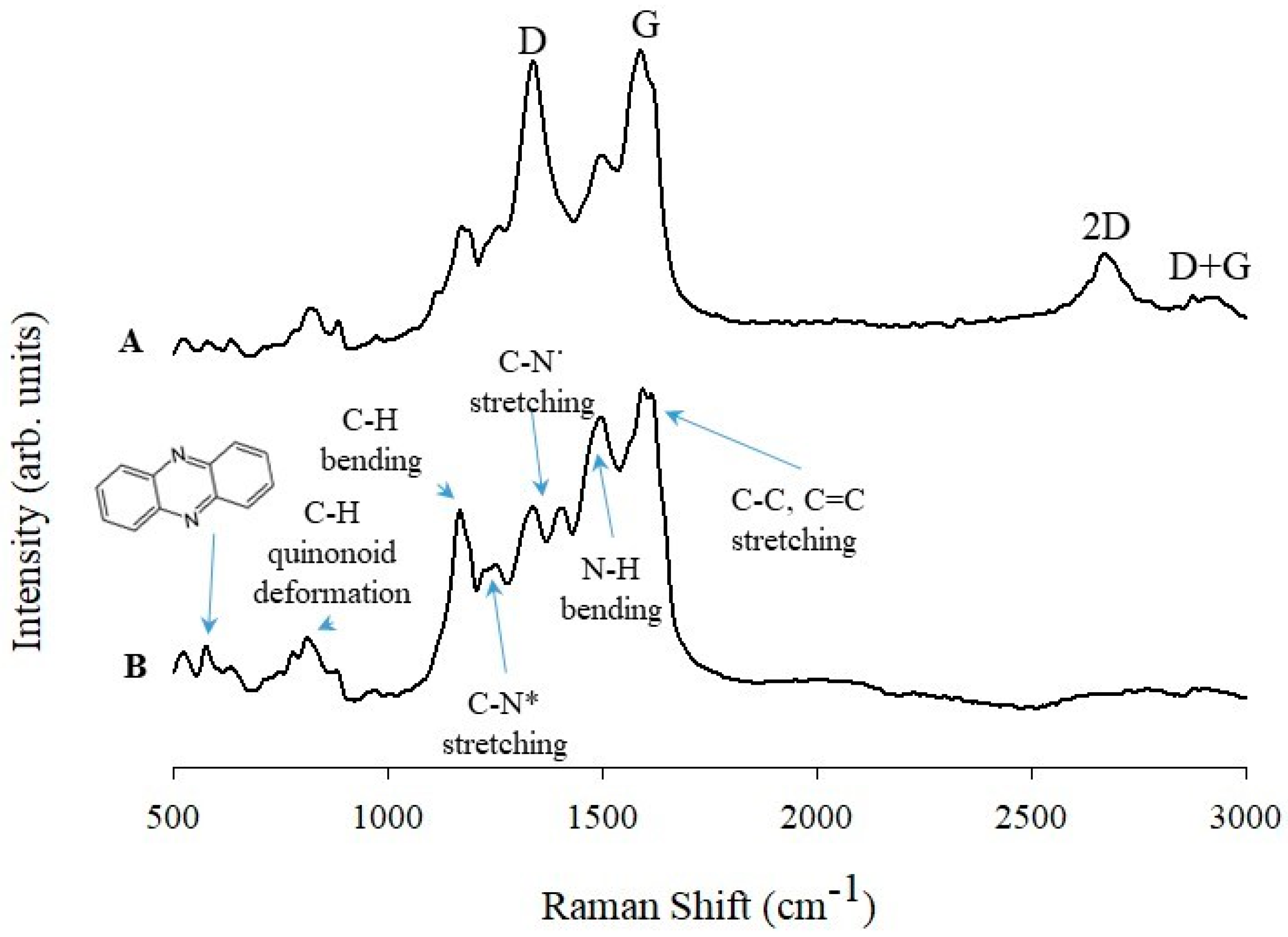
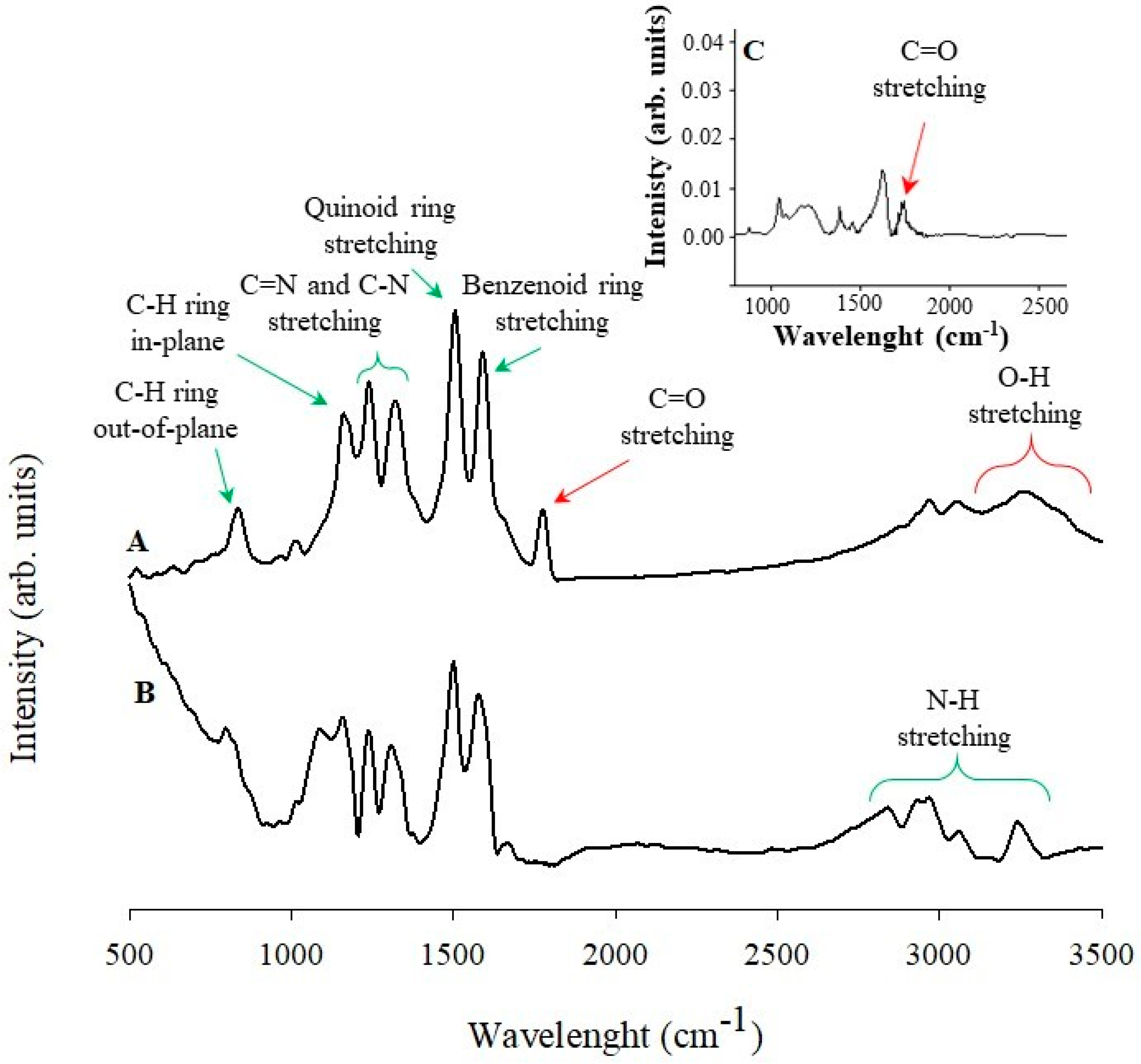
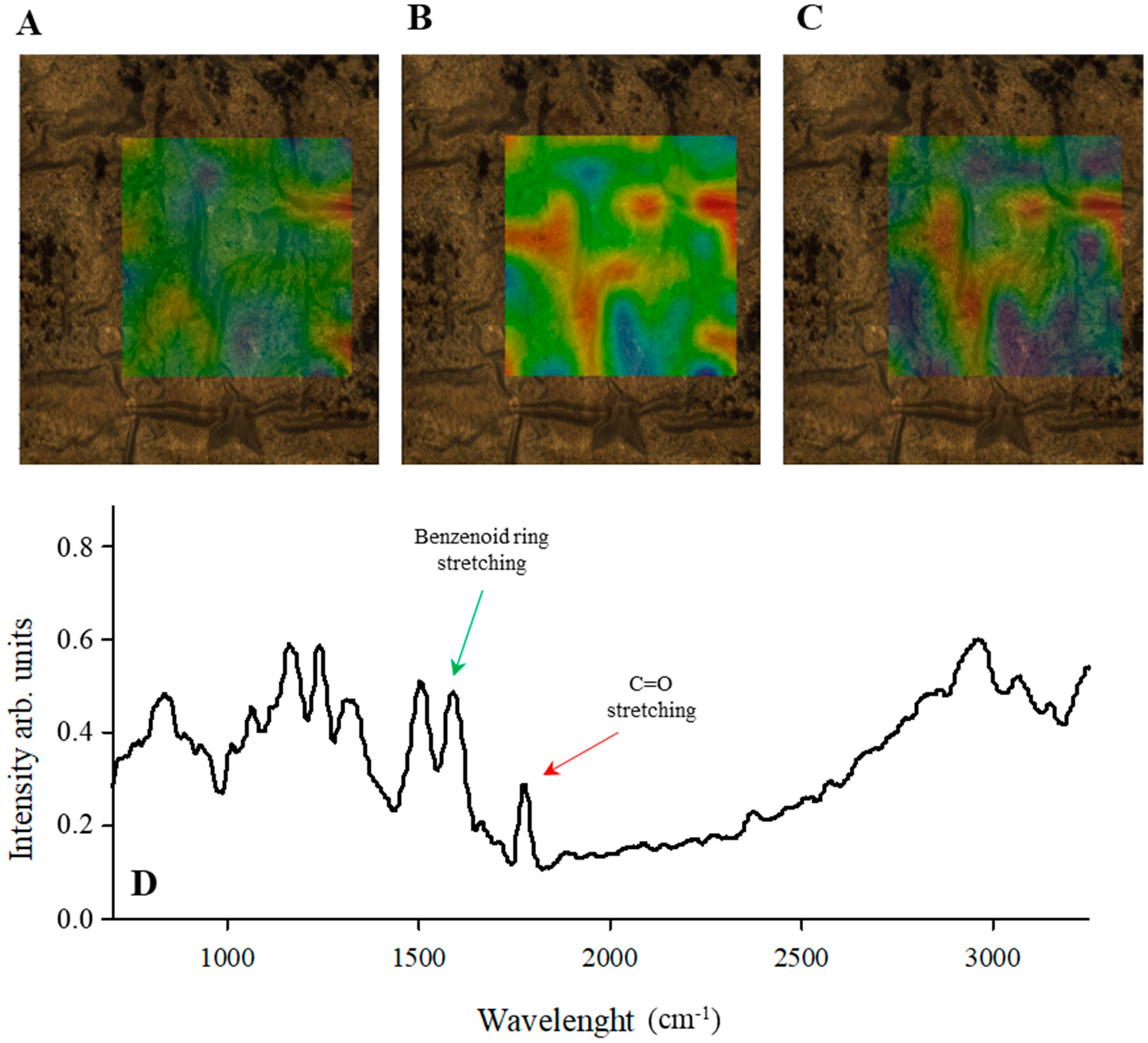
3.2. Raman and Infrared Spectroscopy Studies of PANINT/CNOsox
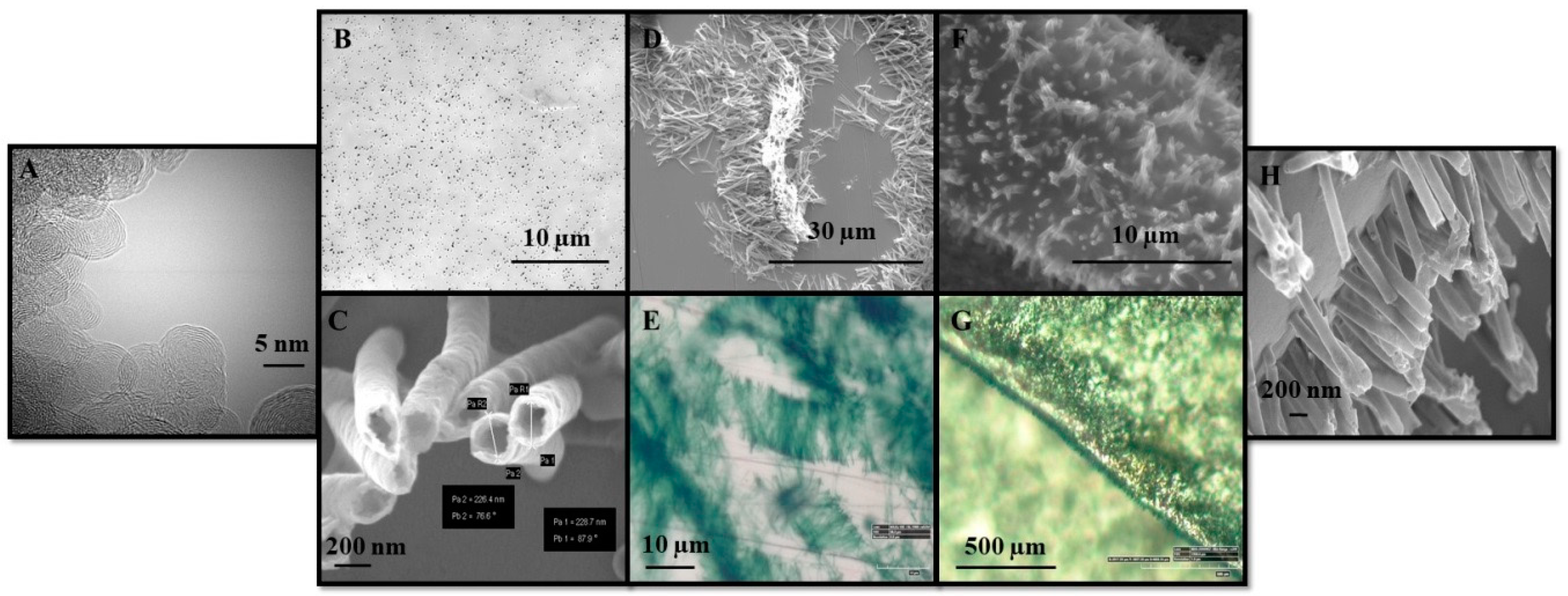
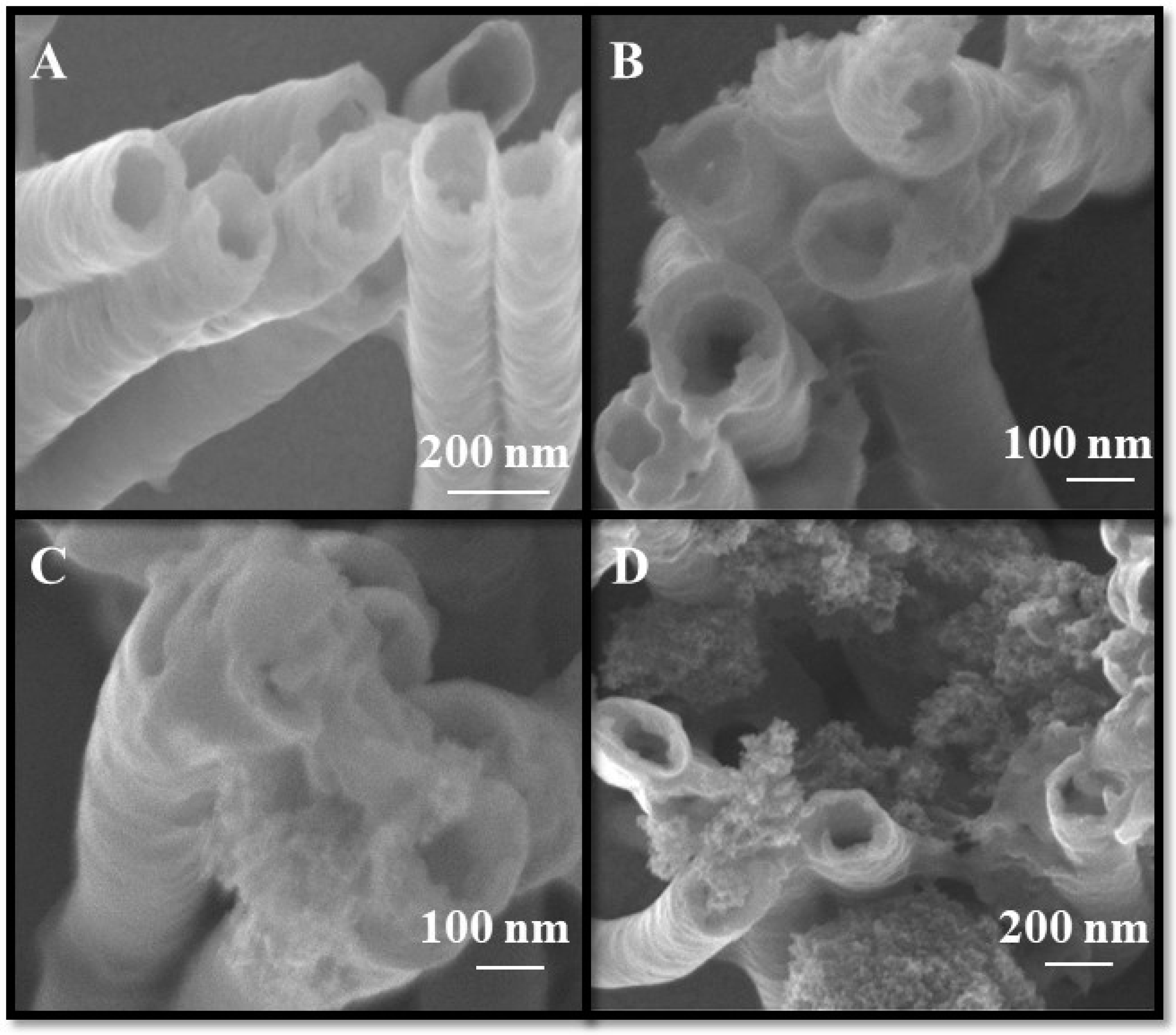
3.3. Nanocomposite Morphology Study
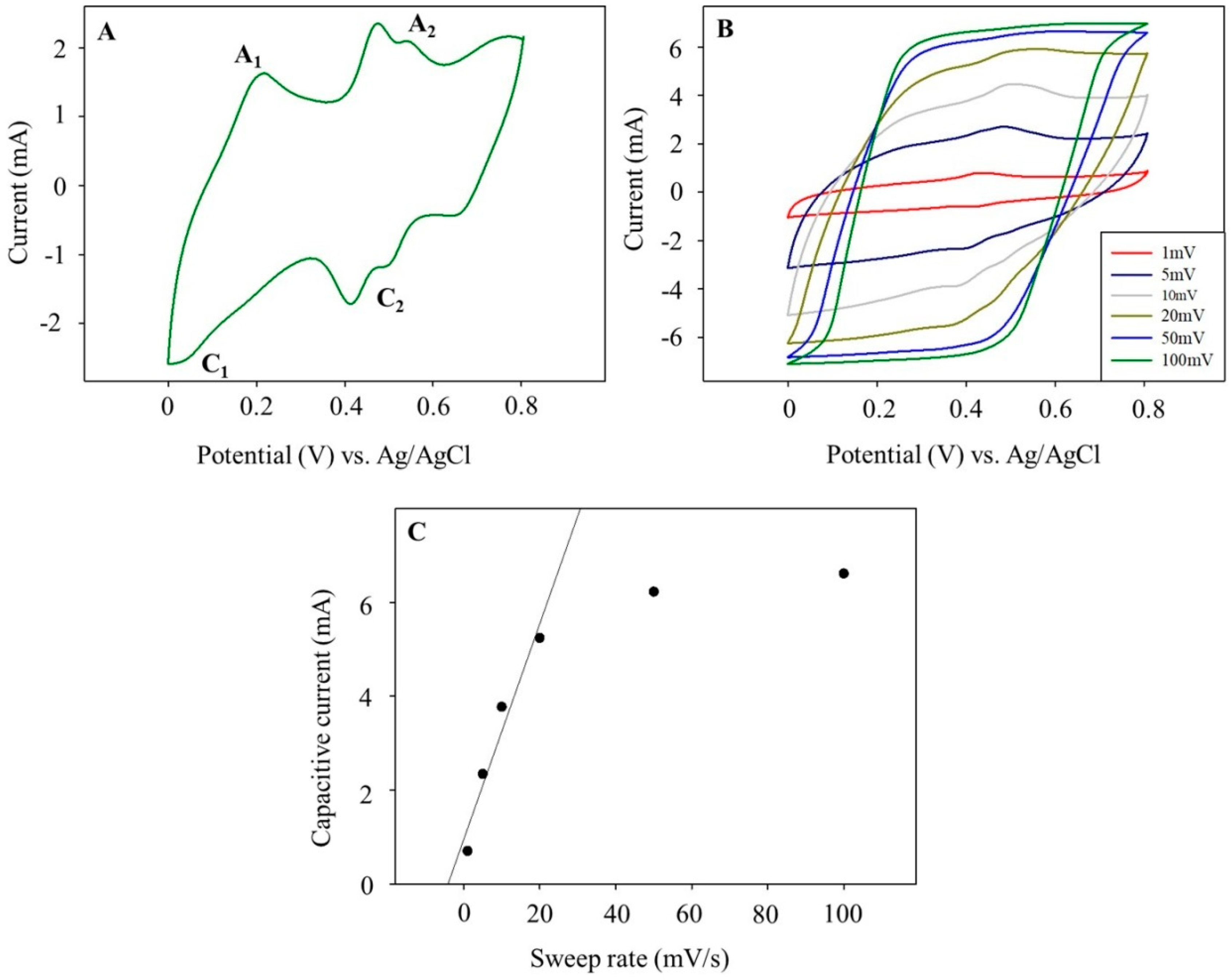
3.4. Voltammetric Studies of the PANINT/CNOsox Nanocomposite
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mosa, I.M.; Pattammattel, A.; Kadimisetty, K.; Pande, P.; El-Kady, M.F.; Bishop, G.W.; Novak, M.; Kaner, R.B.; Basu, A.K.; Kumar, C.V.; et al. Ultrathin Graphene–Protein Supercapacitors for Miniaturized Bioelectronics. Adv. Energy Mater. 2017, 7, 1700358. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Park, S.-M. Electrochemistry of Conductive Polymers 36. pH Dependence of Polyaniline Conductivities Studied by Current-Sensing Atomic Force Microscopy. J. Phys. Chem. B 2005, 109, 9305–9310. [Google Scholar] [CrossRef]
- Prigodin, V.N.; Hsu, F.C.; Park, J.H.; Waldmann, O.; Epstein, A.J. Electron-ion interaction in doped conducting polymers. Phys. Rev. B 2008, 78, 035203. [Google Scholar] [CrossRef]
- Focke, W.W.; Wnek, G.E.; Wei, Y. Influence of oxidation state, pH, and counterion on the conductivity of polyaniline. J. Phys. Chem. 1987, 91, 5813–5818. [Google Scholar] [CrossRef]
- Parthasarathy, R.V.; Martin, C.R. Template-Synthesized Polyaniline Microtubules. Chem. Mater. 1994, 6, 1627–1632. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Grover, S.; Goel, S.; Marichi, R.B.; Sahu, V.; Singh, G.; Sharma, R.K. Polyaniline All Solid-State Pseudocapacitor: Role of Morphological Variations in Performance Evolution. Electrochim. Acta 2016, 196, 131–139. [Google Scholar] [CrossRef]
- Yoon, H.; Jang, J. Conducting-Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Sutar, D.S.; Major, S.S.; Srinivasa, R.S.; Yakhmi, J.V. Conformational morphology of polyaniline grown on self-assembled monolayer modified silicon. Thin Solid Films 2011, 520, 351–355. [Google Scholar] [CrossRef]
