Self-Structuring in Water of Polyamidoamino Acids with Hydrophobic Side Chains Deriving from Natural α-Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
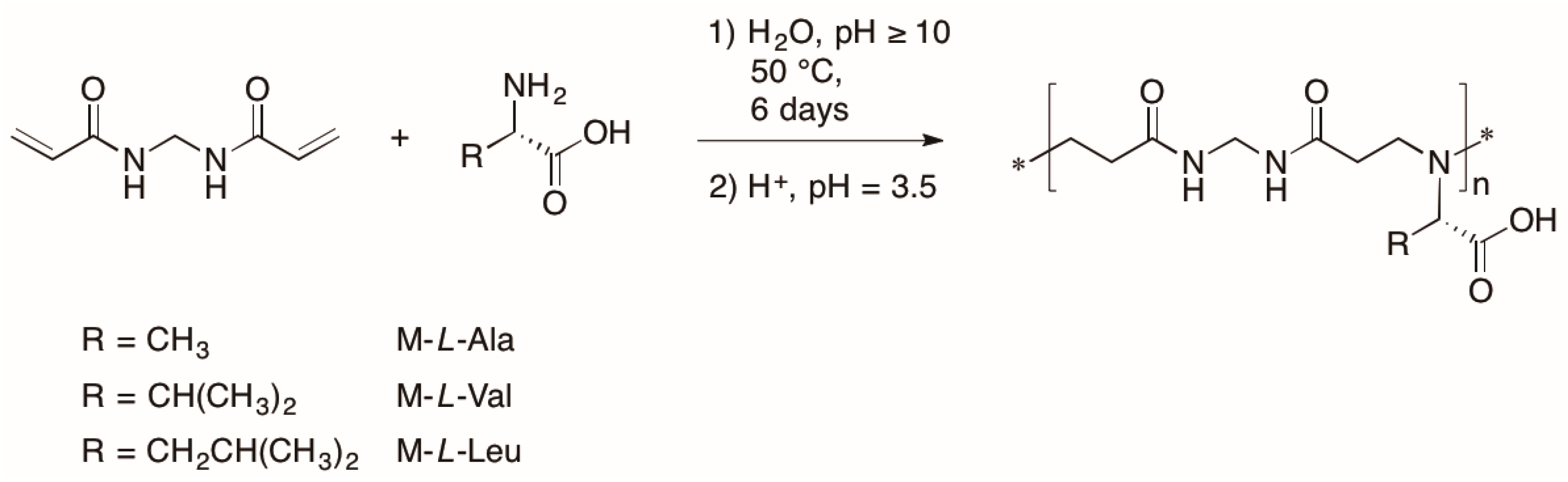
3.1. Synthesis of Polyamidoamino Acids
