Fluorescent Amorphous Distyrylnaphthalene-Based Polymers: Synthesis, Characterization and Thin-Film Nanomolar Sensing of Nitroaromatics in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods and Instrumentation
2.2. Materials and Synthesis
2.3. Molecular Modeling
3. Results and Discussion
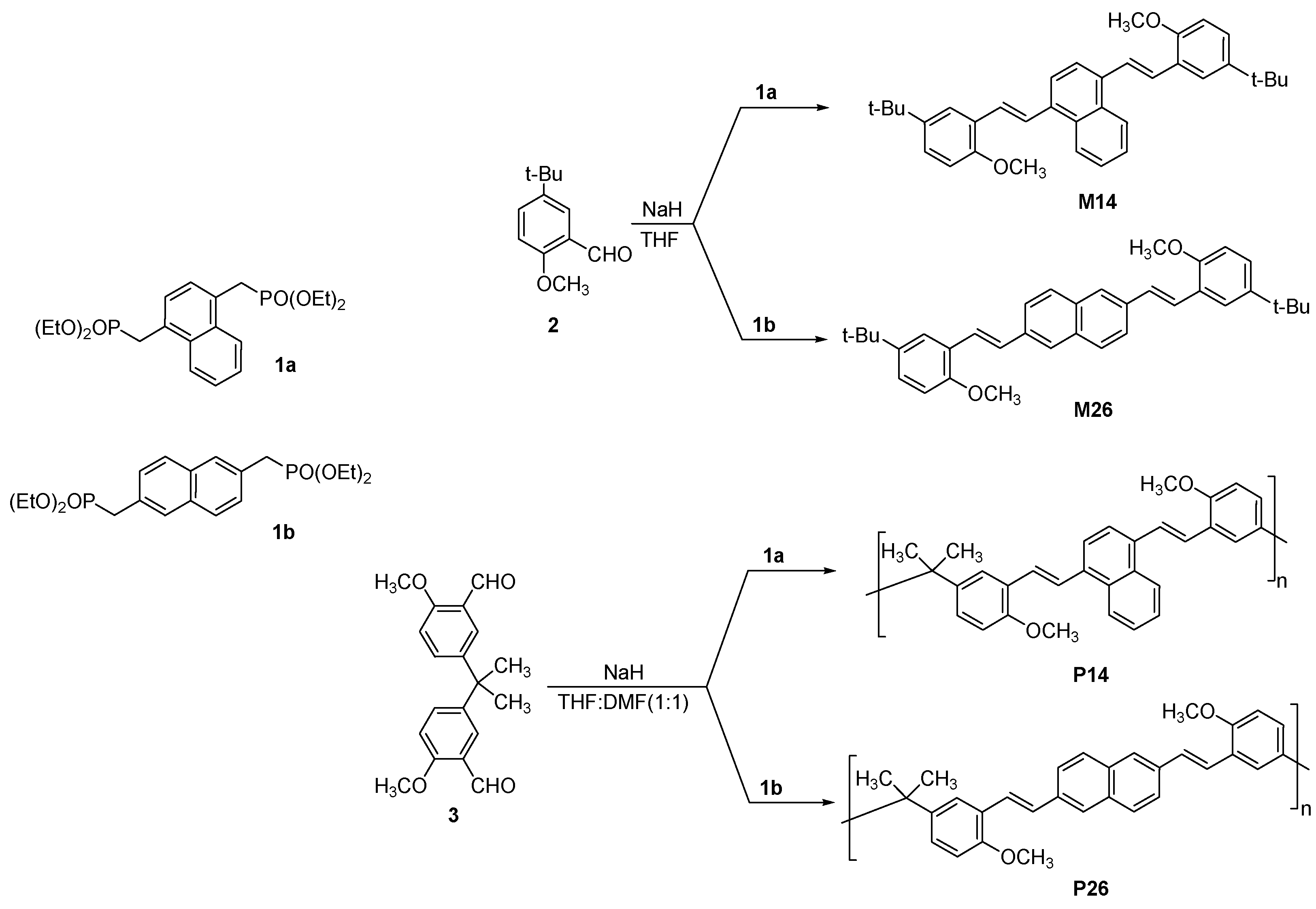
3.1. Synthesis
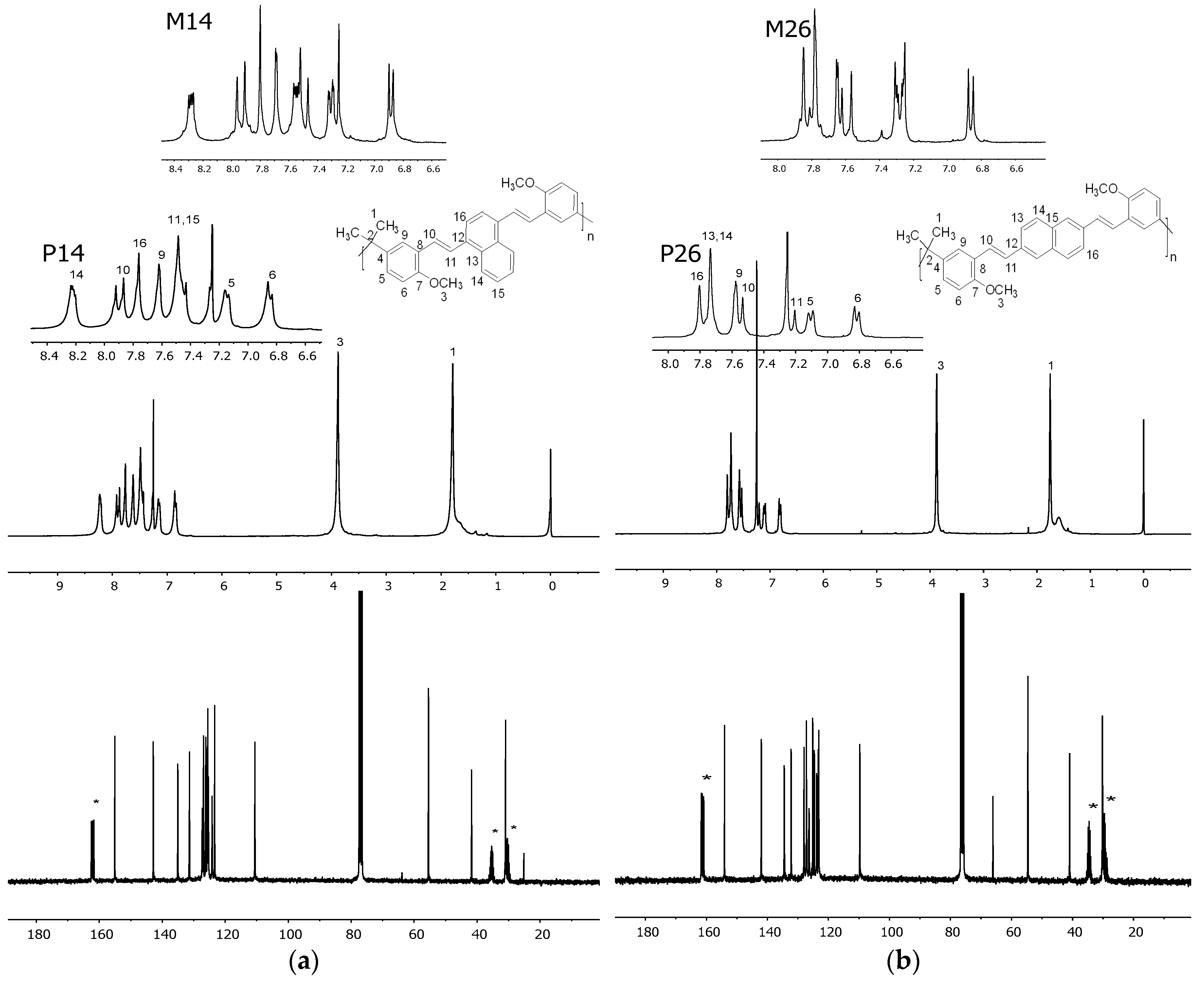
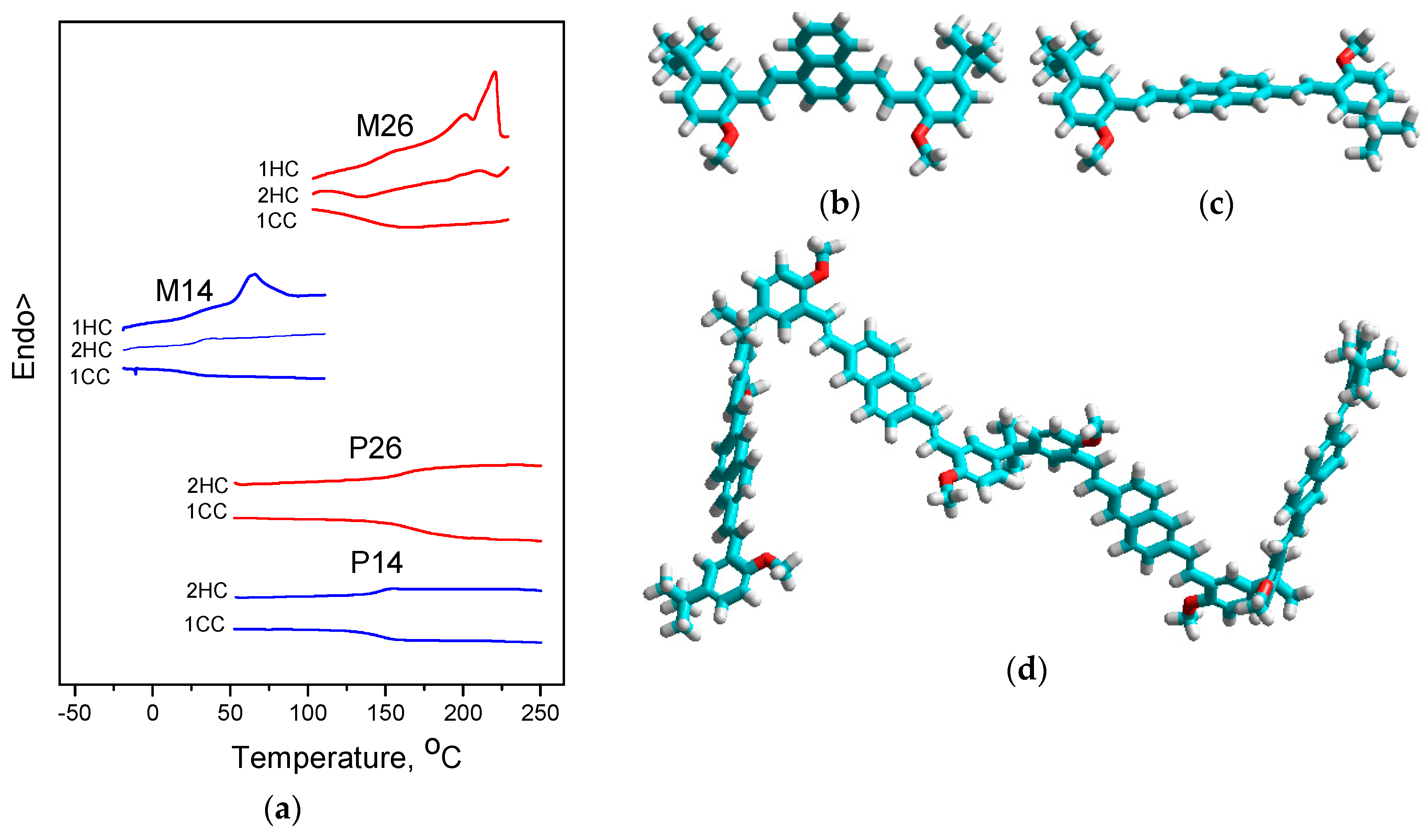
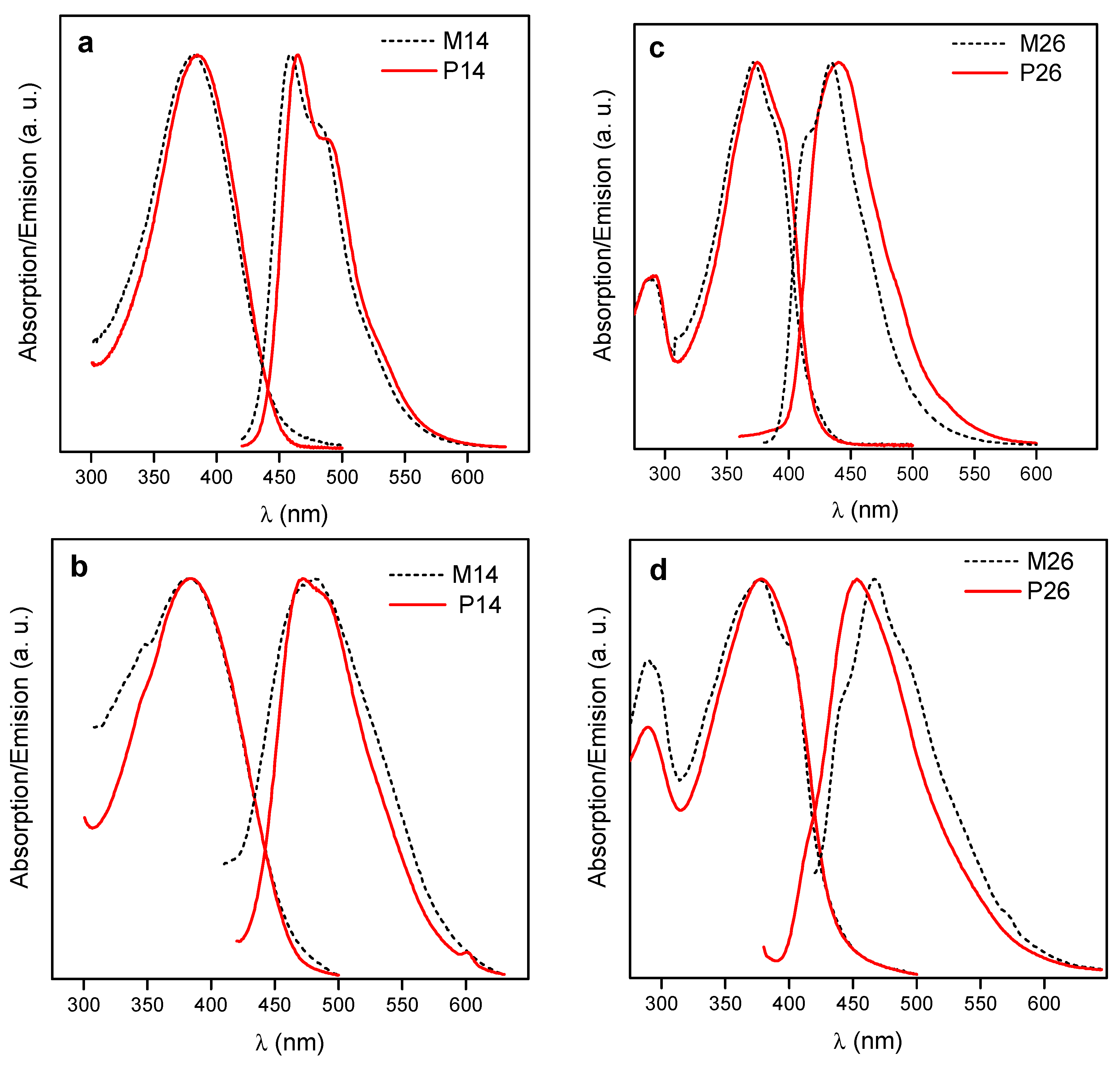
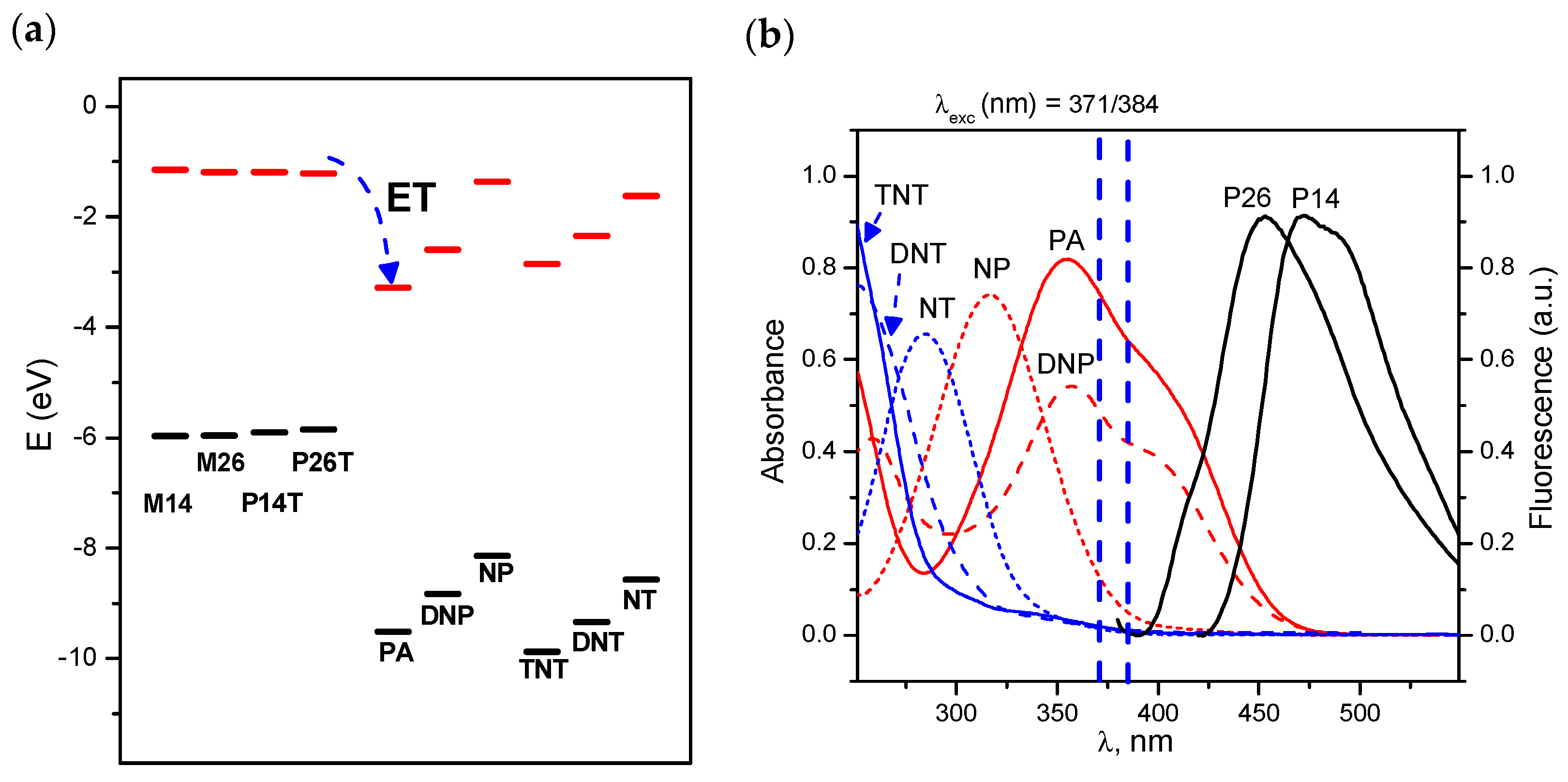
3.2. Morphology. Thermal and Optical Properties
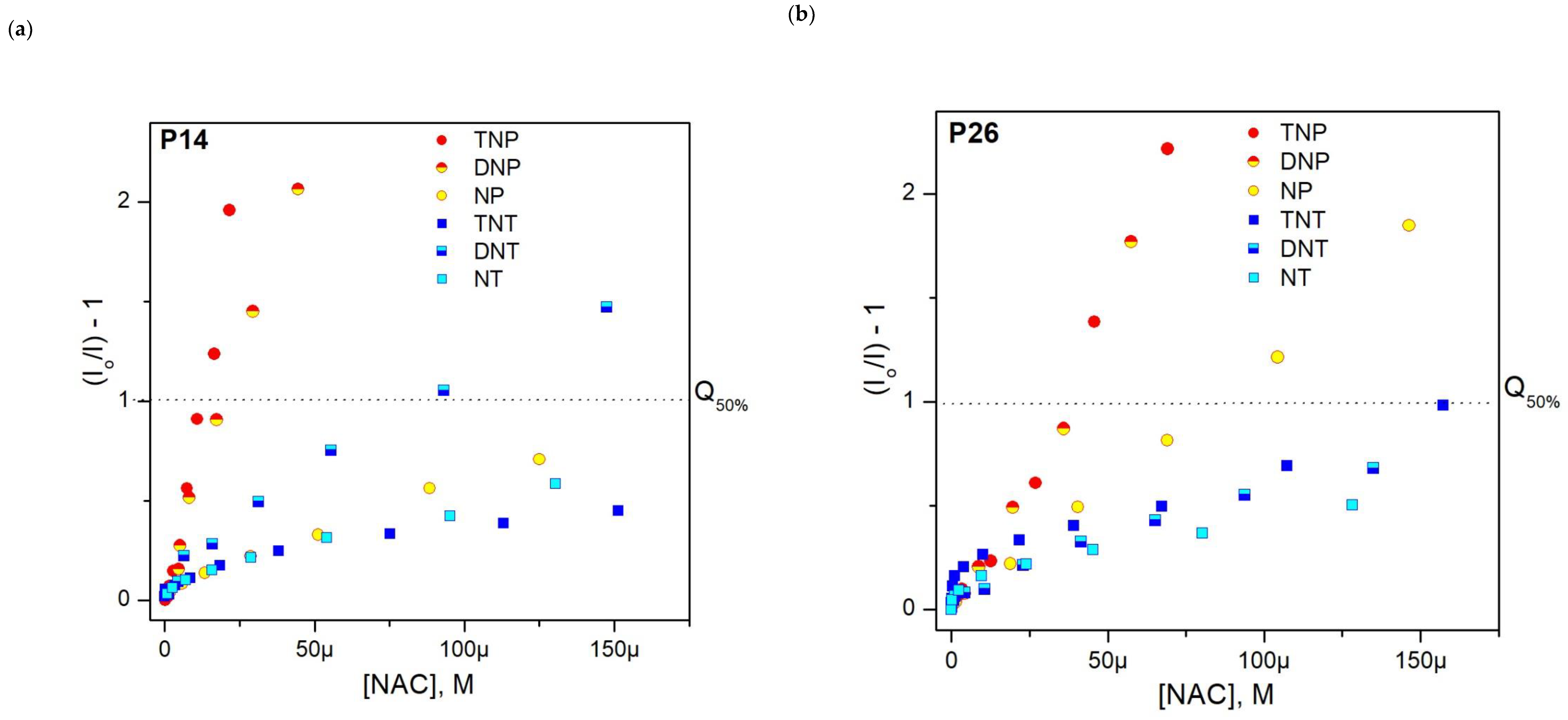
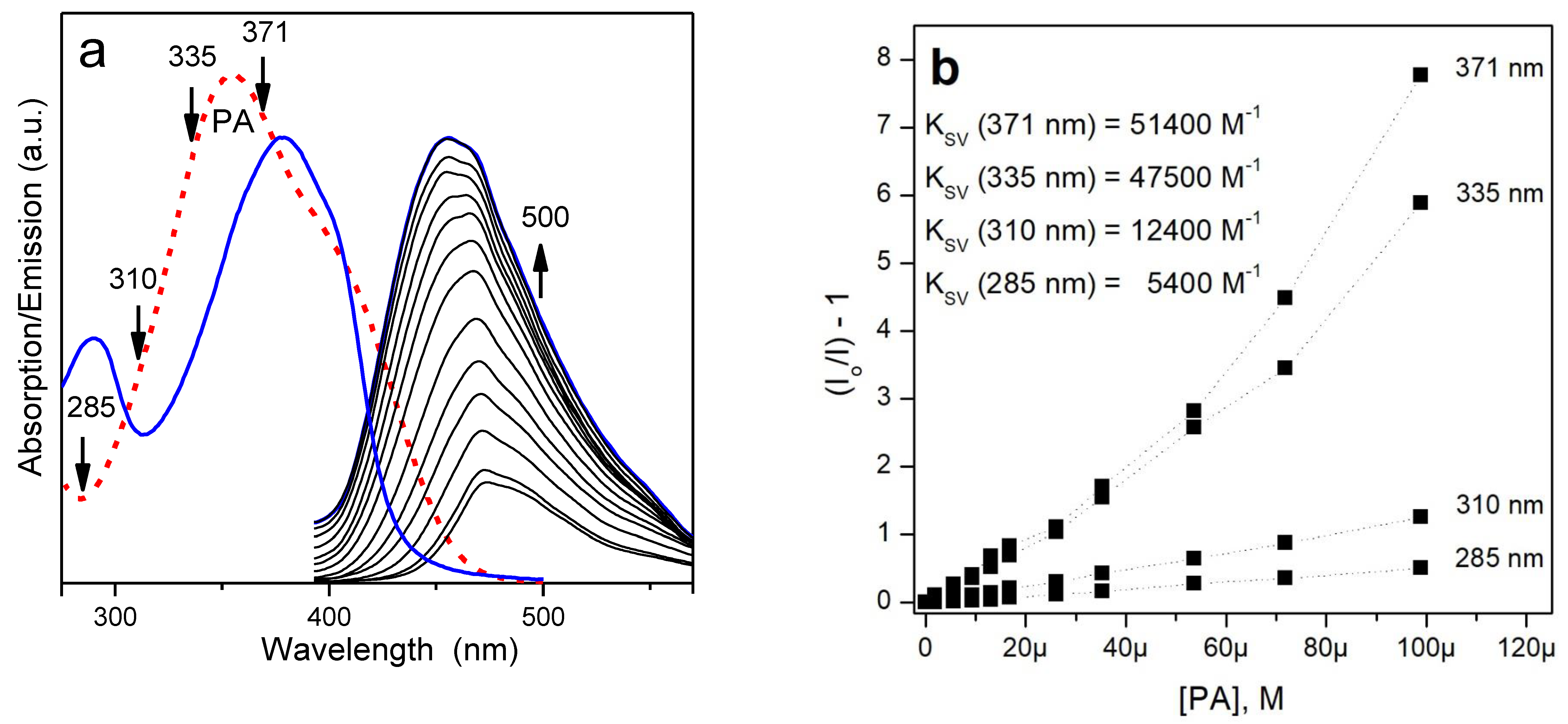
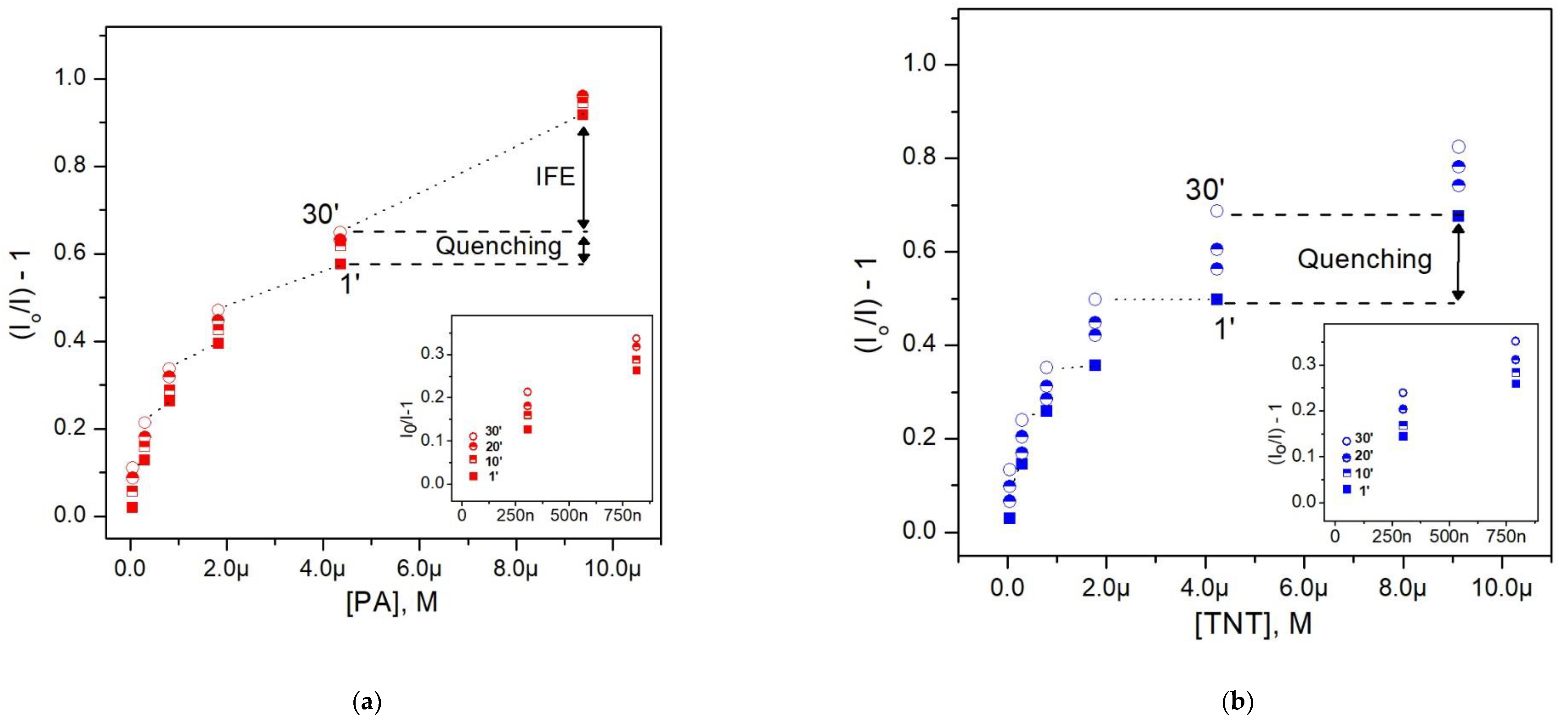
3.3. Fluorescence Response to Nitroaromatic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, M.R.; Lu, Z.; Li, X.; Sarker, A.K.; Hu, L.; Choi, P.; Li, X.; Hakobyan, N.; Serpe, M.J. Responsive polymers for analytical applications: A review. Anal. Chim. Acta 2013, 789, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.-S.; Wen, H.-W.; Chen, M.-T.; Yang, P.-C. Enhanced sensing ability of fluorescent chemosensors with triphenylamine-functionalized conjugated polyfluorene. Sens. Actuators B 2016, 231, 399–411. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; Mukherjee, P.S. π-Electron rich small molecule sensors for the recognition of nitroaromatics. Chem. Commun. 2015, 51, 16014–16032. [Google Scholar] [CrossRef]
