High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries
Abstract
1. Introduction
2. Experimental
2.1. Materials
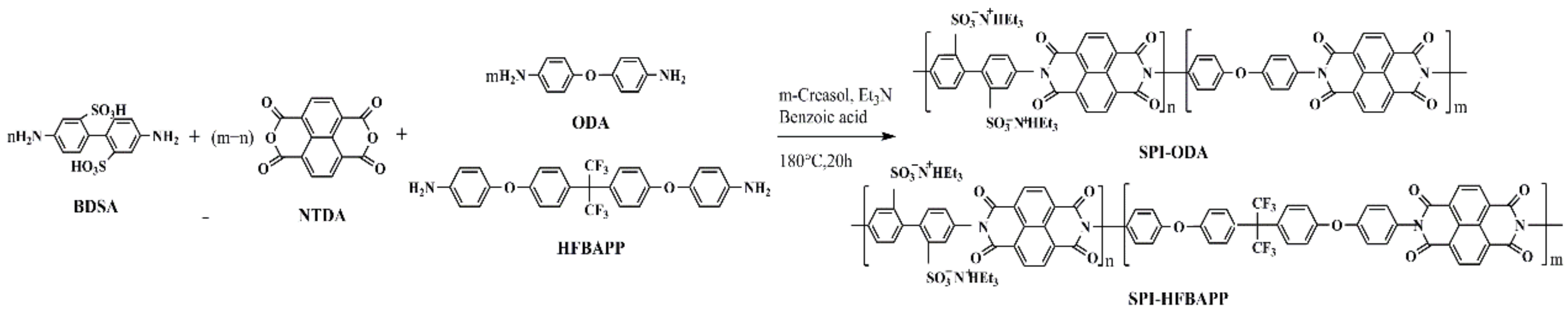
2.2. Synthesis of Polyimides
2.3. Preparation of SPI Membranes
2.4. Characterization of SPI
2.5. Characterization of Membranes
3. Results and Discussion
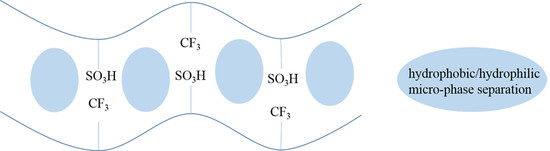
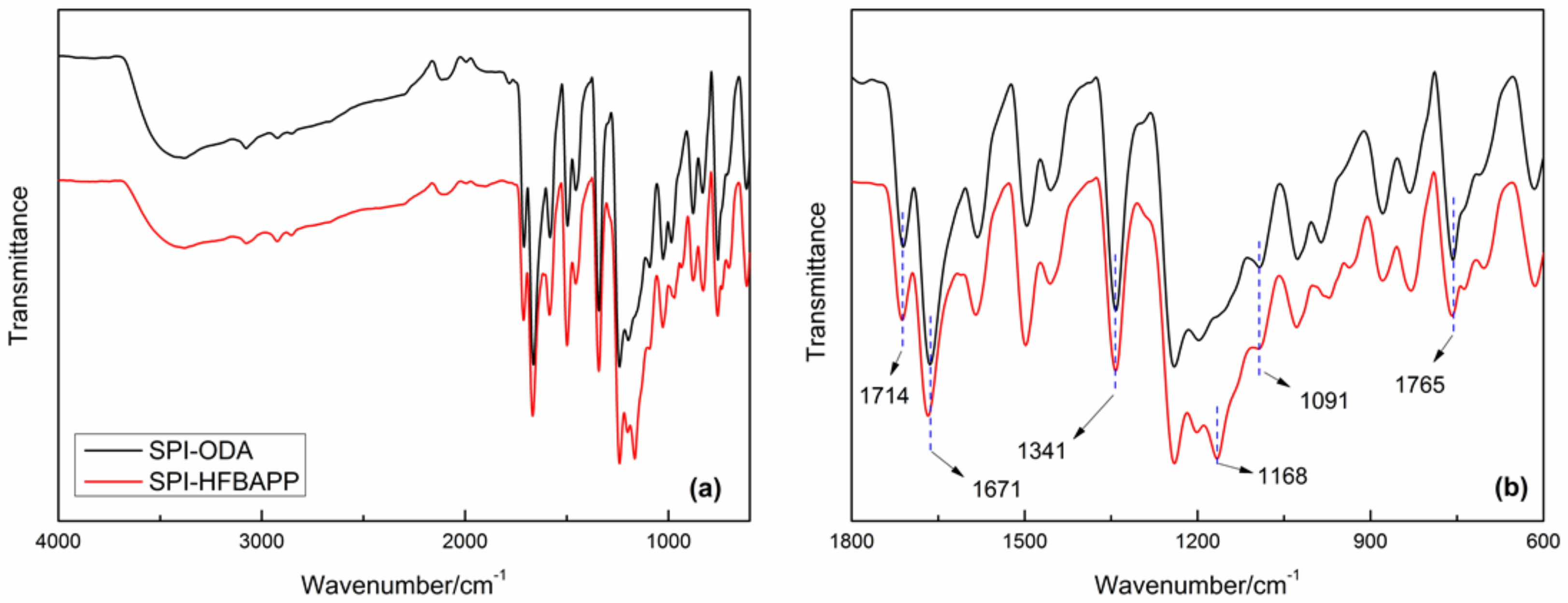
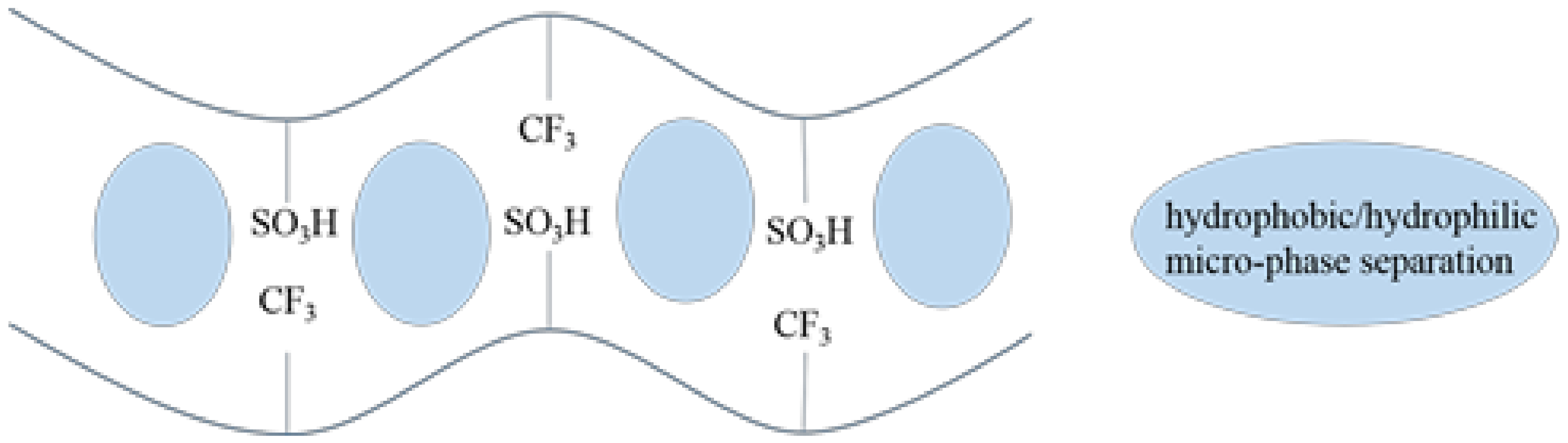
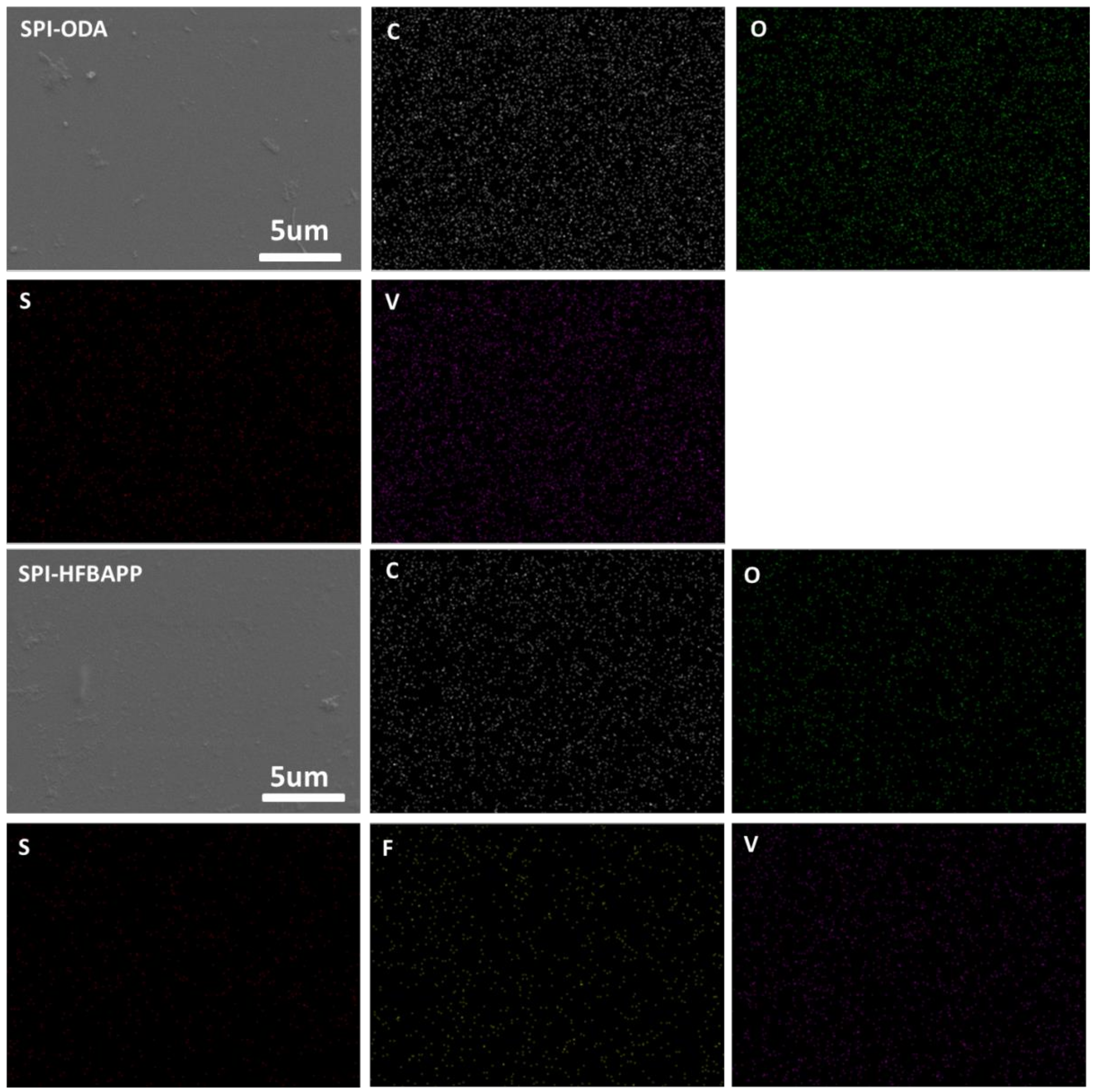
3.1. Structure Characterization of SPI
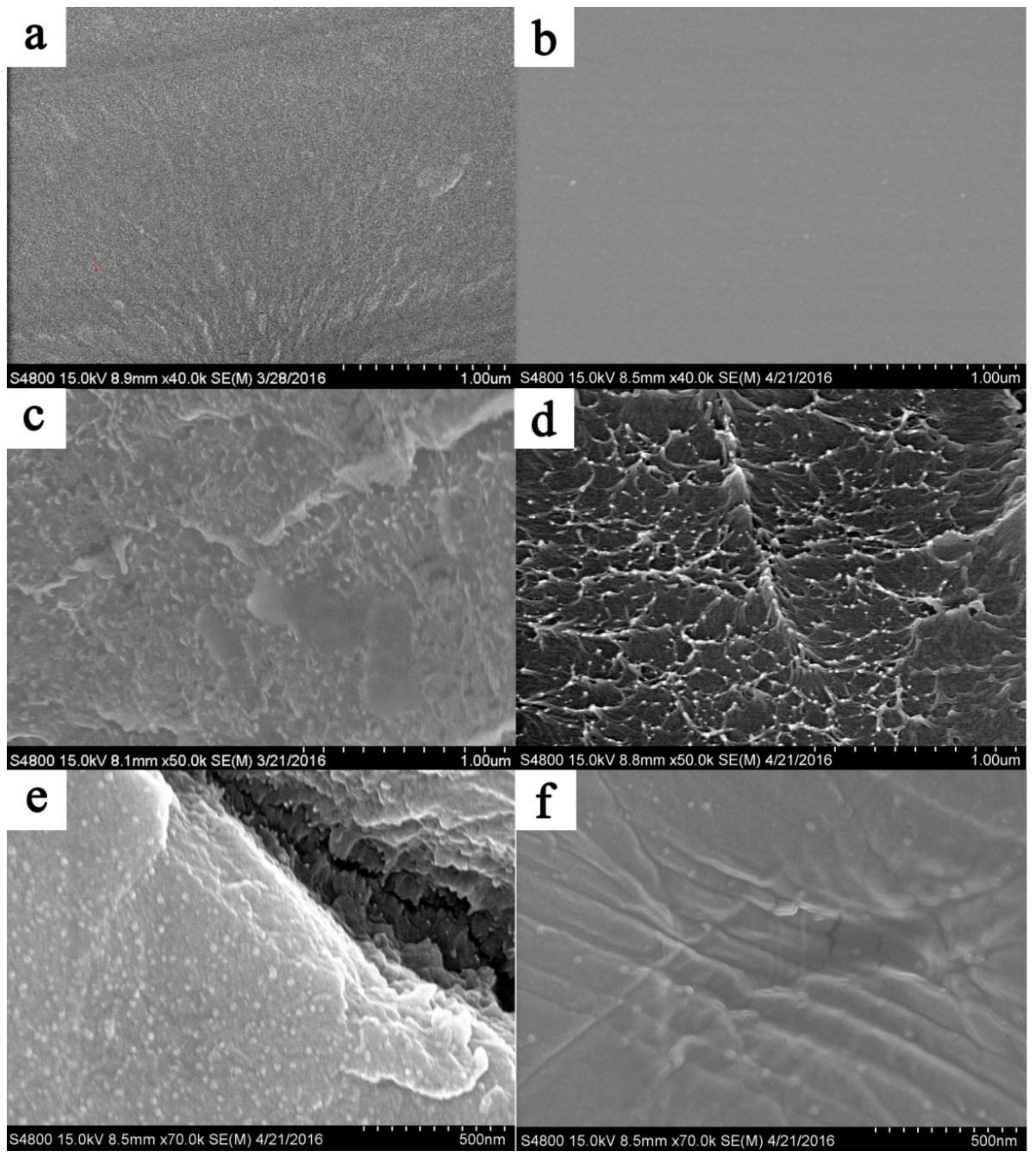
3.2. Physicochemical Properties of SPI Membranes
3.3. Stability of SPI Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramachandra, T.V.; Shruthi, B.V. Spatial mapping of renewable energy potential. Renew. Sustain. Energy Rev. 2007, 11, 1460–1480. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Ling, W.; Sheng, H.; Zhou, Y.; Wu, X.P.; Zeng, X.X.; Wu, X.W.; Guo, Y.G. Heteroatom-doped electrodes for all-vanadium redox flow batteries with ultralong lifespan. J. Mater. Chem. A 2018, 6, 41–44. [Google Scholar] [CrossRef]
- Li, L.Y.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.M.; Chen, B.W.; Zhang, J.L.; Xia, G.G.; Hu, J.Z.; Graff, G.; et al. A Stable Vanadium Redox-Flow Battery with High Energy Density for Large-Scale Energy Storage. Adv. Energy Mater. 2011, 1, 394–400. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, H.M.; Mai, Z.S.; Zhang, H.Z.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Sum, E.; Rychcik, M.; Skyllas-Kazacos, M. Investigation of the V(V)/V(Iv) System for Use in the Positive Half-Cell of a Redox Battery. J. Power Sources 1985, 16, 85–95. [Google Scholar] [CrossRef]
