Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly(NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings
Abstract
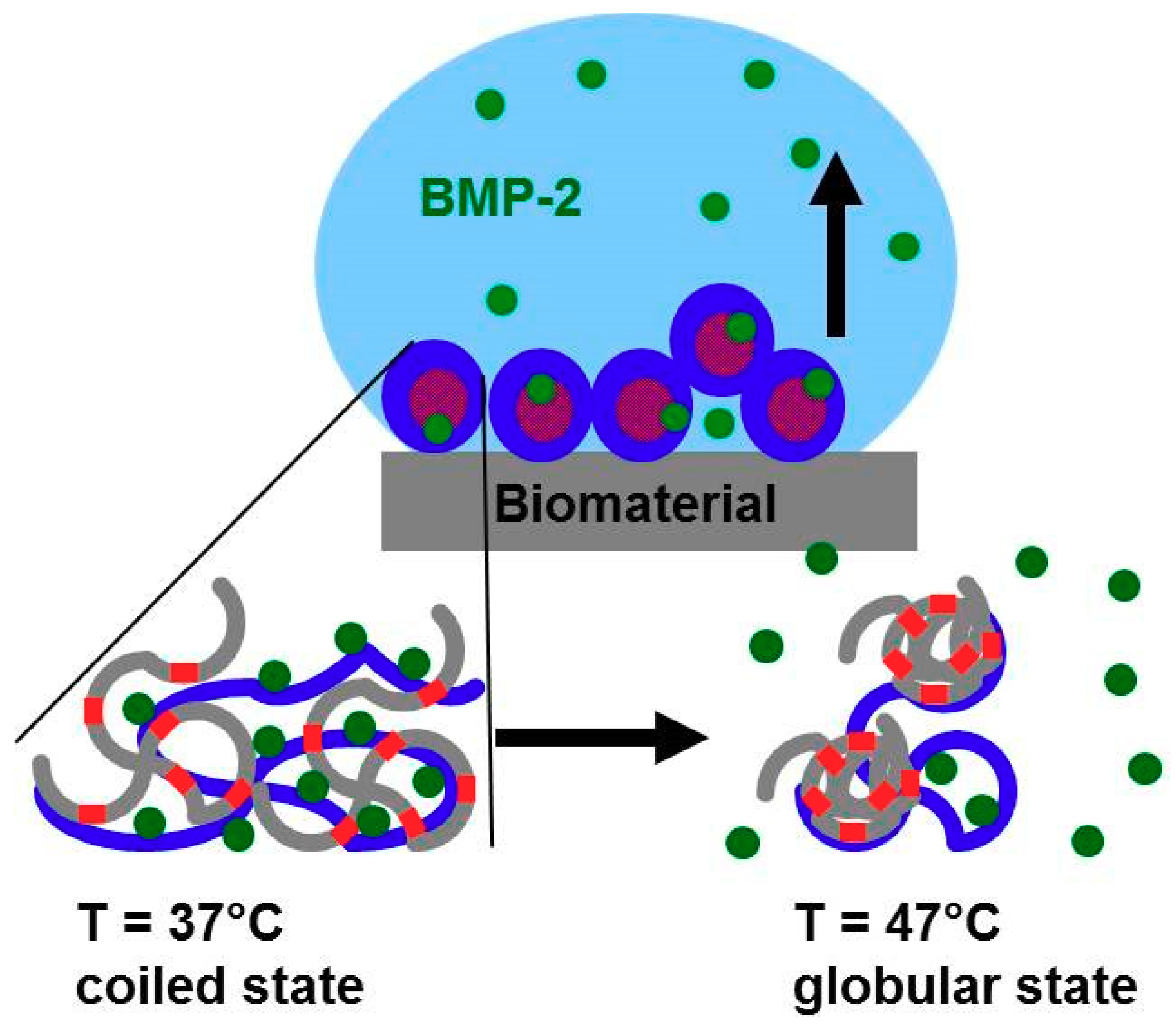
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Polyelectrolyte Complex (PEC) Preparation
2.3. PEC Coating Preparation
2.4. Protein Loading and Release
2.5. Colloid Titration
2.6. Turbidity
2.7. Dynamic Light Scattering (DLS)
2.8. Fourier Transform Infrared (FTIR) spectroscopy
2.9. Wet Adhesion of PNIPAM-DMAEMA/CS Films
2.10. Volume Phase Transition of PNIPAM-DMAEMA/CS Films
2.11. Protein Content in PNIPAM-DMAEMA/CS Films
2.12. Scanning Force Microscopy (SFM)
2.13. Spectroscopic Reflectometry (SR)
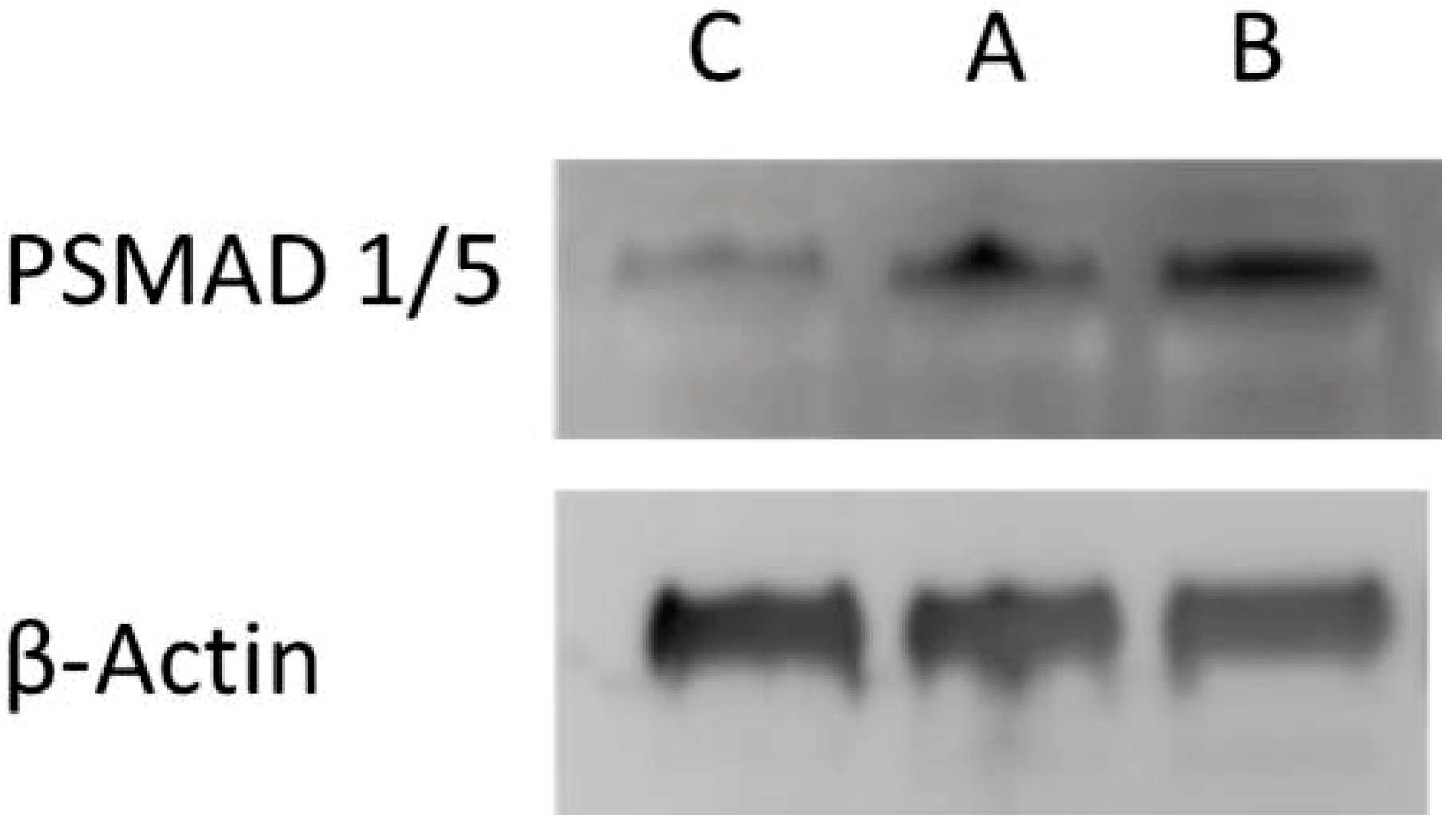
2.14. Cell Culture and Western Blotting
3. Results and Discussion
3.1. Colloid and Thermal Properties of PNIPAM-DMAEMA/CS Dispersions
3.1.1. Turbidity
3.1.2. Particle Size
3.2. Interfacial and Thermal Properties of PNIPAM-DMAEMA/CS Coatings
3.2.1. Wet-Adhesiveness of the Coating
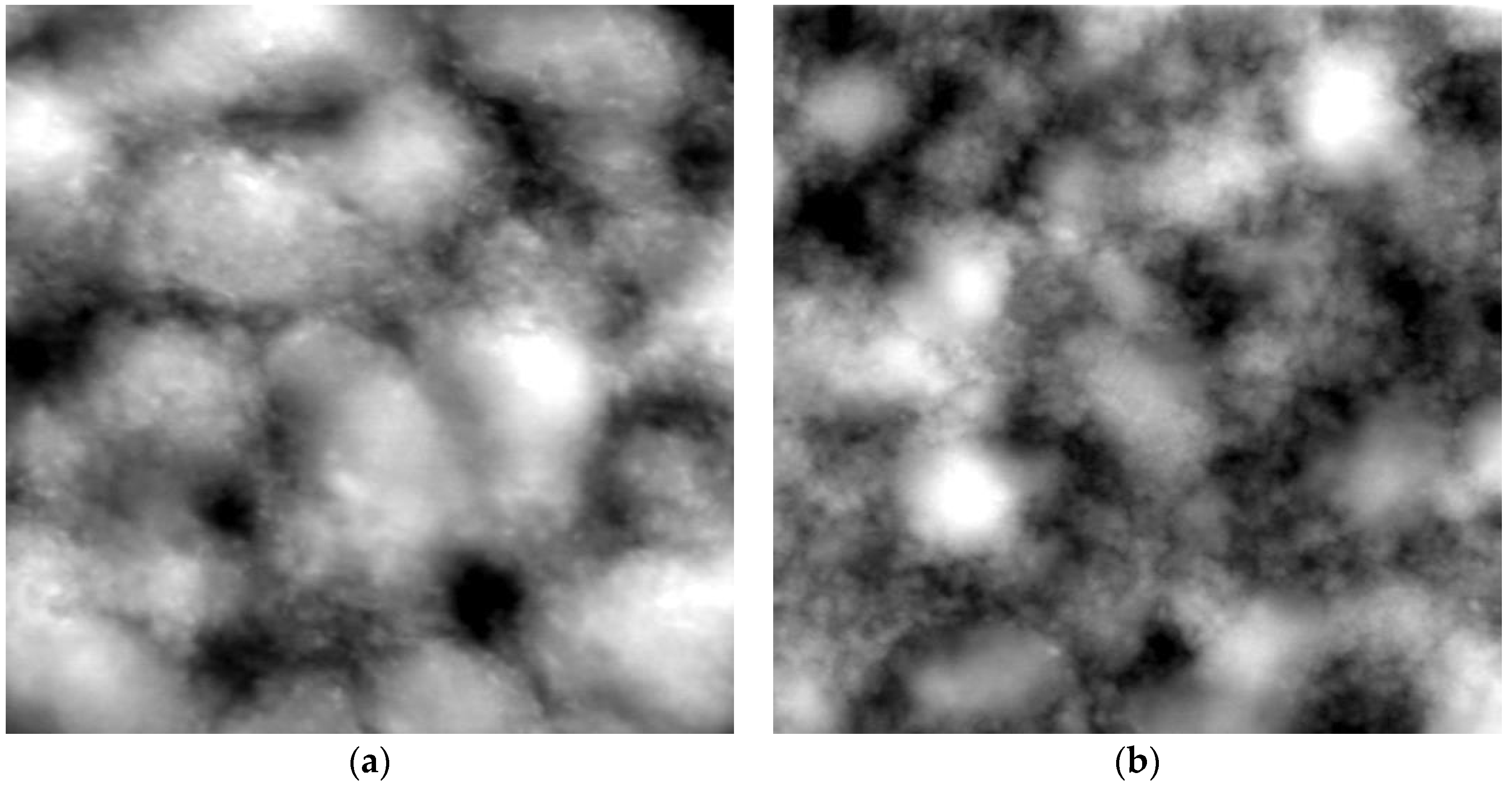
