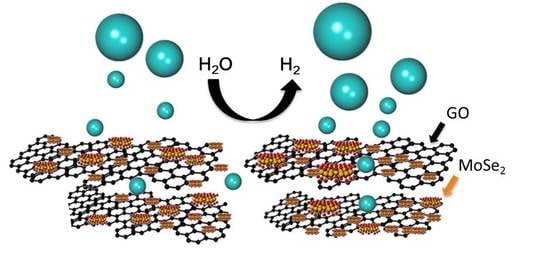
MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO-MoSe2 and rGO-MoSe2 Composites
2.2.1. Synthesis of GO and rGO
2.2.2. Synthesis of MoSe2 Nanosheets
2.2.3. Synthesis of MoSe2-GO and MoSe2-rGO Composites
2.3. Material Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Synthesis of MoSe2 Nanosheets
3.2. Material Characterization
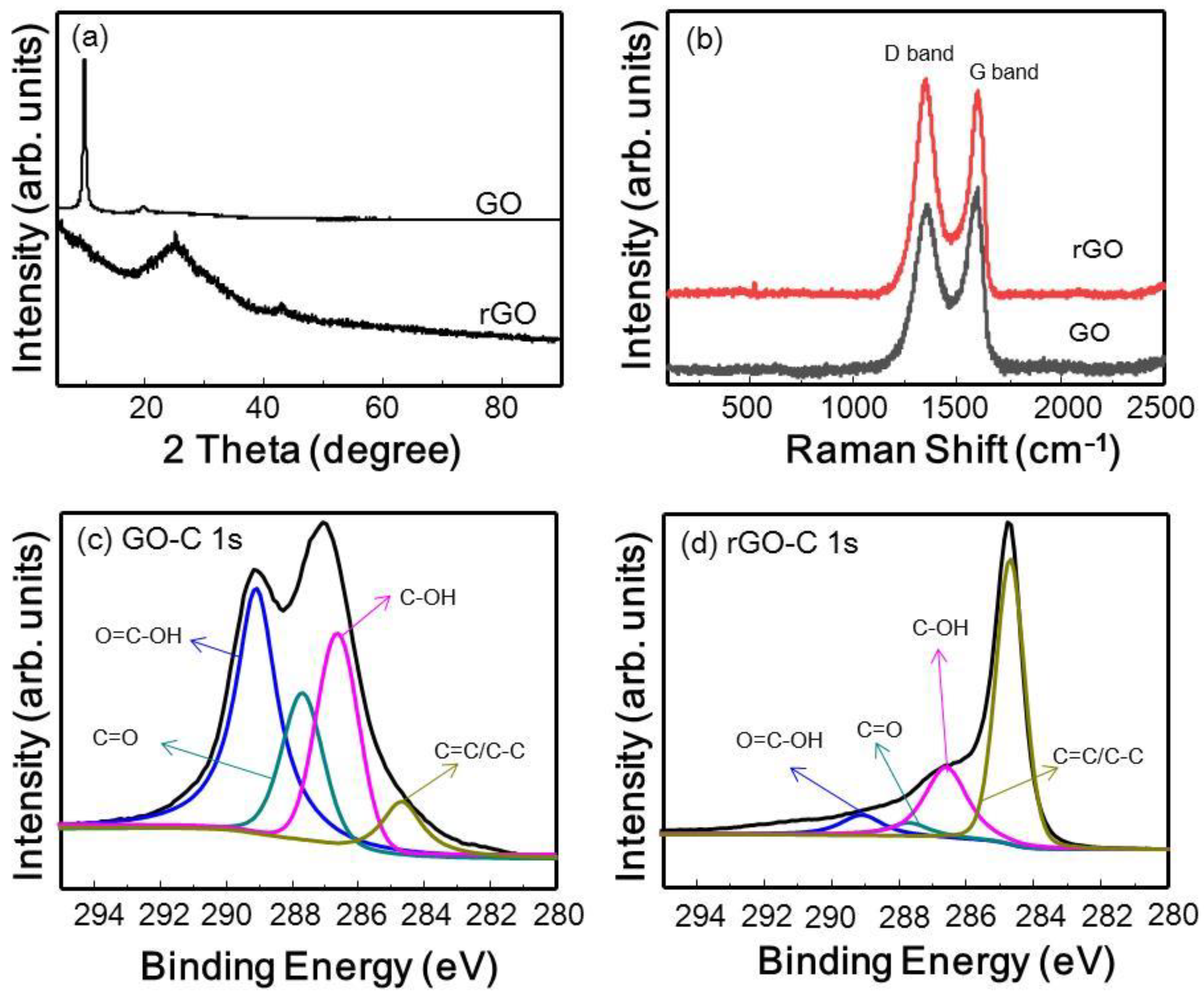
3.2.1. Characterization of GO and rGO
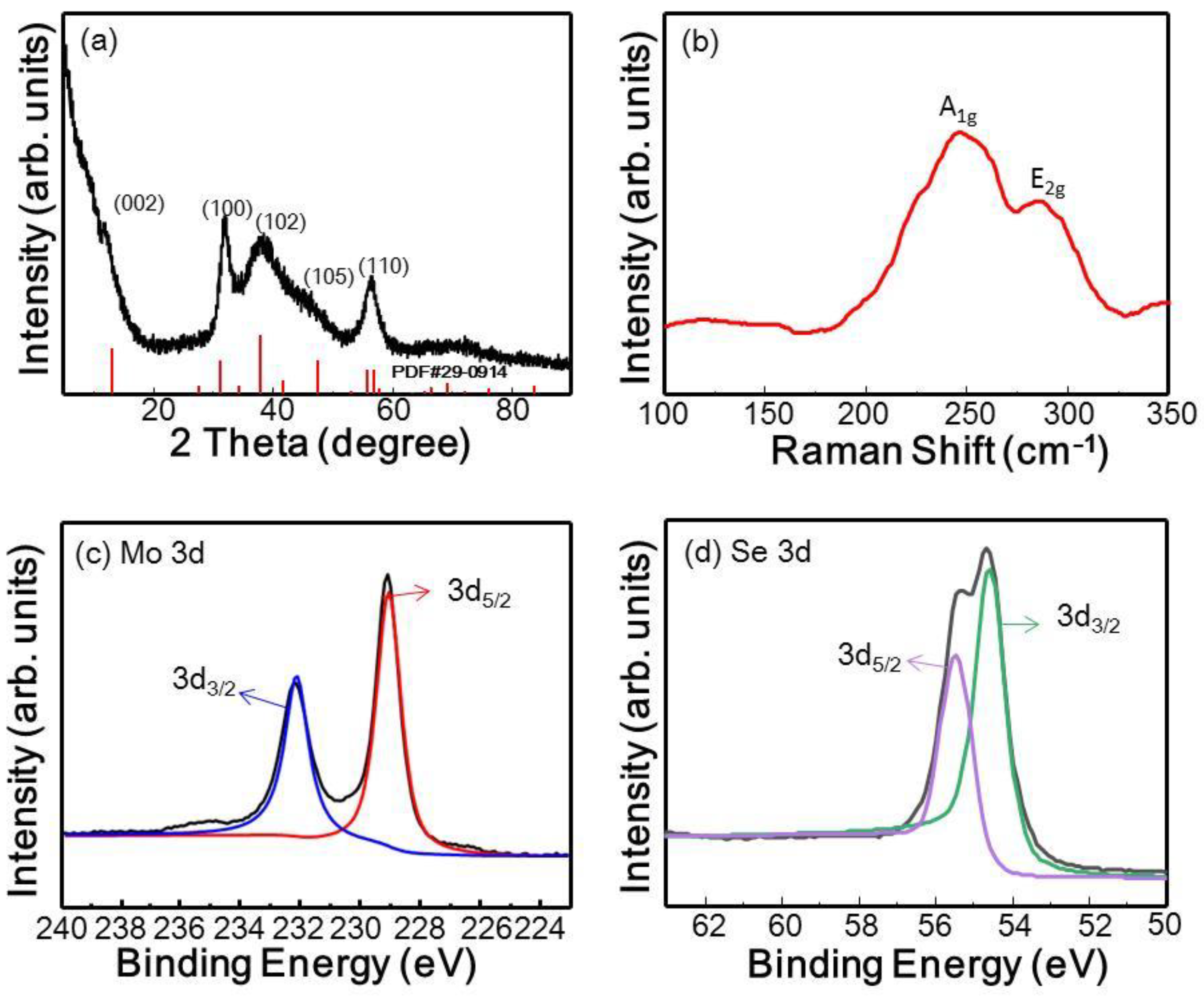
3.2.2. Characterization of MoSe2
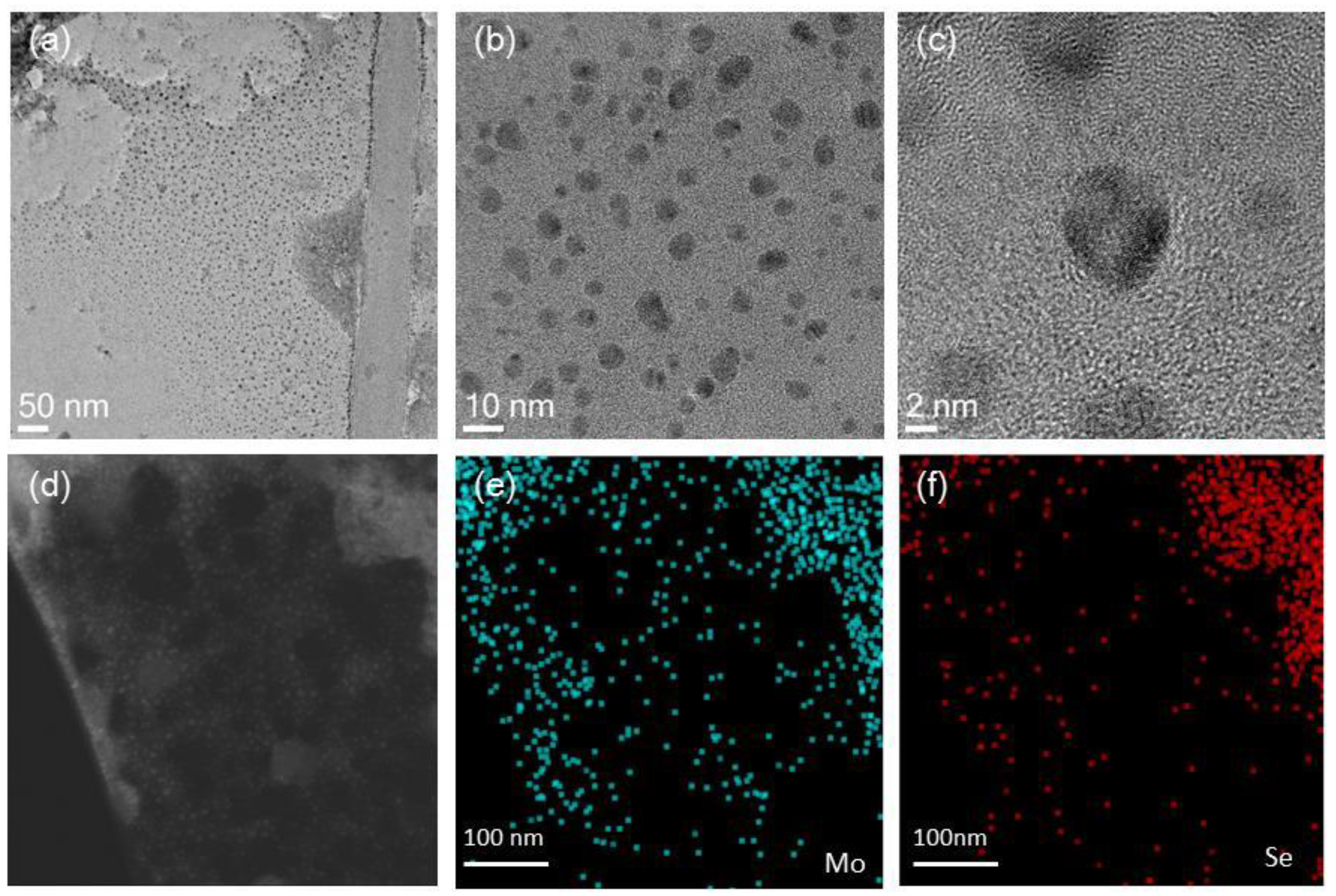
3.2.3. Characterization of MoSe2 Composites
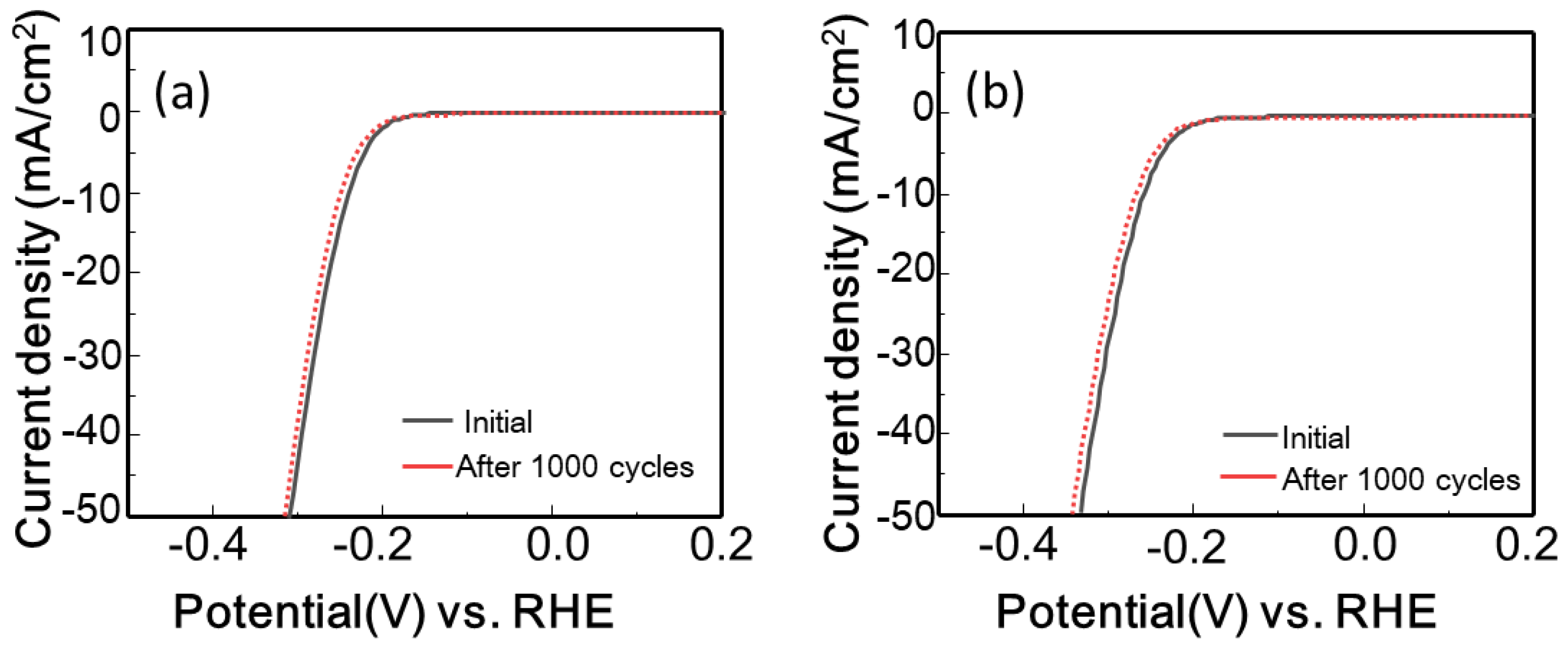
3.3. Electrocatalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, M.; Thomas, I. Alternative energy technologies. Nature 2001, 414, 332. [Google Scholar] [CrossRef] [PubMed]
- Cortright, R.D.; Davda, R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Sun, Y.; Chen, X.; Zhuang, G.; Li, X.; Wang, J.G. Mo Doping Induced More Active Sites in Urchin-Like W18O49 Nanostructure with Remarkably Enhanced Performance for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 5778–5786. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xie, Y. Structural engineering of electrocatalysts for the hydrogen evolution reaction: Order or disorder? ChemCatChem 2015, 7, 2568–2580. [Google Scholar] [CrossRef]