- Sum, E.; Skyllas-Kazacos, M. A Study of the V(Ii)/V(Iii) Redox Couple for Redox Flow Cell Applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Rychcik, M.; Robins, R.G.; Fane, A.G.; Green, M.A. New All-Vanadium Redox Flow Cell. J. Electrochem. Soc. 1986, 133, 1057–1058. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient Vanadium Redox Flow Cell. J. Electrochem. Soc. 1987, 134, 2950–2953. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Cha, S.H. Recent Development of Nanocomposite Membranes for Vanadium Redox Flow Batteries. J. Nanomater. 2015, 2015, 207525. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Hoang, T.K.A.; Chen, P. Recent development of polymer membranes as separators for all-vanadium redox flow batteries. RSC Adv. 2015, 5, 72805–72815. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.L.; Kim, S.; Li, L.Y.; Liu, J.; Yang, Z.G. Membrane Development for Vanadium Redox Flow Batteries. Chemsuschem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.Y.; Wu, Z.H.; Teng, X.G.; Zhao, Y.T.; Chen, L.Q.; Qiu, X.P. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Wang, N.F.; Peng, S.; Lu, D.; Liu, S.Q.; Liu, Y.N.; Huang, K.L. Nafion/TiO2 hybrid membrane fabricated via hydrothermal method for vanadium redox battery. J. Solid State Electr. 2012, 16, 1577–1584. [Google Scholar] [CrossRef]
- Sun, C.X.; Chen, J.; Zhang, H.M.; Han, X.; Luo, Q.T. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