3.2.2. Morphology and Thickness of the Coating
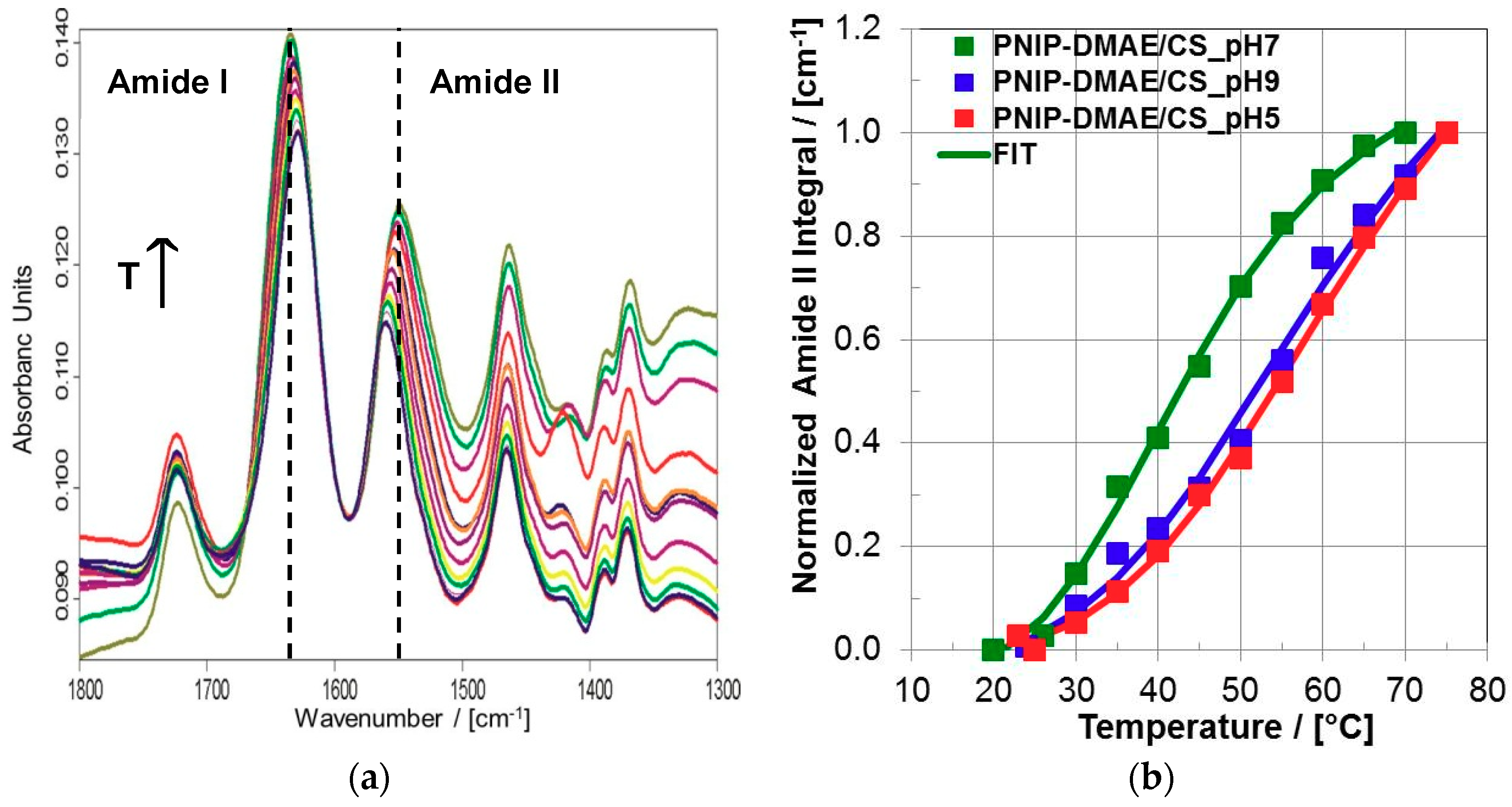
3.2.3. Thermal Characterization of the Coating
3.3. Thermoaddressable Delivery of Bone Therapeutic Proteins
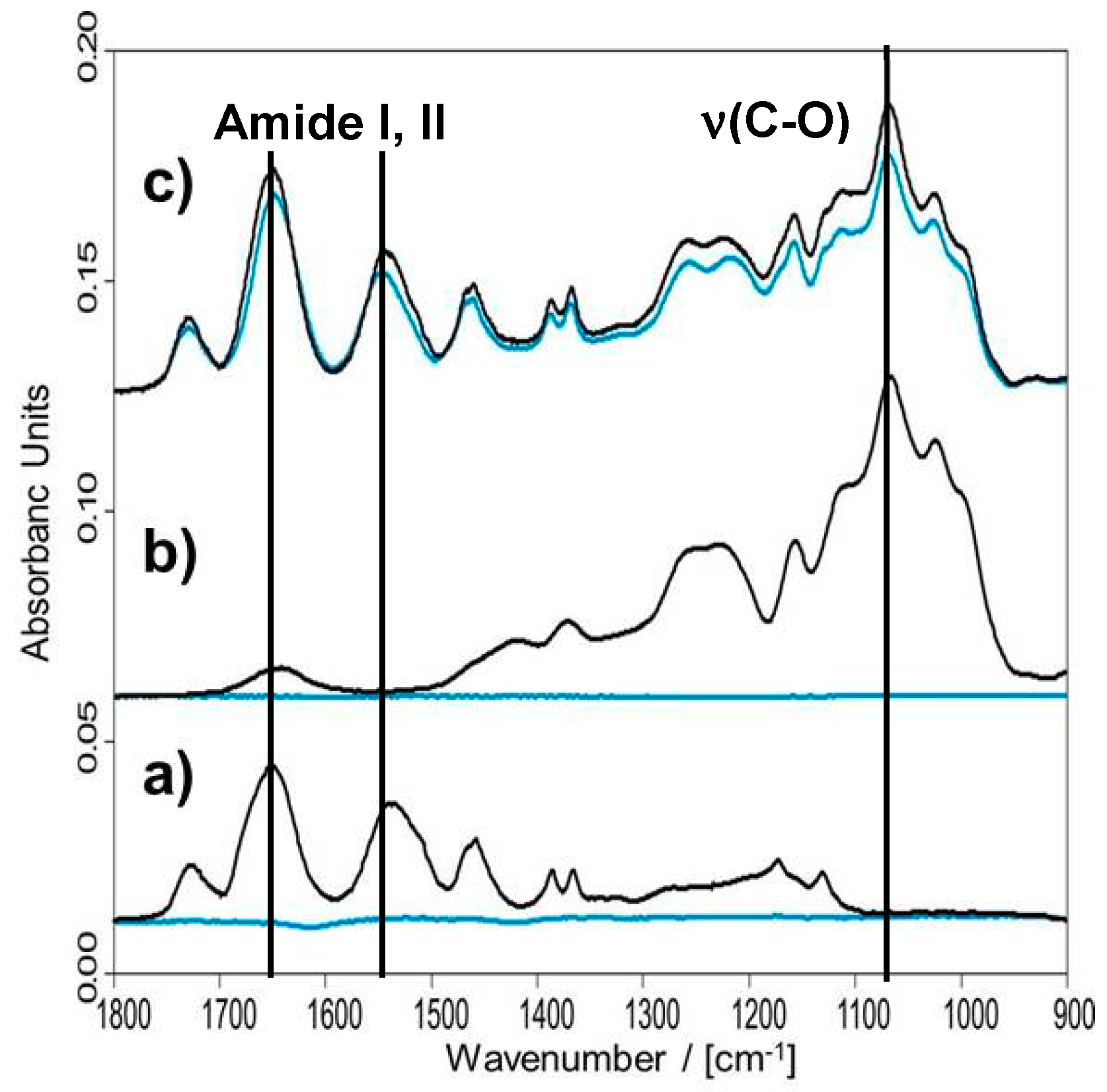
3.3.1. Loading and Elution Profiles of Functional Proteins
Papain
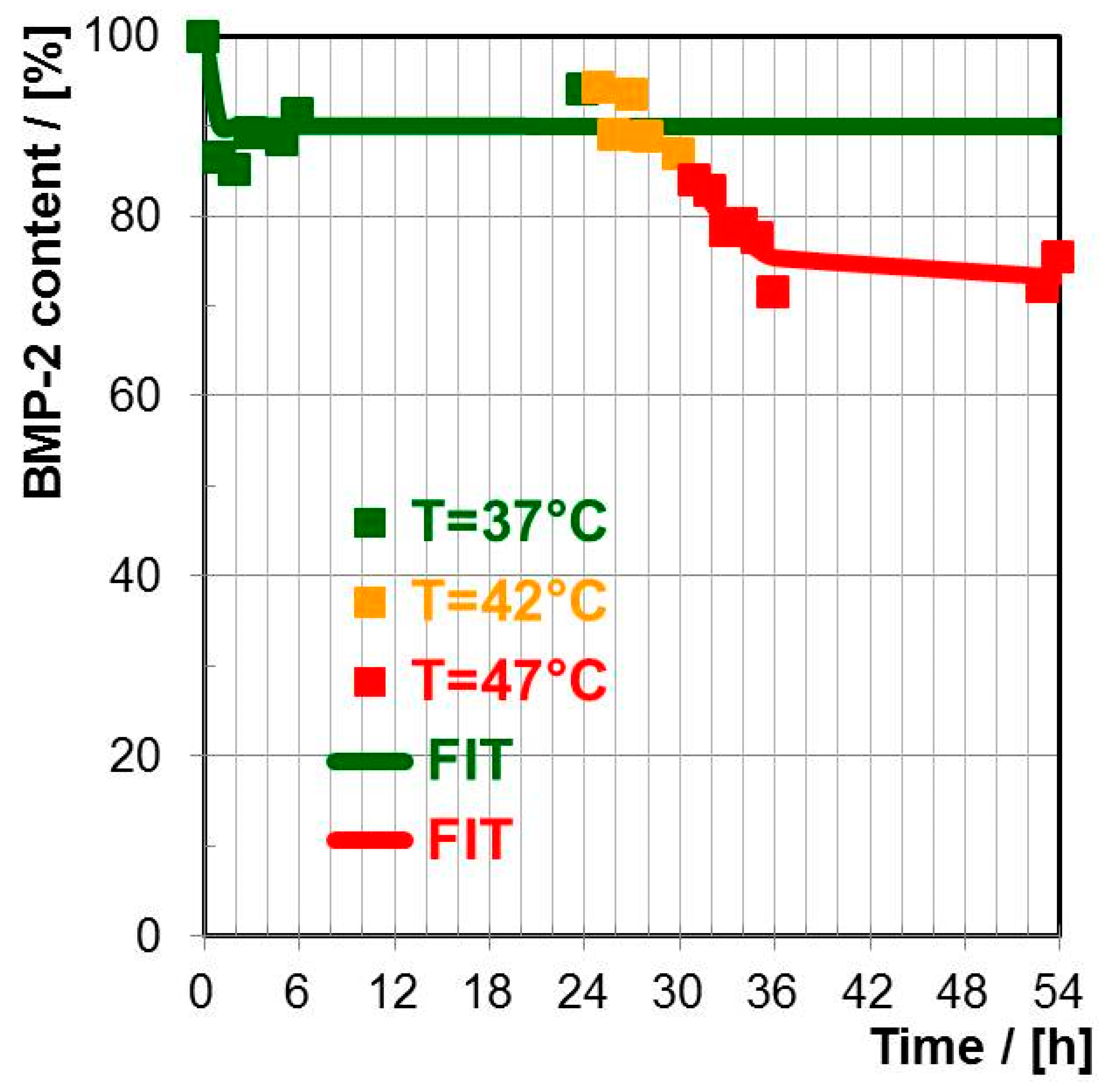
Bone Morphogenetic Protein (BMP-2)
3.3.2. Thermorsponsive Release Mechanism and Physiological Relevance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Müller, M.; Urban, B.; Vehlow, D.; Möller, M.L. Adjusting and switching the elution of bone therapeutics from thermoaddressable coatings of poly(N-isopropylacrylamide-co-acrylic acid)/ethylenediaminocellulose complex particles. Coll. Polym. Sci. 2017, 295, 1187–1199. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Shibayama, M.; Norisuye, T.; Nomura, S. Cross-link density dependence of spatial inhomogeneities and dynamic fluctuations of poly(N-isopropylacrylamide) gels. Macromolecules 1996, 29, 8746–8750. [Google Scholar] [CrossRef]