- Ding, L.; Fang, Y. Chemically assembled monolayers of fluorophores as chemical sensing materials. Chem. Soc. Rev. 2010, 39, 4258–4273. [Google Scholar] [CrossRef] [PubMed]
- Squeo, B.M.; Gregoriou, V.G.; Avgeropoulos, A.; Baysec, S.; Allard, S.; Scherf, U.; Chochos, C.L. BODIPY-based polymeric dyes as emerging horizon materials for biological sensing and organic electronic applications. Prog. Polym. Sci. 2017, 71, 26–52. [Google Scholar] [CrossRef]
- Ali, M.A.; Chen, S.S.Y.; Cavaye, H.; Smith, A.R.G.; Burn, P.L.; Gentle, I.R.; Meredith, P.; Shaw, E. Diffusion of nitroaromatic vapours into fluorescent dendrimer films for explosives detection. Sens. Actuators B 2015, 210, 550–557. [Google Scholar] [CrossRef]
- Guan, W.; Zhou, W.; Lu, J.; Lu, C. Luminescent films for chemo-and biosensing. Chem. Soc. Rev. 2015, 44, 6981–7009. [Google Scholar] [CrossRef] [PubMed]
- Rochat, S.; Swager, T.M. Conjugated amplifying polymers for optical sensing applications. ACS Appl. Mater. Interfaces 2013, 5, 4488–4502. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Rodgers, J.D.; Bunce, N.J. Treatment methods for the remediation of nitroaromatic explosives. Water Res. 2001, 35, 2101–2111. [Google Scholar] [CrossRef]