- Dai, W.J.; Shen, Y.; Li, Z.H.; Yu, L.H.; Xi, J.Y.; Qiu, X.P. SPEEK/Graphene oxide nanocomposite membranes with superior cyclability for highly efficient vanadium redox flow battery. J. Mater. Chem. A 2014, 2, 12423–12432. [Google Scholar] [CrossRef]
- Wei, X.L.; Nie, Z.M.; Luo, Q.T.; Li, B.; Chen, B.W.; Simmons, K.; Sprenkle, V.; Wang, W. Nanoporous Polytetrafl uoroethylene/Silica Composite Separator as a High-Performance All-Vanadium Redox Flow Battery Membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar] [CrossRef]
- Mogelin, H.; Barascu, A.; Krenkel, S.; Enke, D.; Turek, T.; Kunz, U. Effect of the pore size and surface modification of porous glass membranes on vanadium redox-flow battery performance. J. Appl. Electrochem. 2018, 48, 651–662. [Google Scholar] [CrossRef]
- Yan, X.M.; Zhang, C.M.; Dai, Y.; Zheng, W.J.; Ruan, X.H.; He, G.H. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery. J. Membrane Sci. 2017, 544, 98–107. [Google Scholar] [CrossRef]
- Gindt, B.P.; Tang, Z.J.; Watkins, D.L.; Abebe, D.G.; Seo, S.; Tuli, S.; Ghassemi, H.; Zawodzinski, T.A.; Fujiwara, T. Effects of sulfonated side chains used in polysulfone based PEMs for VRFB separator. J. Membrane Sci. 2017, 532, 58–67. [Google Scholar] [CrossRef]
- Li, J.C.; Yuan, X.D.; Liu, S.Q.; He, Z.; Zhou, Z.; Li, A.K. A Low-Cost and High-Performance Sulfonated Polyimide Proton-Conductive Membrane for Vanadium Redox Flow/Static Batteries. ACS Appl. Mater. Interfaces 2017, 9, 32643–32651. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Huang, X.D.; Yang, P.; Zhou, Y.Q.; Xuan, S.S.; Zhang, Y.P. Effect of non-sulfonated diamine monomer on branched sulfonated polyimide membrane for vanadium redox flow battery application. Electrochim. Acta 2017, 241, 50–62. [Google Scholar] [CrossRef]