- Chaudhari, S.; Patil, P.P. Inhibition of nickel coated mild steel corrosion by electrosynthesized polyaniline coatings. Electrochim. Acta 2011, 56, 3049–3059. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, R.; Tian, X. Novel route to polyaniline nanofibers from miniemulsion polymerization. J. Mater. Sci. 2011, 46, 1049–1052. [Google Scholar] [CrossRef]
- Cui, Z.; Coletta, C.; Rebois, R.; Baiz, S.; Gervais, M.; Goubard, F.; Aubert, P.H.; Dazzi, A.; Remita, S. Radiation-induced reduction–polymerization route for the synthesis of PEDOT conducting polymers. Radiat. Phys. Chem. 2016, 119, 157–166. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Vinu, A.; Lokhande, C.D. Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim. Acta 2011, 56, 9482–9487. [Google Scholar] [CrossRef]
- Martin, C.R. Template Synthesis of Electronically Conductive Polymer Nanostructures. Acc. Chem. Res. 1995, 28, 61–68. [Google Scholar] [CrossRef]
- Mazur, M.; Tagowska, M.; Pałys, B.; Jackowska, K. Template synthesis of polyaniline and poly(2-methoxyaniline) nanotubes: Comparison of the formation mechanisms. Electrochem. Commun. 2003, 5, 403–407. [Google Scholar] [CrossRef]
- Olejnik, P.; Gniadek, M.; Palys, B. Layers of polyaniline nanotubes deposited by langmuir–blodgett method. J. Phys. Chem. C 2012, 116, 10424–10429. [Google Scholar] [CrossRef]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, J.; Wan, M. Highly crystalline and thin polyaniline nanofibers oxidized by ferric chloride. Mater. Chem. Phys. 2009, 115, 275–279. [Google Scholar] [CrossRef]
- Rahy, A.; Yang, D.J. Synthesis of highly conductive polyaniline nanofibers. Mater. Lett. 2008, 62, 4311–4314. [Google Scholar] [CrossRef]
- Delvaux, M.; Duchet, J.; Stavaux, P.-Y.; Legras, R.; Demoustier-Champagne, S. Chemical and electrochemical synthesis of polyaniline micro- and nano-tubules. Synth. Met. 2000, 113, 275–280. [Google Scholar] [CrossRef]