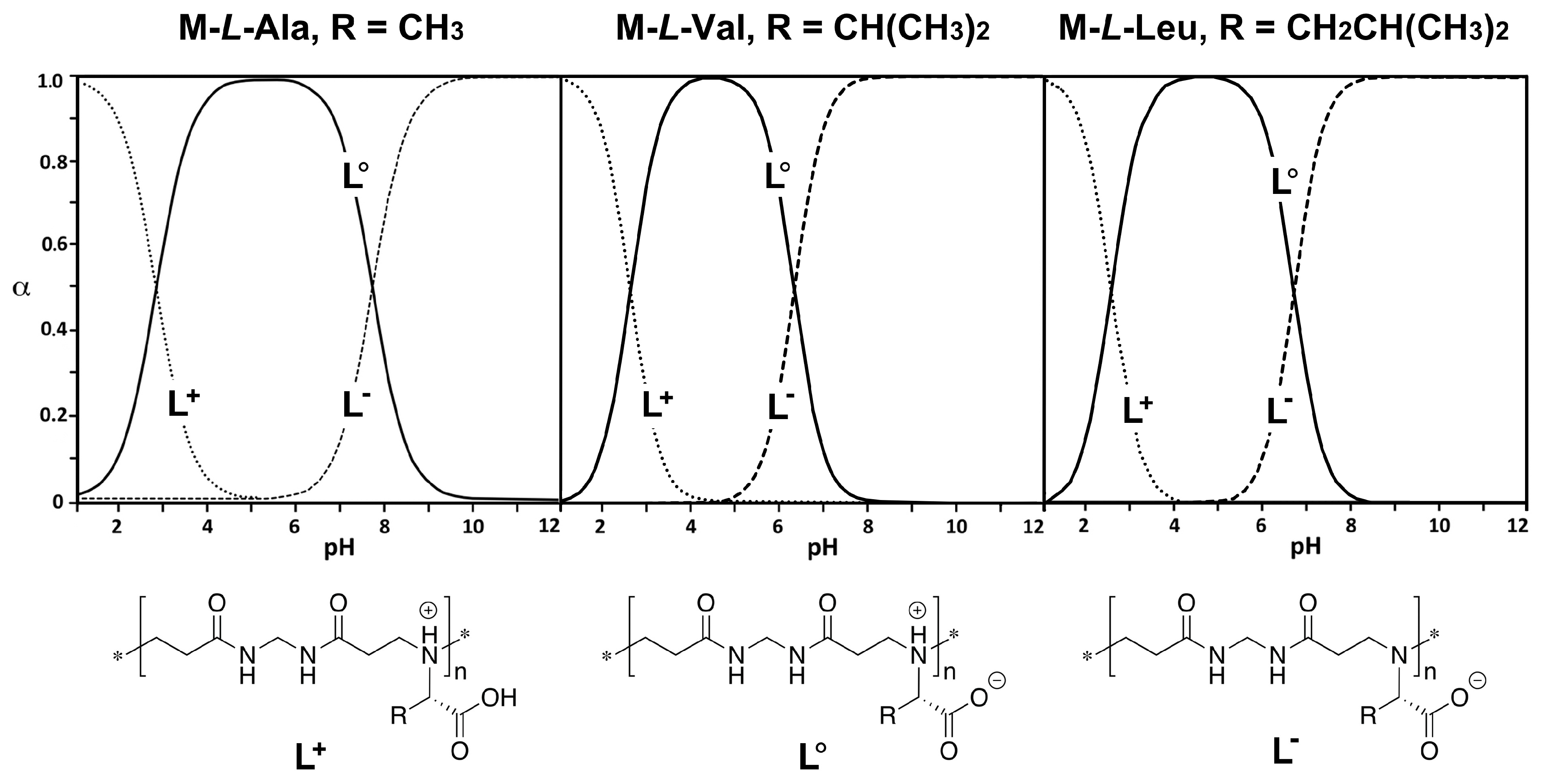
3.2. Acid-Base Properties
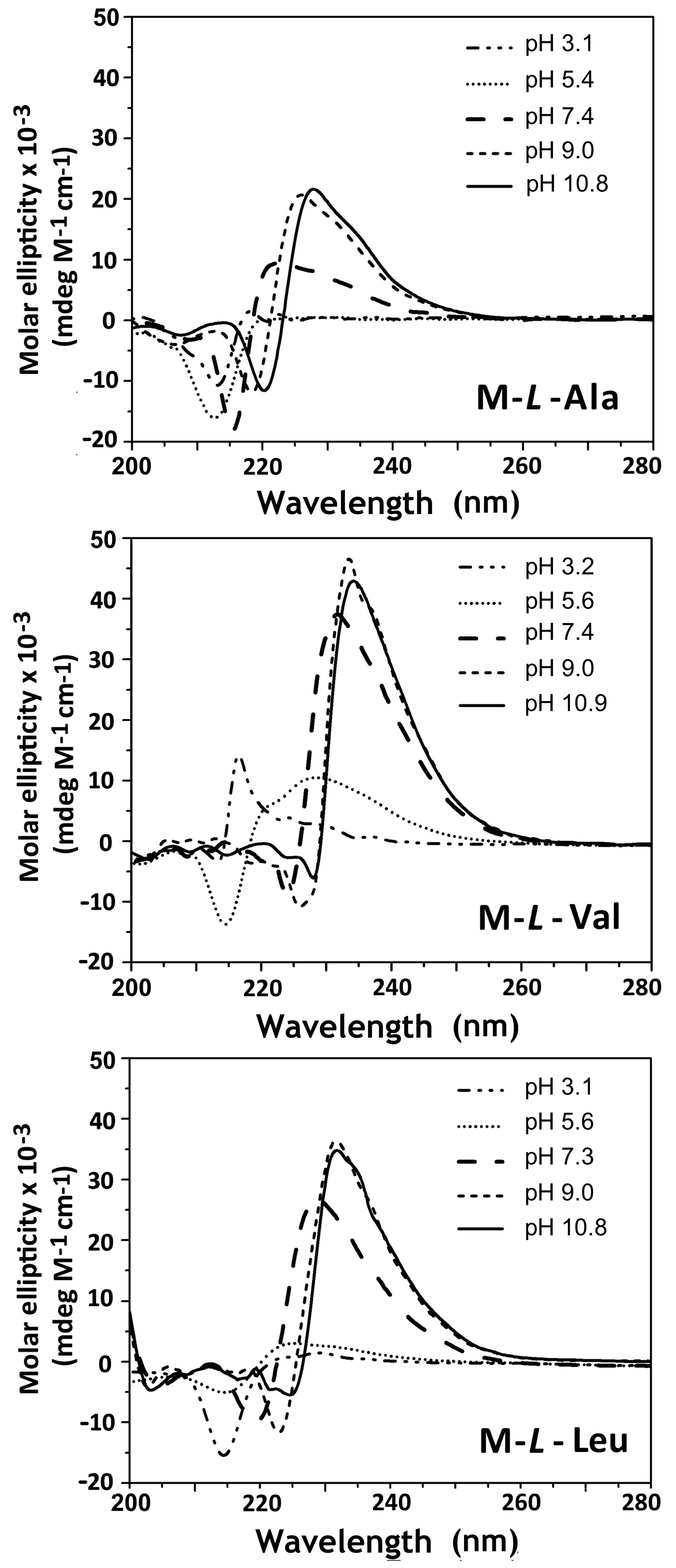