- Cao, L.; Sun, Q.Q.; Gao, Y.H.; Liu, L.T.; Shi, H.F. Novel acid-base hybrid membrane based on amine-functionalized reduced graphene oxide and sulfonated polyimide for vanadium redox flow battery. Electrochim. Acta 2015, 158, 24–34. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.A.; Wang, L.H.; Yang, H.J.; Han, X.T.; Liu, B.Q. Ethylenediarnine-functionalized graphene oxide incorporated acid-base ion exchange membranes for vanadium redox flow battery. Electrochim. Acta 2017, 230, 204–211. [Google Scholar] [CrossRef]
- Luo, Q.T.; Zhang, H.M.; Chen, J.; You, D.J.; Sun, C.X.; Zhang, Y. Preparation and characterization of Nafion/SPEEK layered composite membrane and its application in vanadium redox flow battery. J. Membrane Sci. 2008, 325, 553–558. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Jeon, J.D.; Kwak, S.Y. Ion-exchange composite membranes pore-filled with sulfonated poly(ether ether ketone) and Engelhard titanosilicate-10 for improved performance of vanadium redox flow batteries. J. Power Sources 2018, 383, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.H.; Li, D.; Liu, B.Q.; Wang, J.J.; Song, Y.L. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone)/quaternized poly(ether imide) for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 17590–17597. [Google Scholar] [CrossRef]