- Kemppainen, E.; Bodin, A.; Sebok, B.; Pedersen, T.; Seger, B.; Mei, B.; Bae, D.; Vesborg, P.C.K.; Halme, J.; Hansen, O. Scalability and feasibility of photoelectrochemical H2 evolution: The ultimate limit of Pt nanoparticle as an HER catalyst. Energy Environ. Sci. 2015, 8, 2991–2999. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Huang, Y.; Sun, Y.; Hu, T.; Xu, K.; Ma, F. Hydrothermal synthesis of N-doped RGO/MoSe2 composites and enhanced electro-catalytic hydrogen evolution. J. Mater. Sci. 2017, 52, 13561–13571. [Google Scholar] [CrossRef]
- Elezović, N.R.; Gajić-Krstajić, L.; Radmilović, V.; Vračar, L.; Krstajić, N.V. Effect of chemisorbed carbon monoxide on Pt/C electrode on the mechanism of the hydrogen oxidation reaction. Electrochim. Acta 2009, 54, 1375–1382. [Google Scholar] [CrossRef]
- Choi, G.J.; Van Le, Q.; Choi, K.S.; Kwon, K.C.; Jang, H.W.; Gwag, J.S.; Kim, S.Y. Polarized Light-Emitting Diodes Based on Patterned MoS2 Nanosheet Hole Transport Layer. Adv. Mater. 2017, 29, 1702598. [Google Scholar] [CrossRef] [PubMed]
- Van Le, Q.; Choi, J.-Y.; Kim, S.Y. Recent advances in the application of two-dimensional materials as charge transport layers in organic and perovskite solar cells. FlatChem 2017, 2, 54–66. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kwon, K.C.; Le, Q.V.; Hong, K.; Jang, H.W.; Kim, S.Y. Atomically thin two-dimensional materials as hole extraction layers in organolead halide perovskite photovoltaic cells. J Power Sources 2016, 319, 1–8. [Google Scholar] [CrossRef]
- Kwon, K.C.; Kim, C.; Le, Q.V.; Gim, S.; Jeon, J.-M.; Ham, J.Y.; Lee, J.-L.; Jang, H.W.; Kim, S.Y. Synthesis of Atomically Thin Transition Metal Disulfides for Charge Transport Layers in Optoelectronic Devices. ACS Nano 2015, 9, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Nguyen, T.P.; Kim, S.Y. UV/ozone-treated WS2 hole-extraction layer in organic photovoltaic cells. Phys. Status Solidi Rapid Res. Lett. 2014, 8, 390–394. [Google Scholar] [CrossRef]
- Van Le, Q.; Nguyen, T.P.; Park, M.; Sohn, W.; Jang, H.W.; Kim, S.Y. Bottom-Up Synthesis of MeSx Nanodots for Optoelectronic Device Applications. Adv. Opt. Mater. 2016, 4, 1796–1804. [Google Scholar] [CrossRef]
- Yu, X.Y.; Hu, H.; Wang, Y.; Chen, H.; Lou, X.W. Ultrathin MoS2 Nanosheets Supported on N-doped Carbon Nanoboxes with Enhanced Lithium Storage and Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 7395–7398. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Z.; Xu, S.; Kong, D.; Cha, J.J.; Zheng, G.; Hsu, P.-C.; Yan, K.; Bradshaw, D.; Prinz, F.B. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yamaguchi, H.; Li, J.; Silva, R.; Alves, D.C.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Peng, Z.; Fu, X.; Lian, L.; Luo, W.; Zhang, J.; Li, H.; Wang, Y.; Zhang, D. Phosphine-free synthesis and shape evolution of MoSe2 nanoflowers for electrocatalytic hydrogen evolution reactions. CrystEngComm 2018, 20, 2491–2498. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Le, Q.V.; Choi, S.; Lee, T.H.; Hong, S.-P.; Choi, K.S.; Jang, H.W.; Lee, M.H.; Park, T.J.; Kim, S.Y. Surface extension of MeS2 (Me = Mo or W) nanosheets by embedding MeSx for hydrogen evolution reaction. Electrochim. Acta 2018, 292, 136–141. [Google Scholar] [CrossRef]
- Hasani, A.; Nguyen, T.P.; Tekalgne, M.; Van Le, Q.; Choi, K.S.; Lee, T.H.; Jung Park, T.; Jang, H.W.; Kim, S.Y. The role of metal dopants in WS2 nanoflowers in enhancing the hydrogen evolution reaction. Appl. Catal. A 2018, 567, 73–79. [Google Scholar] [CrossRef]
- Lee, L.T.L.; He, J.; Wang, B.; Ma, Y.; Wong, K.Y.; Li, Q.; Xiao, X.; Chen, T. Few-layer MoSe2 possessing high catalytic activity towards iodide/tri-iodide redox shuttles. Sci. Rep. 2014, 4, 4063. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Y.; Wang, L.; Xu, J.; Zeng, D.; Peng, D.