- McCoy, C.P.; Brady, C.; Cowley, J.F.; McGlinchey, S.M.; McGoldrick, N.; Kinnear, D.J.; Andrews, G.P.; Jones, G.S. Triggered drug delivery from biomaterials. Expert. Opin. Drug Deliv. 2010, 7, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell. Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G.; Caravaggio, T. Isoelectric points and molecular weights of proteins. J. Chromatogr. 1976, 127, 1–28. [Google Scholar] [CrossRef]
- Petzold, R.; Vehlow, D.; Urban, B.; Grab, A.; Cavalcanti, E.A.; Alt, V.; Müller, M. Colloid, adhesive and release properties of nanoparticular ternary complexes between cationic and anionic polysaccharides and basic proteins like bone morphogenetic protein BMP-2. Coll. Surf. B Biointerfaces 2017, 151, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Fringeli, U.P. In situ Infrared Attenuated total reflection (IR ATR) spectroscopy: A complementary analytical tool for drug design and drug delivery. Chimia 1992, 46, 200–214. [Google Scholar]
- Scherzinger, C.; Schwarz, A.; Bardow, A.; Leonhard, K.; Richtering, W. Cononsolvency of poly-N-isopropyl acrylamide (PNIPAM): Microgels versus linear chains and macrogels. Curr. Opin. Coll. Interface Sci. 2014, 19, 84–94. [Google Scholar] [CrossRef]
- Yoo, M.K.; Sung, Y.K.; Cho, C.S.; Lee, Y.M. Effect of polymer complex formation on the cloud-point of poly(isopropylacrylamide) (PNIPAAM) in the poly(NIPAAM-co-acrylic acid)—Polyelectrolyte complex between poly(acrylic acid) and poly(allylamine). Polymer 1997, 38, 2759–2765. [Google Scholar]
- Müller, M.; Urban, B.; Schwarz, S. Biorelated polyelectrolyte coatings studied by in-situ attenuated total reflection-Fourier transform infrared spectroscopy: Deposition, concepts, wet Adhesiveness, and biomedical applications. Langmuir 2018, 34, 8129–8144. [Google Scholar] [CrossRef] [PubMed]
- Starchenko, V.; Müller, M.; Lebovka, N. Growth of Polyelectrolyte Complex Nanoparticles: Computer Simulations and Experiments. J. Phys. Chem. C 2008, 112, 8863–8869. [Google Scholar] [CrossRef]