- Liu, S.; Sang, X.X.; Wang, L.H.; Zhang, J.L.; Song, J.L.; Han, B.X. Incorporation of metal-organic framework in polymer membrane enhances vanadium flow battery performance. Electrochim. Acta 2017, 257, 243–249. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.H.; Zhang, B.; Liu, B.Q.; Wang, J.J.; Song, Y.L. Novel sulfonated polyimide/polyvinyl alcohol blend membranes for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 2072–2081. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, P.; Baker, G.L. Sulfonated polyimide and PVDF based blend proton exchange membranes for fuel cell applications. J. Mater. Chem. A 2015, 3, 3847–3853. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, J.C.; Zhang, H.; Zhang, S.A.; Huang, X.D. Sulfonated polyimide membranes with different non-sulfonated diamines for vanadium redox battery applications. Electrochim. Acta 2014, 150, 114–122. [Google Scholar] [CrossRef]
- Yue, M.Z.; Zhang, Y.P.; Chen, Y. Preparation and properties of sulfonated polyimide proton conductive membrane for vanadium redox flow battery. Adv. Mater. Res. 2011, 239–242, 2779–2784. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, J.C.; Wang, L.; Zhang, S. Sulfonated polyimide/AlOOH composite membranes with decreased vanadium permeability and increased stability for vanadium redox flow battery. J. Solid State Electr. 2014, 18, 3479–3490. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, S.; Huang, X.D.; Zhou, Y.Q.; Pu, Y.; Zhang, H.P. Synthesis and properties of branched sulfonated polyimides for membranes in vanadium redox flow battery application. Electrochim. Acta 2016, 210, 308–320. [Google Scholar] [CrossRef]