- Boulanger, N.; Barbero, D.R. Ordered and Highly Conductive Carbon Nanotube Nano-Networks in a Semiconducting Polymer Film by Solution Processing. Adv. Electron. Mater. 2015, 1, 1400030. [Google Scholar] [CrossRef]
- Li, L.; Qiu, J.; Wang, S. Three-dimensional ordered nanostructures for supercapacitor electrode. Electrochim. Acta 2013, 99, 278–284. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Sata, S.A.; Buniran, S.; Sharif Zein, S.H. Remarkable Stability of Supercapacitor Material Synthesized by Manganese Oxide Filled in Multiwalled Carbon Nanotubes. Curr. Nanosci. 2010, 6, 553–559. [Google Scholar] [CrossRef]
- Mondal, C.; Ghosh, D.; Aditya, T.; Sasmal, A.K.; Pal, T. Mn3O4 nanoparticles anchored to multiwall carbon nanotubes: A distinctive synergism for high-performance supercapacitors. New J. Chem. 2015, 39, 8373–8380. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Papathanassiou, A.N.; Mykhailiv, O.; Echegoyen, L.; Sakellis, I.; Plonska-Brzezinska, M.E. Electric properties of carbon nano-onion/polyaniline composites: A combined electric modulus and ac conductivity study. J. Phys. Appl. Phys. 2016, 49, 285305. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Imierska, M.; Petelczyc, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Chemical versus Electrochemical Synthesis of Carbon Nano-onion/Polypyrrole Composites for Supercapacitor Electrodes. Chem. Eur. J. 2015, 21, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Papathanassiou, A.N.; Plonska-Brzezinska, M.E.; Mykhailiv, O.; Echegoyen, L.; Sakellis, I. Combined high permittivity and high electrical conductivity of carbon nano-onion/polyaniline composites. Synth. Met. 2015, 209, 583–587. [Google Scholar] [CrossRef]
- Grądzka, E.; Winkler, K.; Borowska, M.; Plonska-Brzezinska, M.E.; Echegoyen, L. Comparison of the electrochemical properties of thin films of MWCNTs/C60-Pd, SWCNTs/C60-Pd and ox-CNOs/C60-Pd. Electrochim. Acta 2013, 96, 274–284. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Z.Y.; Li, L.; Zhang, Y.; Wei, Y.L.; Wang, L.F.; Zhu, M.F. Polyaniline/multi-walled carbon nanotube composites with core–shell structures as supercapacitor electrode materials. Electrochim. Acta 2010, 55, 3904–3908. [Google Scholar] [CrossRef]