- Starchenko, V.; Müller, M.; Lebovka, N. Sizing of PDADMAC/PSS Complex Aggregates by Polyelectrolyte and Salt Concentration and PSS Molecular Weight. J. Phys. Chem. B 2012, 116, 14961–14967. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; Gerlach, G.; Corten, C.; Kuckling, D.; Müller, M.; Shi, Z.; Sorber, J.; Arndt, K.F. Application of polyelectrolytic temperature-responsive hydrogels in Chemical Sensors. Macromol. Symp. 2007, 254, 314–321. [Google Scholar] [CrossRef]
- Czeslik, C.; Jansen, R.; Ballauff, M.; Wittemann, A.; Royer, C.A.; Gratton, E.; Hazlett, T. Mechanism of protein binding to spherical polyelectrolyte brushes studied in situ using two-photon excitation fluorescence fluctuation spectroscopy. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004, 69, 021401. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.C.J.; Spencer, R.F. The role of PMMA bone cement in modern orthopaedic surgery. J. Bone Joint Surg. Br. 2007, 89, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.H.; Su, Y.C. Iron-oxide embedded solid lipid nanoparticles for magnetically controlled heating and drug delivery. Biomed. Microdevices 2008, 10, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estrade, S.; Peiro, F.; et al. Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci. Rep. 2013, 3, 165217. [Google Scholar] [CrossRef] [PubMed]
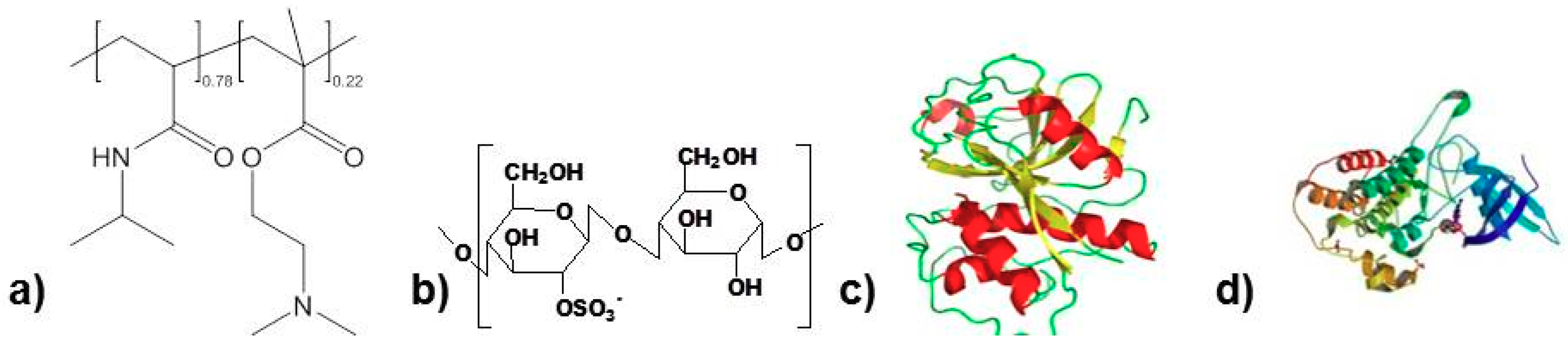


| Mn/[g/mol] | M/[g/mol] | F (pH = 4.0) | F (pH = 7.0) | |
|---|---|---|---|---|
| PNIPAM-DMAEMA | 7.200 | 123 (average) | 0.28–0.33 (+) | 0.22–0.26 (+) |
| CS | 100.000 | 213 | 0.36 (−) | 0.39 (−) |
| PAP | 24.000 | 100 | 0.07 * | 0.04 * |
| BMP-2 | 30.000 | 100 | --- ** | --- ** |
| Samples | VPTT | P |
|---|---|---|
| PNIPAM, pH = 7.0 | 34.1 ± 0.2 °C | 34.5 ± 8.2 |
| PNIPAM-DMAEMA, pH = 7.0 | 55.8 ± 0.3 °C | 9.7 ± 0.4 |
| PNIPAM-DMAEMA/CS-1.2, pH = 7.0 | 47.1 ± 0.5 °C | 6.2 ± 0.3 |
| PNIPAM-DMAEMA, pH = 5.0 | 59.6 ± 2.3 °C | 4.2 ± 0.8 |
| PNIPAM-DMAEMA, pH = 9.0 | 35.3 ± 0.3 °C | 17.2 ± 2.2 |
| PEC Dispersions | RH/[nm] | |
|---|---|---|
| T = 25 °C | T = 60 °C | |
| PNIPAM-DMAEMA/CS-0.9, pH = 7.0 | 171 ± 13 | 103 ± 38 |
| PNIPAM-DMAEMA/CS-1.2, pH = 7.0 | 170 ± 45 | 76 ± 26 |
| PNIPAM-DMAEMA/CS-0.9, pH = 4.0 | --- * | --- * |