- Cao, L.; Kong, L.; Kong, L.Q.; Zhang, X.X.; Shi, H.F. Novel sulfonated polyimide/zwitterionic polymer-functionalized graphene oxide hybrid membranes for vanadium redox flow battery. J. Power Sources 2015, 299, 255–264. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, Y.P.; Zhang, S.; Huang, X.D. Sulfonated polyimide/s-MoS2 composite membrane with high proton selectivity and good stability for vanadium redox flow battery. J. Membrane Sci. 2015, 490, 179–189. [Google Scholar] [CrossRef]
- Li, J.C.; Zhang, Y.P.; Zhang, S.; Huang, X.D.; Wang, L. Novel sulfonated polyimide/ZrO2 composite membrane as a separator of vanadium redox flow battery. Polym. Adv. Technol. 2014, 25, 1610–1615. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.W.; Zhang, J.; Wang, G.; Zhang, J.C.; Zhu, X.J.; Wang, R.L. Sulfonated poly(ether ether ketone)/poly(vinylidene fluoride)/tungstophosphoric acid membrane for vanadium redox flow battery application. High Perform. Polym. 2016, 28, 735–740. [Google Scholar] [CrossRef]
- Han, P.X.; Wang, H.B.; Liu, Z.H.; Chen, X.A.; Ma, W.; Yao, J.H.; Zhu, Y.W.; Cui, G.L. Graphene oxide nanoplatelets as excellent electrochemical active materials for VO2+/VO2+ and V2+/V3+ redox couples for a vanadium redox flow battery. Carbon 2011, 49, 693–700. [Google Scholar] [CrossRef]
- Han, P.X.; Yue, Y.H.; Liu, Z.H.; Xu, W.; Zhang, L.X.; Xu, H.X.; Dong, S.M.; Cui, G.L. Graphene oxide nanosheets/multi-walled carbon nanotubes hybrid as an excellent electrocatalytic material towards VO2+/VO2+ redox couples for vanadium redox flow batteries. Energy Environ. Sci. 2011, 4, 4710–4717. [Google Scholar] [CrossRef]