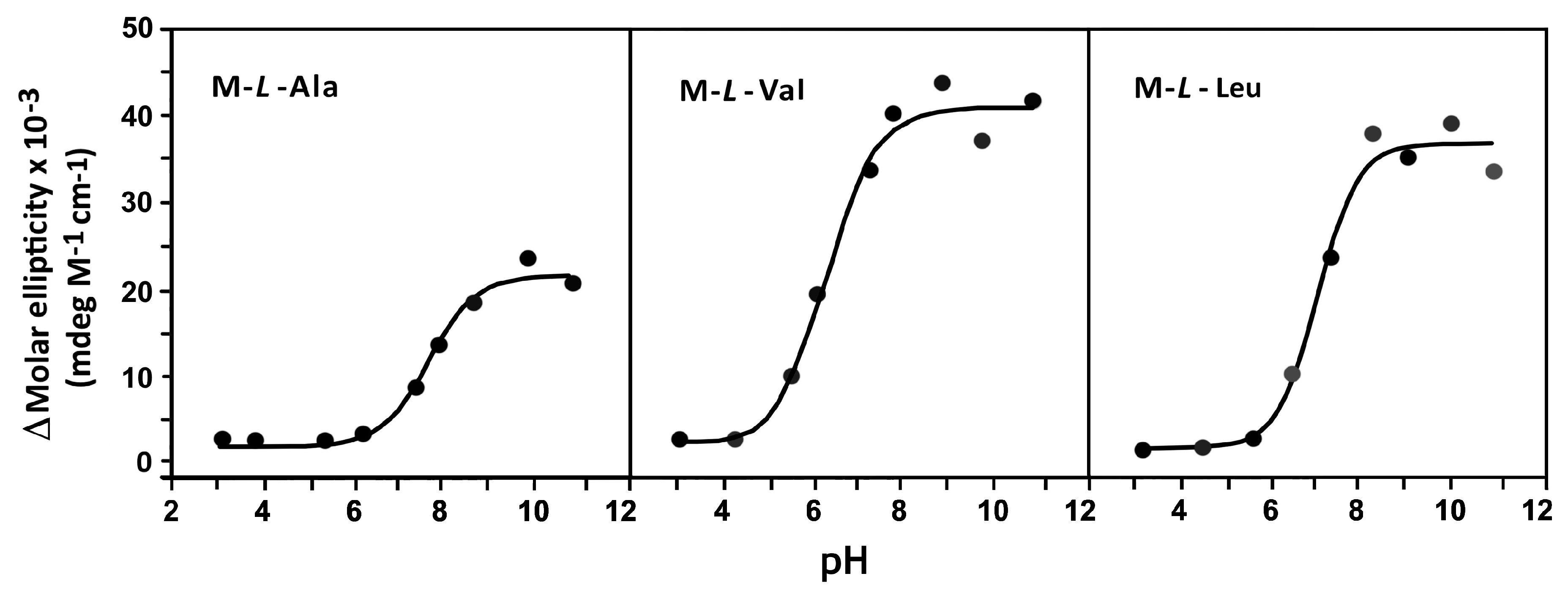
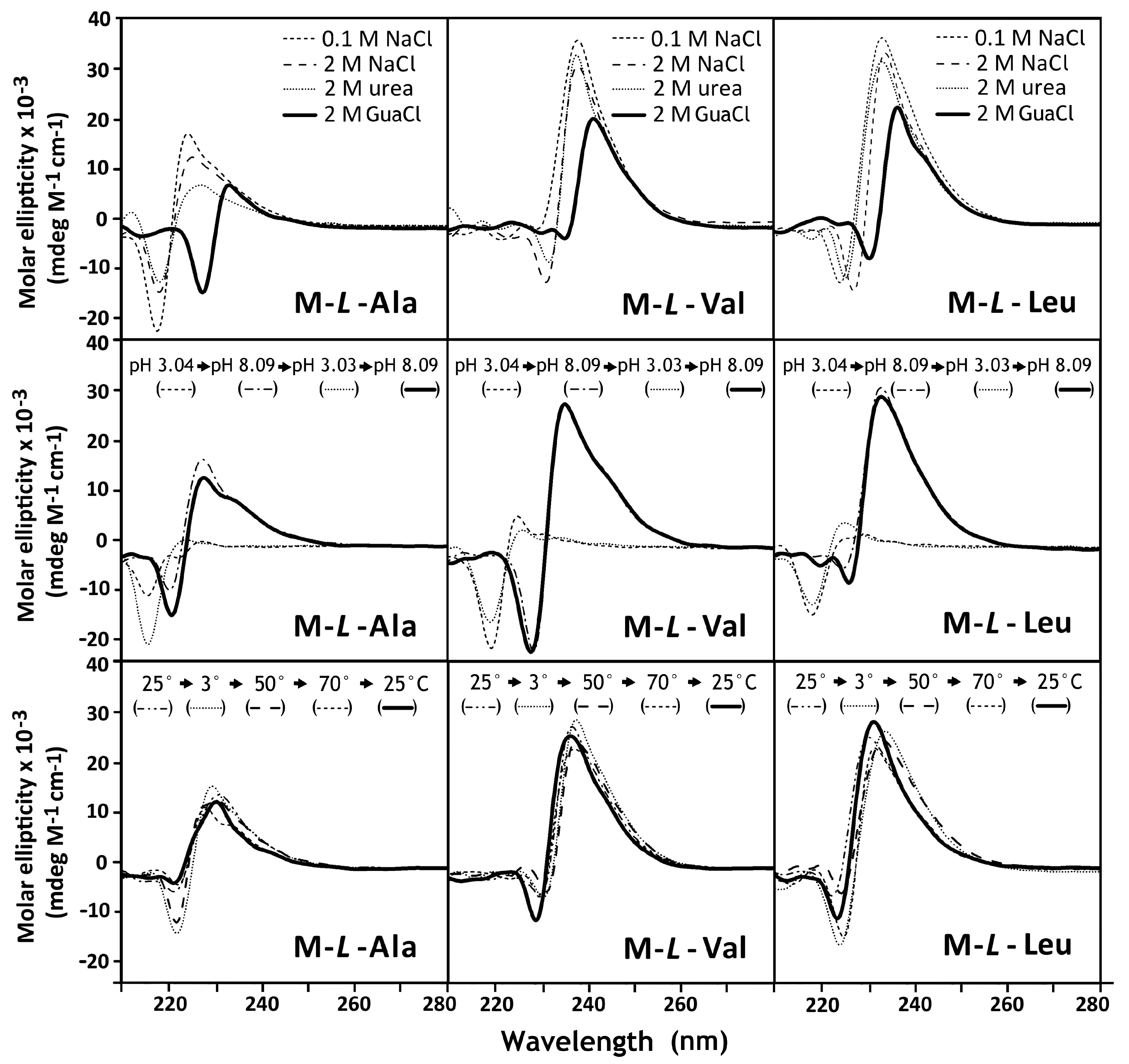
3.3. Circular Dichroism Spectroscopy