-L. Colloidal synthesis of MoSe2 nanonetworks and nanoflowers with efficient electrocatalytic hydrogen-evolution activity. Electrochim. Acta 2017, 231, 69–76. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Peng, S.; Tan, C.; Ang, H.; Tan, H.; Zhang, H.; Yan, Q. Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J. Mater. Chem. A 2014, 2, 5597–5601. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 2013, 13, 3426–3433. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Li, C.; Zhu, C.; Wang, S.; Zhang, X.; Chen, Y. Growth of MoSe2 nanosheets with small size and expanded spaces of (002) plane on the surfaces of porous N-doped carbon nanotubes for hydrogen production. Nanoscale 2016, 8, 16886–16893. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Kim, J.H.; Park, S.-K.; Kang, Y.C. MoSe2 embedded CNT-reduced graphene oxide composite microsphere with superior sodium ion storage and electrocatalytic hydrogen evolution performances. ACS Appl. Mater. Interfaces 2017, 9, 10673–10683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core–shell MoO3–MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via l-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Feng, S.; Terrones, M.; Schaak, R.E. Formation and interlayer decoupling of colloidal MoSe2 nanoflowers. Chem. Mater. 2015, 27, 3167–3175. [Google Scholar] [CrossRef]
- Huang, J.; Kelley, D. Synthesis and characterization of MoSe2 and WSe2 nanoclusters. Chem. Mater. 2000, 12, 2825–2828. [Google Scholar] [CrossRef]
- Pol, V.G.; Pol, S.V.; George, P.P.; Gedanken, A. Combining MoS2 or MoSe2 nanoflakes with carbon by reacting Mo(CO)6 with S or Se under their autogenic pressure at elevated temperature. J. Mater. Sci. 2008, 43, 1966–1973. [Google Scholar] [CrossRef]
- Tang, H.; Dou, K.; Kaun, C.-C.; Kuang, Q.; Yang, S. MoSe2 nanosheets and their graphene hybrids: Synthesis, characterization and hydrogen evolution reaction studies. J. Mater.Chem. A 2014, 2, 360–364. [Google Scholar] [CrossRef]
- Sekine, T.; Izumi, M.; Nakashizu, T.; Uchinokura, K.; Matsuura, E. Raman scattering and infrared reflectance in 2H-MoSe2. J. Phys. Soc. Jpn. 1980, 49, 1069–1077. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ye, Z.; Liu, C.; Xi, D.; Zhou, C.; Shi, Z.; Xia, H.; Liu, G.; Qiao, G. Morphology-controllable synthesis of cobalt telluride branched nanostructures on carbon fiber paper as electrocatalysts for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 2910–2916. [Google Scholar] [CrossRef] [PubMed]

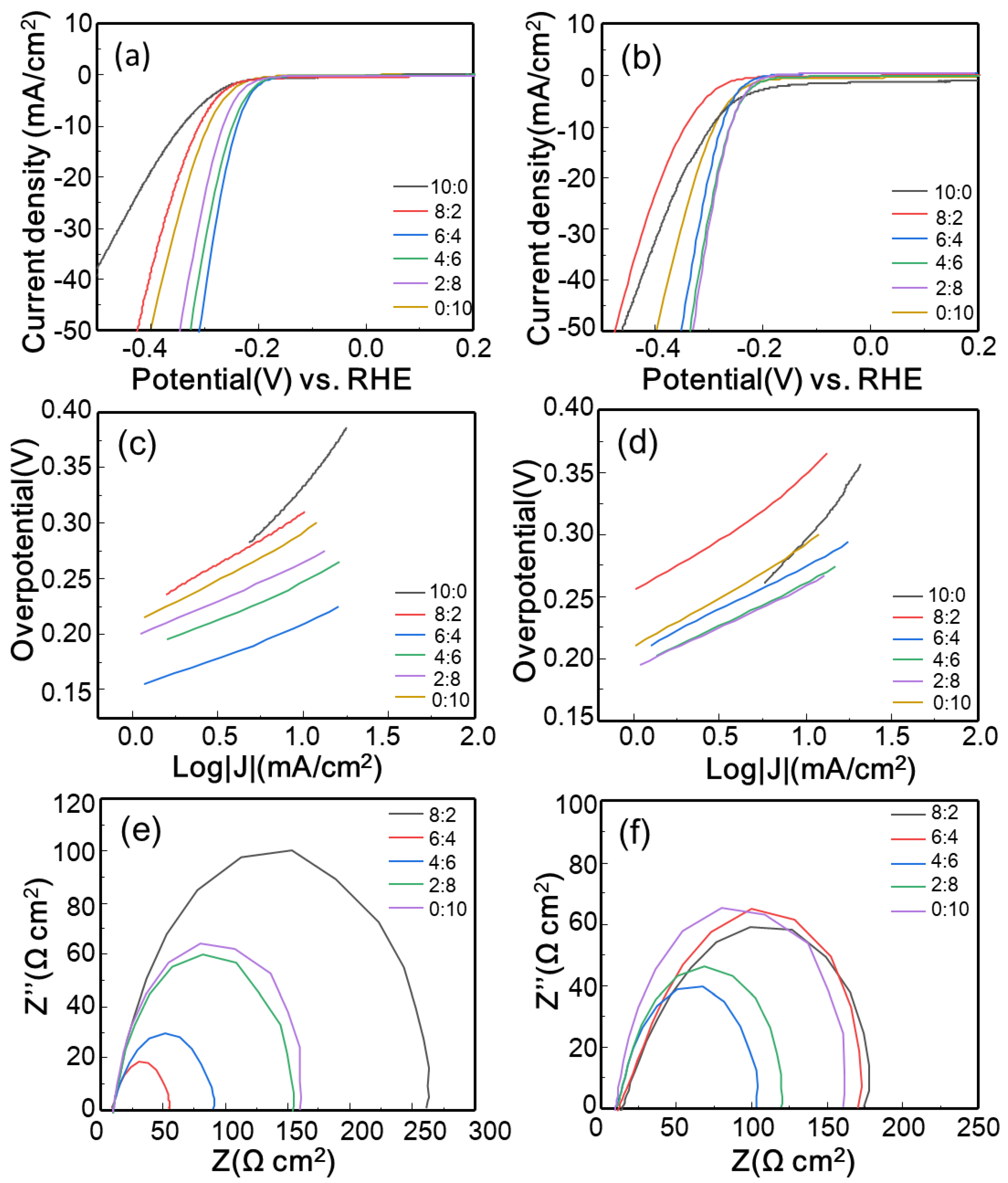
| GO-MoSe2 | rGO-MoSe2 | |||||||
|---|---|---|---|---|---|---|---|---|