- Del Rosso, P.G.; Romagnolli, M.J.; Almassio, M.F.; Barbero, C.A.; Garay, R.O. Diphenylanthrylene and diphenylfluorene-based segmented conjugated polymer films as fluorescent chemosensors for nitroaromatics in aqueous solution. Sens. Actuators B 2014, 203, 612–620. [Google Scholar] [CrossRef]
- Almassio, M.F.; Romagnoli, M.J.; Schvval, A.B.; Del Rosso, P.G.; Garay, R.O. Distyrylbenzene-based segmented conjugated polymers: Synthesis, thin film morphology and chemosensing of hydrophobic and hydrophilic nitroaromatics in aqueous media. Polymer 2017, 113, 167–179. [Google Scholar] [CrossRef]
- Mazumdar, P.; Maity, S.; Shyamal, M.; Das, D.; Sahoo, G.P.; Misra, A. Proton triggered emission and selective sensing of picric acid by the fluorescent aggregates of 6, 7-dimethyl-2, 3-bis-(2-pyridyl)-quinoxaline. Phys. Chem. Chem. Phys. 2016, 18, 7055–7067. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Zhu, Z.; Wang, X.; Xia, H.; Wang, Y.; Li, D. Poly{tris[4-(2-Thienyl)phenyl] amine} fluorescent conjugated microporous polymer for selectively sensing picric acid. Sens. Actuators B 2017, 244, 334–343. [Google Scholar] [CrossRef]