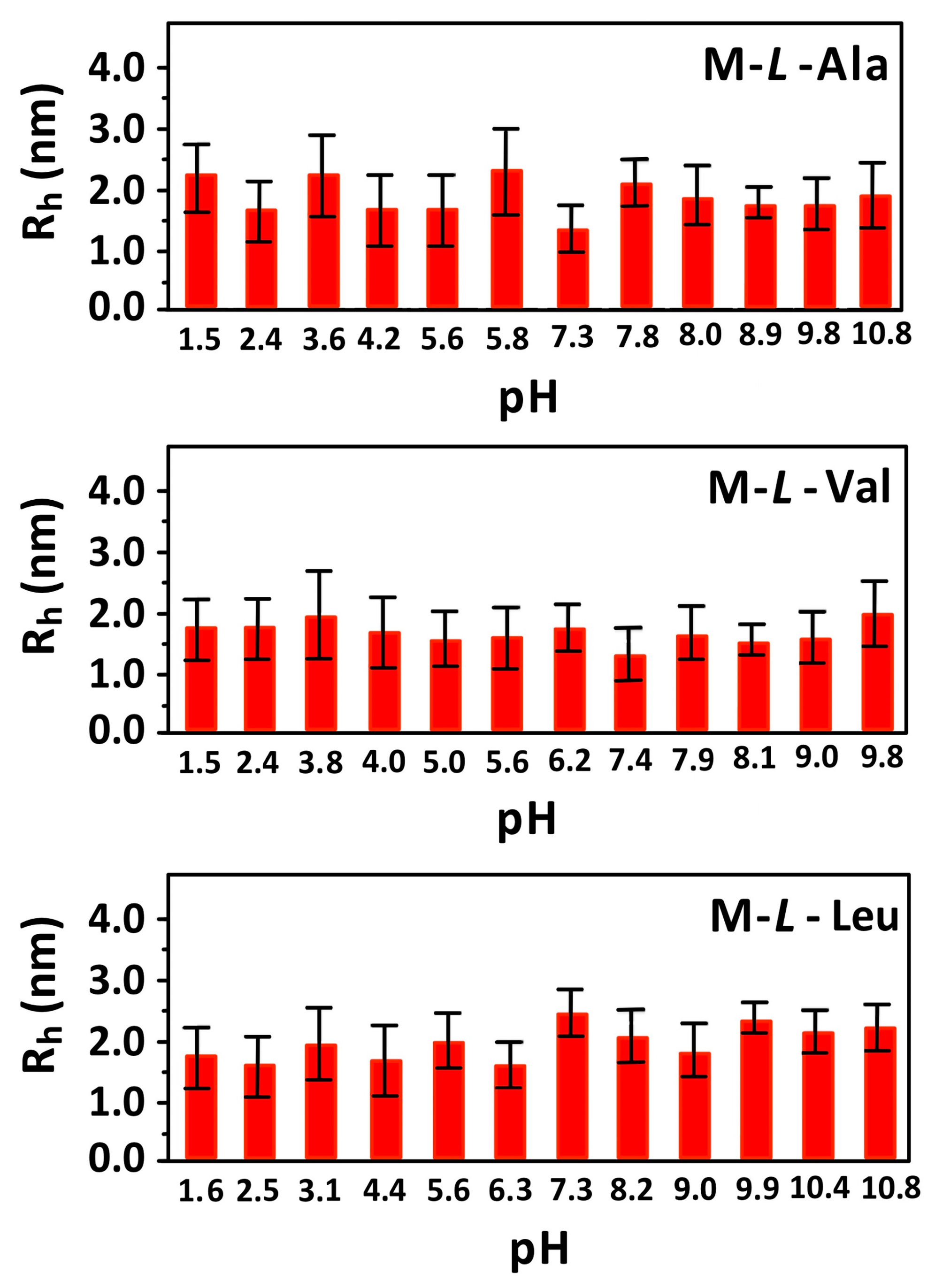
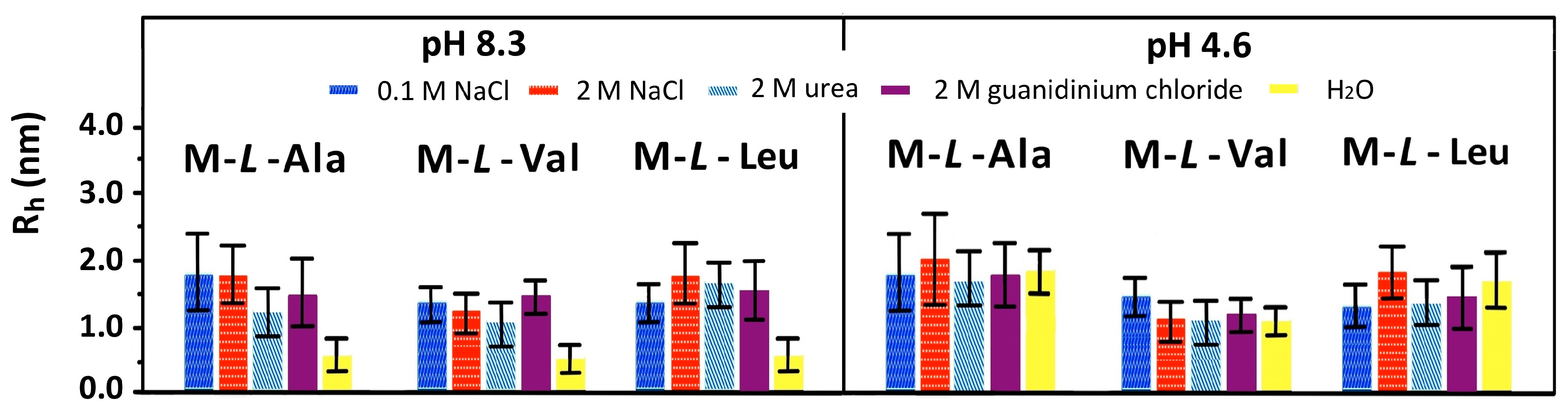
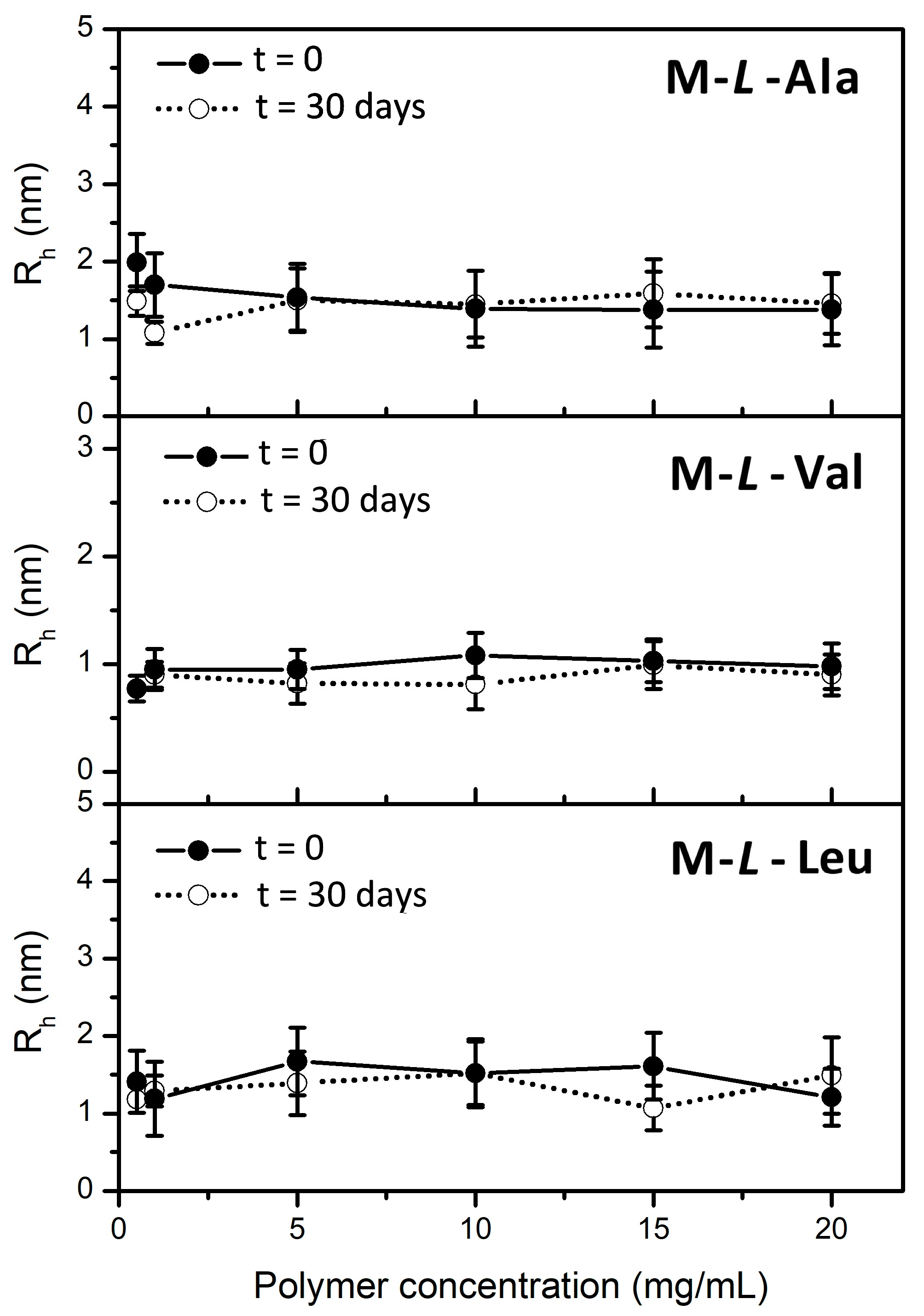