| η1 (mV) | η10 (mV) | Tafel Slope (mV/dec) | Rct (Ω cm2) | η1 (mV) | η10 (mV) | Tafel Slope (mV/dec) | Rct (Ω cm2) | |
| 10:0 | −43 | 333 | 164 | 819 | −304 | 295 | 166 | 476 |
| 8:2 | 210 | 293 | 89 | 176 | 255 | 349 | 88 | 148 |
| 6:4 | 180 | 238 | 57 | 35.4 | 202 | 274 | 70 | 134 |
| 4:6 | 190 | 248 | 63 | 54.7 | 194 | 261 | 66 | 79.1 |
| 2:8 | 195 | 264 | 67 | 118 | 194 | 256 | 67 | 89.6 |
| 0:10 | 210 | 295 | 80 | 134 | 210 | 295 | 80 | 134 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Le, Q.V.; Hasani, A.; Lee, T.H.; Jang, H.W.; Luo, Z.; Kim, S.Y. MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction. Polymers 2018, 10, 1309. https://doi.org/10.3390/polym10121309
Guo W, Le QV, Hasani A, Lee TH, Jang HW, Luo Z, Kim SY. MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction. Polymers. 2018; 10(12):1309. https://doi.org/10.3390/polym10121309
Chicago/Turabian StyleGuo, Wenwu, Quyet Van Le, Amirhossein Hasani, Tae Hyung Lee, Ho Won Jang, Zhengtang Luo, and Soo Young Kim. 2018. "MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction" Polymers 10, no. 12: 1309. https://doi.org/10.3390/polym10121309
APA StyleGuo, W., Le, Q. V., Hasani, A., Lee, T. H., Jang, H. W., Luo, Z., & Kim, S. Y. (2018). MoSe2-GO/rGO Composite Catalyst for Hydrogen Evolution Reaction. Polymers, 10(12), 1309. https://doi.org/10.3390/polym10121309