- Ning, G.; Li, T.; Yan, J.; Xu, C.; Wei, T.; Fan, Z. Three-dimensional hybrid materials of fish scale-like polyaniline nanosheet arrays on graphene oxide and carbon nanotube for high-performance ultracapacitors. Carbon 2013, 54, 241–248. [Google Scholar] [CrossRef]
- Lee, T.; Yun, T.; Park, B.; Sharma, B.; Song, H.-K.; Kim, B.-S. Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: Importance of establishing intimate electronic contact through nanoscale blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef]
- Al-Jishi, R.; Dresselhaus, G. Lattice-dynamical model for graphite. Phys. Rev. B 1982, 26, 4514–4522. [Google Scholar] [CrossRef]
- Bacon, R. Growth, Structure, and Properties of Graphite Whiskers. J. Appl. Phys. 1960, 31, 283–290. [Google Scholar] [CrossRef]
- Banhart, F. Structural transformations in carbon nanoparticles induced by electron irradiation. Phys. Solid State 2002, 44, 399–404. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Rummeli, M.H.; Gemming, T.; Lange, H.; Huczko, A. Catalyst-free synthesis of onion-like carbon nanoparticles. New Carbon Mater. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Cabioc’h, T.; Jaouen, M.; Rivière, J.P.; Delafond, J.; Hug, G. Characterization and growth of carbon phases synthesized by high temperature carbon ion implantation into copper. Diam. Relat. Mater. 1997, 6, 261–265. [Google Scholar] [CrossRef]
- Palkar, A.; Melin, F.; Cardona, C.M.; Elliott, B.; Naskar, A.K.; Edie, D.D.; Kumbhar, A.; Echegoyen, L. Reactivity Differences between Carbon Nano Onions (CNOs) Prepared by Different Methods. Chem. Asian J. 2007, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Gruen, D.M.; Shenderova, O.A.; Vul’, A.Y. (Eds.) Synthesis, Properties and Applications of Ultrananocrystalline Diamond; Springer: Berlin/Heidelberg, Germany, 2005; Volume 192. [Google Scholar]