- Han, P.X.; Wang, X.G.; Zhang, L.X.; Wang, T.S.; Yao, J.H.; Huang, C.S.; Gu, L.; Cui, G.L. RuSe/reduced graphene oxide: an efficient electrocatalyst for VO2+/VO2+ redox couples in vanadium redox flow batteries. RSC Adv. 2014, 4, 20379–20381. [Google Scholar] [CrossRef]
- Yin, B.B.; Yu, L.H.; Jiang, B.; Wang, L.; Xi, J.Y. Nano oxides incorporated sulfonated poly(ether ether ketone) membranes with improved selectivity and stability for vanadium redox flow battery. J. Solid State Electr. 2016, 20, 1271–1283. [Google Scholar] [CrossRef]
- Jia, C.K.; Liu, J.G.; Yan, C.W. A multilayered membrane for vanadium redox flow battery. J. Power Sources 2012, 203, 190–194. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.H.; Ding, Y.; Liu, B.Q.; Han, X.T.; Song, Y.L. Novel sulfonated poly (ether ether keton)/polyetherimide acid-base blend membranes for vanadium redox flow battery applications. Electrochim. Acta 2014, 130, 90–96. [Google Scholar] [CrossRef]
- Jang, W.; Sundar, S.; Choi, S.; Shul, Y.G.; Han, H. Acid-base polyimide blends for the application as electrolyte membranes for fuel cells. J. Membrane Sci. 2006, 280, 321–329. [Google Scholar] [CrossRef]
- Yue, M.Z.; Zhang, Y.P.; Wang, L. Sulfonated polyimide/chitosan composite membrane for vanadium redox flow battery: Influence of the infiltration time with chitosan solution. Solid State Ionics 2012, 217, 6–12. [Google Scholar] [CrossRef]