- Chowdhury, A.; Mukherjee, P.S. Electron-rich triphenylamine-based sensors for picric acid detection. J. Org. Chem. 2015, 80, 4064–4075. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cao, D.; Yun, J.; Zeng, X. Highly selective detection of picric acid from multicomponent mixtures of nitro explosives by using COP luminescent probe. Sens. Actuators B 2017, 243, 753–760. [Google Scholar] [CrossRef]
- Tian, X.; Qi, X.; Liu, X.; Zhang, Q. Selective detection of picric acid by a fluorescent ionic liquid chemosensor. Sens. Actuators B 2016, 229, 520–527. [Google Scholar] [CrossRef]
- Duraimurugan, K.; Balasaravanan, R.; Siva, A. Electron rich triphenylamine derivatives (D-π-D) for selective sensing of picric acid in aqueous media. Sens. Actuators B 2016, 231, 302–312. [Google Scholar] [CrossRef]
- Wang, B.; Mu, Y.; Zhang, C.; Li, J. Blue photoluminescent carbon nanodots prepared from zeolite as efficient sensors for picric acid detection. Sens. Actuators B 2017, 253, 911–917. [Google Scholar] [CrossRef]
- Sun, X.; He, J.; Meng, Y.; Zhang, L.; Zhang, S.; Ma, X.; Lei, Y. Microwave-assisted ultrafast and facile synthesis of fluorescent carbon nanoparticles from a single precursor: Preparation, characterization and their application for the highly selective detection of explosive picric acid. J. Mater. Chem. A 2016, 4, 4161–4171. [Google Scholar] [CrossRef]