3.4. Dynamic Light Scattering (DLS) Measurements
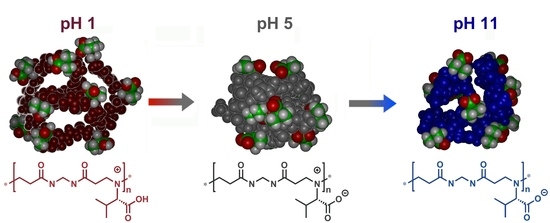
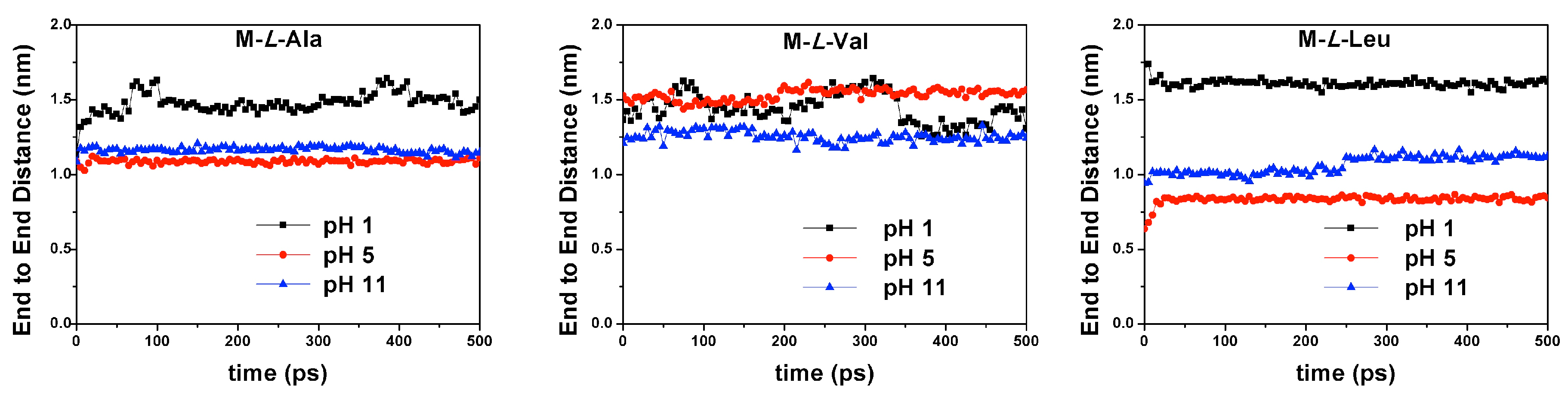
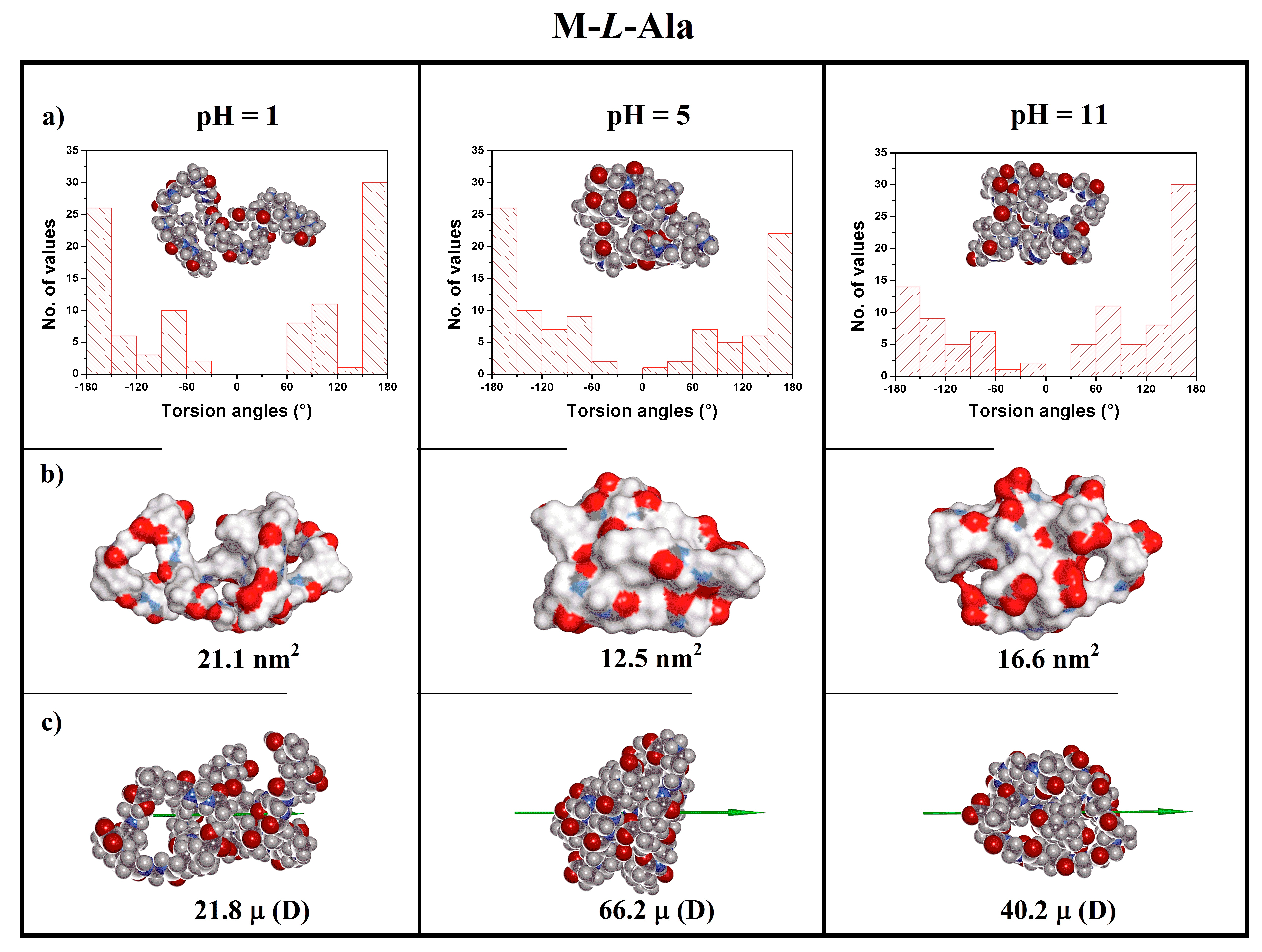
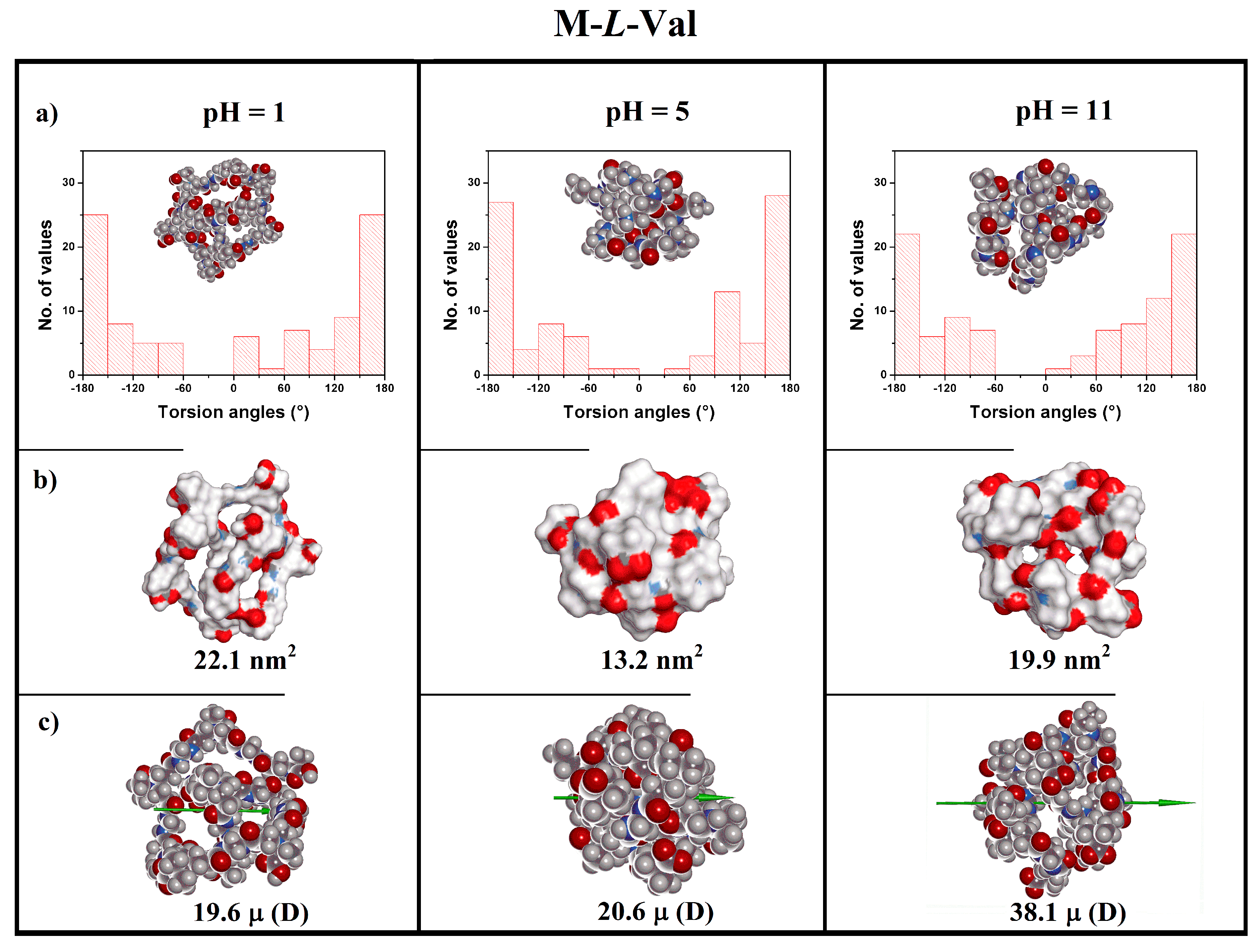