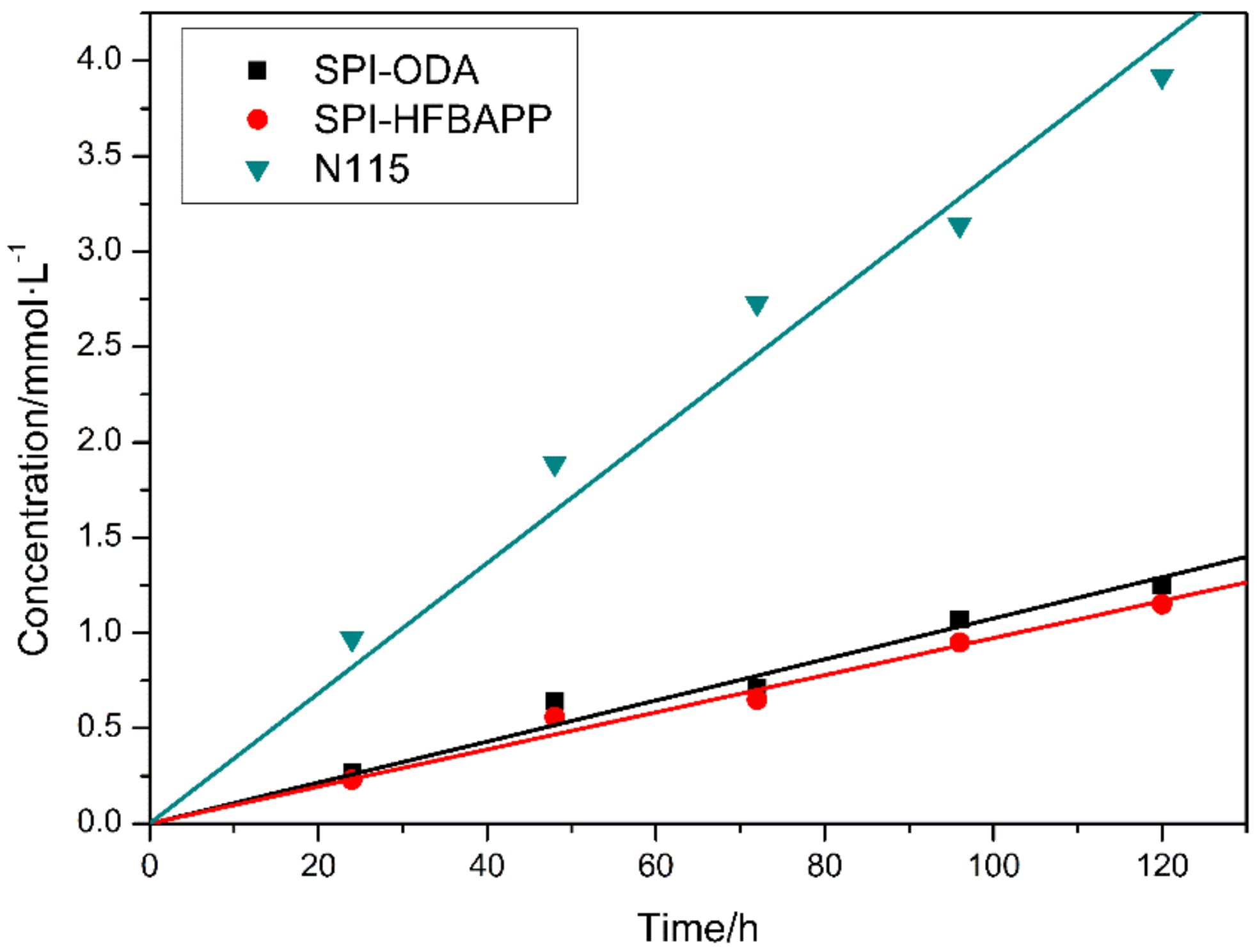
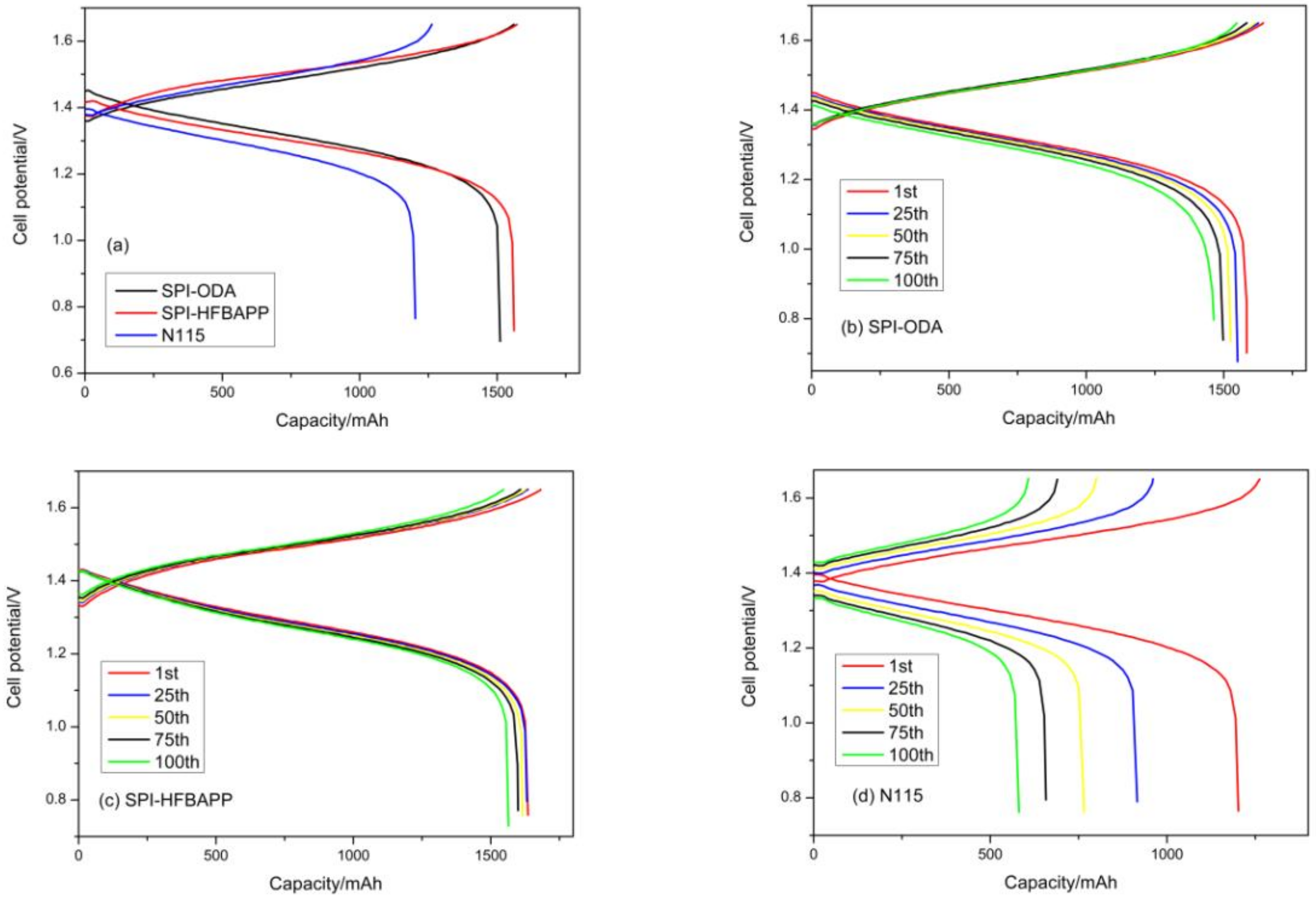

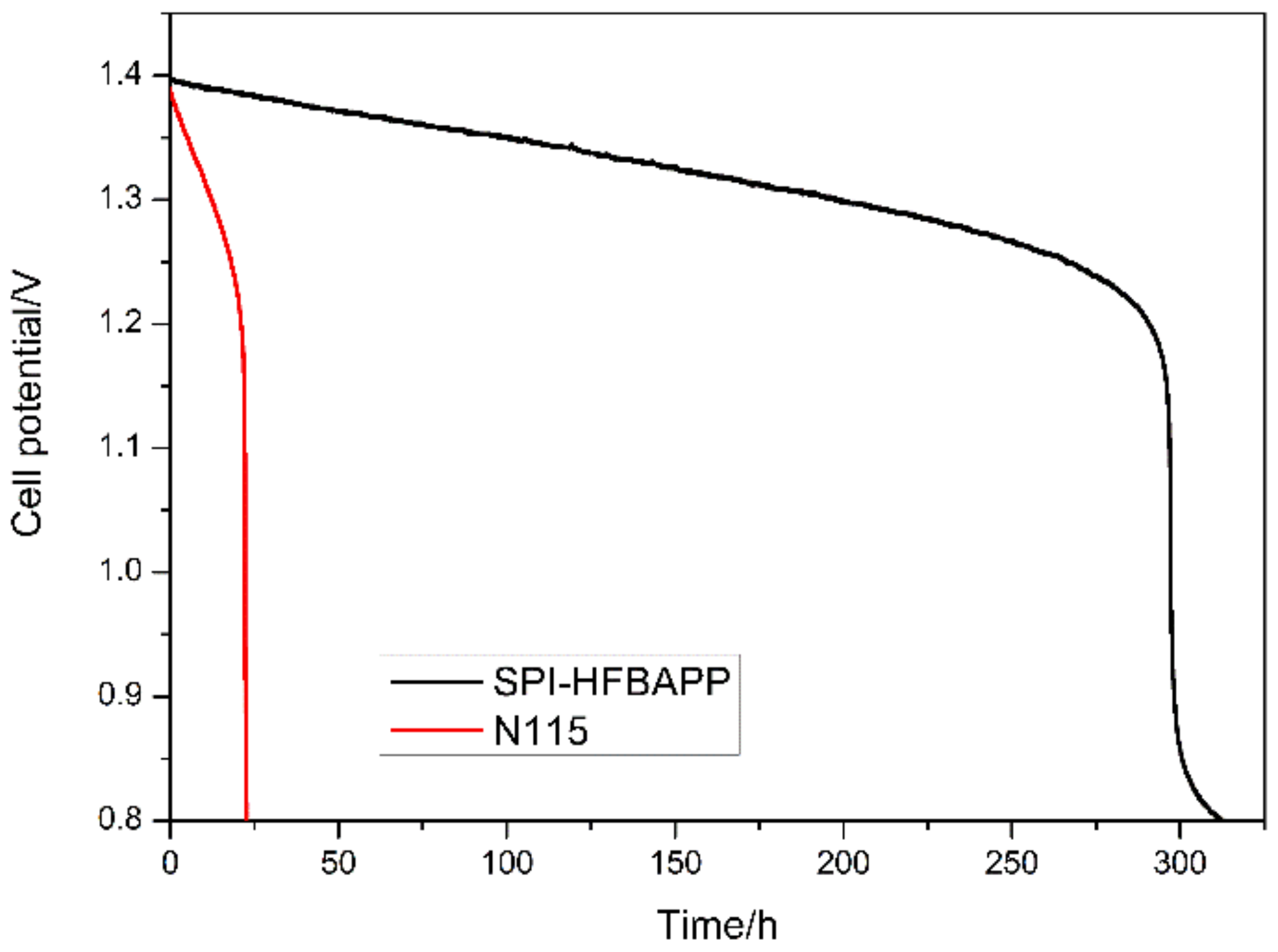
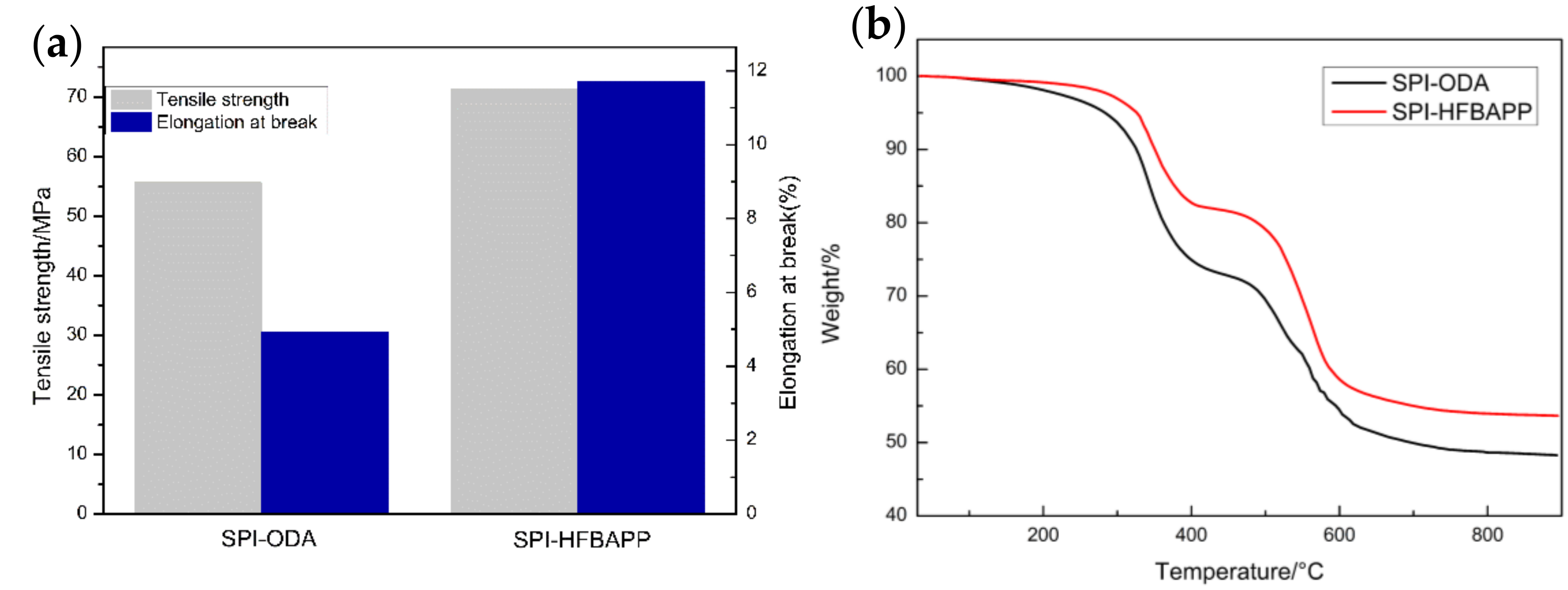
| Membranes | Thickness (μm) | WU (%) | IEC (mmol·g−1) | Area Resistance (Ω·cm2) | Proton Conductivity (mS·cm−1) | VO2+ Permeation (·10−7 cm2·min−1) | Proton Selectivity (104 S·min·cm−3) |
|---|---|---|---|---|---|---|---|
| N115 | 140 | 13.17 | 0.669 | 0.285 | 49.12 | 9.69 | 5.07 |
| SPI-ODA | 58 | 15.68 | 1.468 | 0.183 | 31.69 | 1.61 | 19.68 |
| SPI-HFBAPP | 58 | 6.66 | 1.232 | 0.154 | 37.66 | 0.97 | 38.82 |
| Membranes | CE (%) | VE (%) | EE (%) |
|---|---|---|---|
| SPI-ODA | 94.2 | 86.7 | 81.7 |
| SPI-HFBAPP | 99.7 | 85.3 | 85.0 |
| N115 | 95.5 | 84.5 | 80.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Ding, L.; Wang, L.; Yang, H.; Yu, X. High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries. Polymers 2018, 10, 1315. https://doi.org/10.3390/polym10121315
Chen Q, Ding L, Wang L, Yang H, Yu X. High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries. Polymers. 2018; 10(12):1315. https://doi.org/10.3390/polym10121315
Chicago/Turabian StyleChen, Qi, Liming Ding, Lihua Wang, Haijun Yang, and Xinhai Yu. 2018. "High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries" Polymers 10, no. 12: 1315. https://doi.org/10.3390/polym10121315
APA StyleChen, Q., Ding, L., Wang, L., Yang, H., & Yu, X. (2018). High Proton Selectivity Sulfonated Polyimides Ion Exchange Membranes for Vanadium Flow Batteries. Polymers, 10(12), 1315. https://doi.org/10.3390/polym10121315