- Liang, H.; Yao, Z.; Ge, W.; Qiao, Y.; Zhang, L.; Cao, Z.; Wu, H.C. Selective and sensitive detection of picric acid based on a water-soluble fluorescent probe. RSC Adv. 2016, 6, 38328–38331. [Google Scholar] [CrossRef]
- Sahoo, J.; Waghmode, S.B.; Subramanian, P.S.; Albrecht, M. Specific Detection of Picric Acid and Nitrite in Aqueous Medium Using Flexible Eu (III)-Based Luminescent Probe. ChemistrySelect 2016, 1, 1943–1948. [Google Scholar] [CrossRef]
- Bagheri, M.; Masoomi, M.Y.; Morsali, A.; Schoedel, A. Two Dimensional Host–Guest Metal–Organic Framework Sensor with High Selectivity and Sensitivity to Picric Acid. ACS Appl. Mater. Interfaces 2016, 8, 21472–21479. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.S.; Hussain, S.; Malik, A.H.; Afroz, M.A.; Iyer, P.K. Inner filter effect based selective detection of nitroexplosive-picric acid in aqueous solution and solid support using conjugated polymer. ACS Sens. 2016, 1, 1070–1077. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Han, D.; Sun, R.; Zhang, J.; Feng, S. New Polyhedral Oligomeric Silsesquioxanes-Based Fluorescent Ionic Liquids: Synthesis, Self-Assembly and Application in Sensors for Detecting Nitroaromatic Explosives. Polymers 2018, 10, 917. [Google Scholar] [CrossRef]