- Plonska-Brzezinska, M.E.; Echegoyen, L. Carbon nano-onions for supercapacitor electrodes: Recent developments and applications. J. Mater. Chem. A 2013, 1, 13703–13714. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, D.M.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. A new perspective on carbon nano-onion/nickel hydroxide/oxide composites: Physicochemical properties and application in hybrid electrochemical systems. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 193–203. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Czyrko, J.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon nano-onion composites: Physicochemical characteristics and biological activity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 185–192. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Chuvilin, A.L.; Moroz, E.M.; Kolomiichuk, V.N.; Shaikhutdinov, S.K.; Butenko, Y.V.; Mal’kov, I.Y. Effect of explosion conditions on the structure of detonation soots: Ultradisperse diamond and onion carbon. Carbon 1994, 32, 873–882. [Google Scholar] [CrossRef]
- Wong, S.S.; Woolley, A.T.; Joselevich, E.; Cheung, C.L.; Lieber, C.M. Covalently-Functionalized Single-Walled Carbon Nanotube Probe Tips for Chemical Force Microscopy. J. Am. Chem. Soc. 1998, 120, 8557–8558. [Google Scholar] [CrossRef]
- Salzmann, C.G.; Llewellyn, S.A.; Tobias, G.; Ward, M.A.H.; Huh, Y.; Green, M.L.H. The Role of Carboxylated Carbonaceous Fragments in the Functionalization and Spectroscopy of a Single-Walled Carbon-Nanotube Material. Adv. Mater. 2007, 19, 883–887. [Google Scholar] [CrossRef]
- Pujals, D.C.; de Fuentes, O.A.; García, L.F.D.; Cazzanelli, E.; Caputi, L.S. Raman spectroscopy of polyhedral carbon nano-onions. Appl. Phys. A 2015, 120, 1339–1345. [Google Scholar] [CrossRef]
- Bogdanov, K.; Fedorov, A.; Osipov, V.; Enoki, T.; Takai, K.; Hayashi, T.; Ermakov, V.; Moshkalev, S.; Baranov, A. Annealing-induced structural changes of carbon onions: High-resolution transmission electron microscopy and Raman studies. Carbon 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.; Torres, J.; Lee, S.H.; Yun, M. Facile and template-free method toward chemical synthesis of polyaniline film/nanotube structures. J. Polym. Sci. Part Polym. Chem. 2017, 55, 3973–3979. [Google Scholar] [CrossRef]