| PNIPAM-DMAEMA/CS-1.2, pH = 4.0 | 288 ± 55 | 74 ± 6 |
| W/[%] | ||||
|---|---|---|---|---|
| T = 25 °C | T = 60 °C | |||
| PEC Coatings | PNIPAM-DMAEMA | CS | PNIPAM-DMAEMA | CS |
| PNIPAM-DMAEMA/CS-1.2, pH = 5.0 | 90 ± 4 | 79 ± 3 | 90 ± 4 | 79 ± 2 |
| PNIPAM-DMAEMA/CS-1.2, pH = 7.0 | 85 ± 6 | 79 ± 6 | 91 ± 7 | 82 ± 5 |
| PNIPAM-DMAEMA/CS-1.2, pH = 9.0 | 81 ± 14 | 66 ± 13 | 83 ± 6 | 67 ± 8 |
| PNIPAM-DMAEMA/CS-1.2, NaCl | 78 ± 4 | 74 ± 3 | 93 ± 4 | 79 ± 3 |
| PEC Coating Samples | VPTT (FTIR) | P |
|---|---|---|
| PNIPAM-DMAEMA/CS-1.2, pH = 5.0 | 64.1 ± 3.5 °C | 4.1 ± 0.4 |
| PNIPAM-DMAEMA/CS-1.2, pH = 7.0 | 41.8 ± 1.6 °C | 5.8 ± 0.4 |
| PNIPAM-DMAEMA/CS-1.2, pH = 9.0 | 61.3 ± 7.0 °C | 3.8 ± 0.9 |
| Temperature | IB/[%] | RC/[%] |
|---|---|---|
| PAP, T = 37 °C | 9% | 66% |
| PAP, T = 42 °C | 3% | 50% |
| BMP-2, T = 37 °C | 10% | 90% |
| BMP-2, T = 42 °C | ≈0% | 87% |
| BMP-2, T = 47 °C | 3% | 74% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Urban, B.; Reis, B.; Yu, X.; Grab, A.L.; Cavalcanti-Adam, E.A.; Kuckling, D. Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly(NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings. Polymers 2018, 10, 1314. https://doi.org/10.3390/polym10121314
Müller M, Urban B, Reis B, Yu X, Grab AL, Cavalcanti-Adam EA, Kuckling D. Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly(NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings. Polymers. 2018; 10(12):1314. https://doi.org/10.3390/polym10121314
Chicago/Turabian StyleMüller, Martin, Birgit Urban, Berthold Reis, Xiaoqian Yu, Anna Luise Grab, Elisabetta Ada Cavalcanti-Adam, and Dirk Kuckling. 2018. "Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly(NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings" Polymers 10, no. 12: 1314. https://doi.org/10.3390/polym10121314
APA StyleMüller, M., Urban, B., Reis, B., Yu, X., Grab, A. L., Cavalcanti-Adam, E. A., & Kuckling, D. (2018). Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly(NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings. Polymers, 10(12), 1314. https://doi.org/10.3390/polym10121314