- Ganiga, M.; Mani, N.P.; Cyriac, J. Synthesis of Organophilic Carbon Dots, Selective Screening of Trinitrophenol and a Comprehensive Understanding of Luminescence Quenching Mechanism. ChemistrySelect 2018, 3, 4663–4668. [Google Scholar] [CrossRef]
- Benfaremo, N.; Sandman, D.J.; Tripathy, S.; Kumar, J.; Yang, K.; Rubner, M.F.; Lyons, C. Synthesis and characterization of luminescent polymers of distyrylbenzenes with oligo (ethylene glycol) spacers. Macromolecules 1998, 31, 3595–3599. [Google Scholar] [CrossRef]
- Neese, F.; The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J. Chem. Phys. 2015, 143, 054107. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, F.; Duché, D.; Ruiz, C.M.; Simon, J.J.; Escoubas, L. Study of Optical Properties and Molecular Aggregation of Conjugated Low Band Gap Copolymers: PTB7 and PTB7-Th. J. Phys. Chem. C 2015, 119, 24643–24648. [Google Scholar] [CrossRef]
- Lampert, Z.E.; Reynolds, C.L., Jr.; Papanikolas, J.M.; Aboelfotoh, M.O. Controlling morphology and chain aggregation in semiconducting conjugated polymers: The role of solvent on optical gain in MEH-PPV. J. Phys. Chem. B 2012, 116, 12835–12841. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Schibilla, F.; Stegemann, L.; Strassert, C.A.; Rizzo, F.; Ravoo, B.J. Fluorescence quenching in β-cyclodextrin vesicles: Membrane confinement and host–guest interactions. Photochem. Photobiol. Sci. 2016, 15, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, T.K.; Palai, A.K.; Rath, S.K.; Sharma, S.K.; Sudarshan, K.; Pujari, P.K.; Patri, M.; Mishra, S.P. Pentiptycene-tbutylpyrene based poly (arylene-ethynylene) s: Highly sensitive and selective TNT sensor in aqueous as well as vapor phase. Sens. Actuators B 2017, 252, 901–911. [Google Scholar] [CrossRef]
- Dong, W.; Fei, T.; Scherf, U. Conjugated polymers containing tetraphenylethylene in the backbones and side-chains for highly sensitive TNT detection. RSC Adv. 2018, 8, 5760–5767. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Y.; He, B.; Lam, J.; Tang, B. Functional Poly (dihalopentadiene) s: Stereoselective Synthesis, Aggregation-Enhanced Emission and Sensitive Detection of Explosives. Polymers 2018, 10, 821. [Google Scholar] [CrossRef]
- Mondal, S.; Jana, A.; Bera, R.; Das, N. Design and synthesis of triptycene based fluorescent polymer with pendent triazole: Effect of functionality on host–guest interaction. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3725–3735. [Google Scholar] [CrossRef]
- Borissevitch, I.E. More about the inner filter effect: Corrections of Stern–Volmer fluorescence quenching constants are necessary at very low optical absorption of the quencher. J. Lumin. 1999, 81, 219–224. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garay, R.O.; Schvval, A.B.; Almassio, M.F.; Del Rosso, P.G.; Romagnoli, M.J.; Montani, R.S. Fluorescent Amorphous Distyrylnaphthalene-Based Polymers: Synthesis, Characterization and Thin-Film Nanomolar Sensing of Nitroaromatics in Water. Polymers 2018, 10, 1366. https://doi.org/10.3390/polym10121366
Garay RO, Schvval AB, Almassio MF, Del Rosso PG, Romagnoli MJ, Montani RS. Fluorescent Amorphous Distyrylnaphthalene-Based Polymers: Synthesis, Characterization and Thin-Film Nanomolar Sensing of Nitroaromatics in Water. Polymers. 2018; 10(12):1366. https://doi.org/10.3390/polym10121366
Chicago/Turabian StyleGaray, Raúl O., Ana B. Schvval, Marcela F. Almassio, Pablo G. Del Rosso, Maria J. Romagnoli, and Rosana S. Montani. 2018. "Fluorescent Amorphous Distyrylnaphthalene-Based Polymers: Synthesis, Characterization and Thin-Film Nanomolar Sensing of Nitroaromatics in Water" Polymers 10, no. 12: 1366. https://doi.org/10.3390/polym10121366
APA StyleGaray, R. O., Schvval, A. B., Almassio, M. F., Del Rosso, P. G., Romagnoli, M. J., & Montani, R. S. (2018). Fluorescent Amorphous Distyrylnaphthalene-Based Polymers: Synthesis, Characterization and Thin-Film Nanomolar Sensing of Nitroaromatics in Water. Polymers, 10(12), 1366. https://doi.org/10.3390/polym10121366