- Do Nascimento, G.M.; Temperini, M.R.L. Studies on the resonance Raman spectra of polyaniline obtained with near-IR excitation. J. Raman Spectrosc. 2008, 39, 772–778. [Google Scholar] [CrossRef]
- Tucceri, R.; Arnal, P.M.; Scian, A.N. Spectroscopic Characterization of Poly(ortho-Aminophenol) Film Electrodes: A Review Article. J. Spectrosc. 2013, 2013, 951604. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. The chemical oxidative polymerization of aniline in water: Raman spectroscopy. J. Raman Spectrosc. 2008, 39, 1375–1387. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water—FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Jabłońska, A.; Gniadek, M.; Pałys, B. Enhancement of Direct Electrocatalytic Activity of Horseradish Peroxidase on Polyaniline Nanotubes. J. Phys. Chem. C 2015, 119, 12514–12522. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- Morsi, R.E.; Khamis, E.A.; Al-Sabagh, A.M. Polyaniline nanotubes: Facile synthesis, electrochemical, quantum chemical characteristics and corrosion inhibition efficiency. J. Taiwan Inst. Chem. Eng. 2016, 60, 573–581. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E.; Dubis, A.T.; Lapinski, A.; Villalta-Cerdas, A.; Echegoyen, L. Electrochemical Properties of Oxidized Carbon Nano-Onions: DRIFTS-FTIR and Raman Spectroscopic Analyses. ChemPhysChem 2011, 12, 2659–2668. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Gan, M.; Fu, S.; Dai, W.; Zhou, T.; Sun, X.; Wang, H.; Wang, H. Free-standing 3D graphene/polyaniline composite film electrodes for high-performance supercapacitors. J. Power Sources 2015, 299, 347–355. [Google Scholar] [CrossRef]
- Kim, B.; Kwon, J.; Ko, J.; Park, J.; Too, C.; Wallace, G. Preparation and enhanced stability of flexible supercapacitor prepared from nafion/polyaniline nano-fiber. Synth. Met. 2010, 160, 94–98. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Dubal, D.P.; Jamadade, V.S.; Salunkhe, R.R.; Lokhande, C.D. Fuzzy nanofibrous network of polyaniline electrode for supercapacitor application. Synth. Met. 2010, 160, 519–522. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Salunkhe, R.R.; Jamadade, V.S.; Dubal, D.P.; Pawar, S.M.; Lokhande, C.D. Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr. Appl. Phys. 2010, 10, 904–909. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Chang, J.; Shin, S.R.; Mane, R.S.; Han, S.-H.; Kim, S.J. Hydrogel-Assisted Polyaniline Microfiber as Controllable Electrochemical Actuatable Supercapacitor. J. Electrochem Soc. 2009, 156, A313–A317. [Google Scholar] [CrossRef]
- Bélanger, D.; Ren, X.; Davey, J.; Uribe, F.; Gottesfeld, S. Characterization and Long-Term Performance of Polyaniline-Based Electrochemical Capacitors. J. Electrochem. Soc. 2000, 147, 2923–2929. [Google Scholar] [CrossRef]
- Otrokhov, G.; Pankratov, D.; Shumakovich, G.; Khlupova, M.; Zeifman, Y.; Vasil’eva, I.; Morozova, O.; Yaropolov, A. Enzymatic synthesis of polyaniline/multi-walled carbon nanotube composite with core shell structure and its electrochemical characterization for supercapacitor application. Electrochim. Acta 2014, 123, 151–157. [Google Scholar] [CrossRef]
- Zhou, S.; Mo, S.; Zou, W.; Jiang, F.; Zhou, T.; Yuan, D. Preparation of polyaniline/2-dimensional hexagonal mesoporous carbon composite for supercapacitor. Synth. Met. 2011, 161, 1623–1628. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J.; Choi, M.; Yoon, S.-H. Fabrication and characterization of polyaniline coated carbon nanofiber for supercapacitor. Carbon 2005, 43, 2730–2736. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Feng, Y.; Feng, W. Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor. Electrochim. Acta 2013, 90, 95–100. [Google Scholar] [CrossRef]
- Xia, X.; Hao, Q.; Lei, W.; Wang, W.; Sun, D.; Wang, X. Nanostructured ternary composites of graphene/Fe2O3/polyaniline for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16844–16850. [Google Scholar] [CrossRef]


| Specific Capacitance (F g−1) | ||||
|---|---|---|---|---|
| Pristine PANINT | PANINT/CNOsox Composite | |||
| Sweep rate (mV s−1) | C1 | C2 | C1 | C2 |
| 1 | 237 | 269 | 795 | 946 |
| 5 | - | - | 741 | 681 |
| 10 | - | - | 616 | 614 |
| 20 | - | - | 431 | 441 |
| 50 | - | - | 213 | 200 |
| 100 | 53 | 70 | 115 | 169 |
| Material | Sweep Rate (mV s−1) | Potential Range (V) | Electrolyte | Specific Capacitance (F g−1) | References |
|---|---|---|---|---|---|
| PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 503 | [15]. |
| PANI | 10 | −0.2–0.6 | Nafion | 269 | [64] |
| Nanofibrous PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 839 | [65] |
| Nanofibrous PANI | 10 | −0.1–0.8 | 1 M H2SO4 | 861 | [66] |
| Hydrogel-assisted PANI microfiber | 10 | −0.2–0.8 | 1 M methane sulfonic acid | 703 | [67] |
| BF4-doped PANI | 50 | 0–0.75 | 4 M HBF | 74 | [68] |
| PANI/CNT | 5 | −0.1–0.7 | PVA/H3PO4 | 440 | [69] |
| PANI/MWCNT | 1 | 0–1.0 | 0.1 M H2SO4 | 560 | [33] |
| Mesoporous C/PANI | 2 | −0.1–1.0 | 1 M H2SO4 | 470 | [70] |
| PANI on CNF | 5 | 0–0.8 | 1 M H2SO4 | 264 | [71] |
| PANI/GO | 1 | −0.1–0.9 | 1 M H2SO4 | 1136 | [72] |
| G/Fe2O3/PANI | 1 | −1.0–0.1 | 1 M KOH | 638 | [73] |
| PANINT/CNOsox | 1 | 0–0.8 | 1 M H2SO4 | 946 | this work |
| PANINT/CNOsox | 10 | 0–0.8 | 1 M H2SO4 | 614 | this work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers 2018, 10, 1408. https://doi.org/10.3390/polym10121408
Olejnik P, Gniadek M, Echegoyen L, Plonska-Brzezinska ME. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers. 2018; 10(12):1408. https://doi.org/10.3390/polym10121408
Chicago/Turabian StyleOlejnik, Piotr, Marianna Gniadek, Luis Echegoyen, and Marta E. Plonska-Brzezinska. 2018. "Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors" Polymers 10, no. 12: 1408. https://doi.org/10.3390/polym10121408
APA StyleOlejnik, P., Gniadek, M., Echegoyen, L., & Plonska-Brzezinska, M. E. (2018). Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers, 10(12), 1408. https://doi.org/10.3390/polym10121408