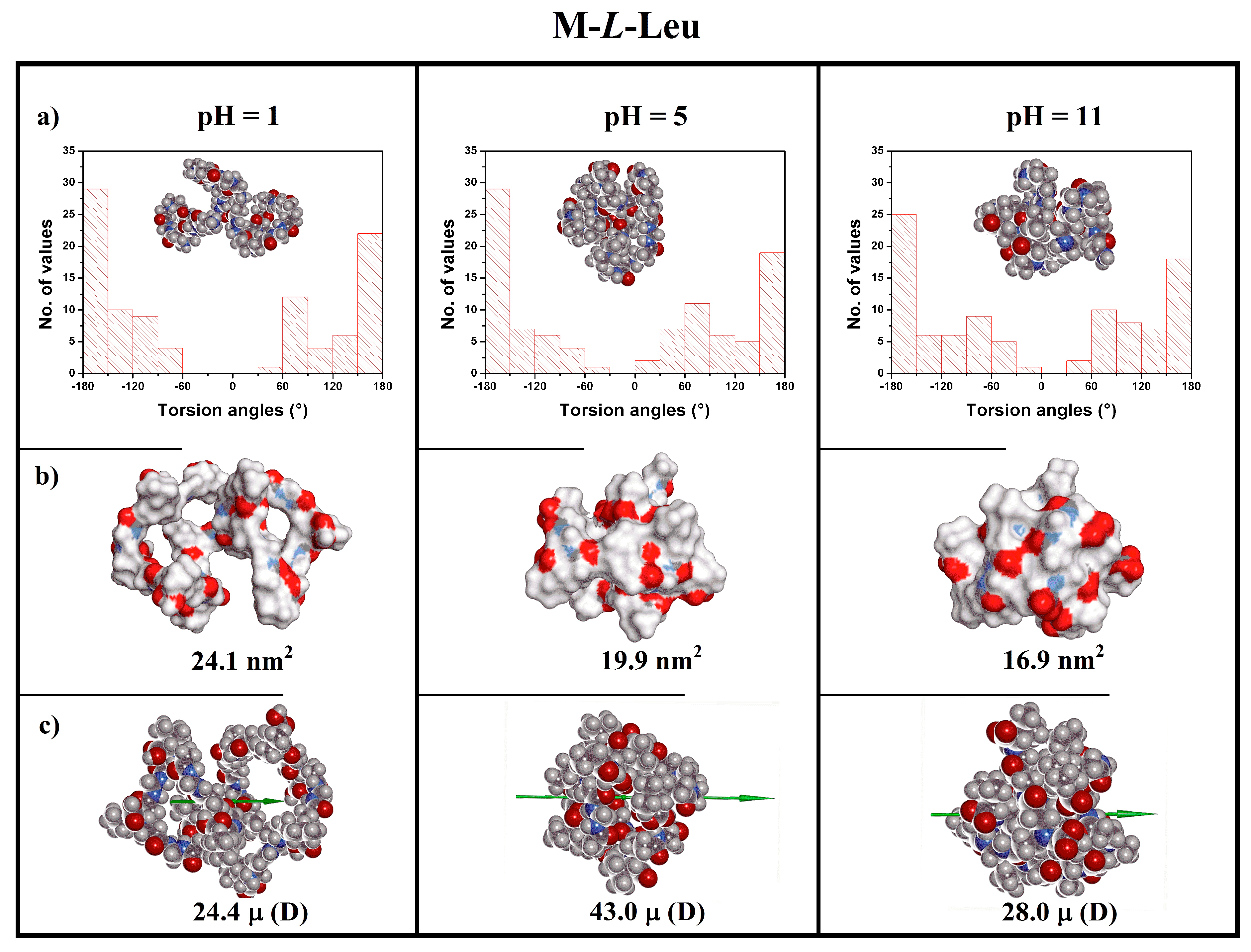
3.5. Theoretical Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- van Hest, J.C.M. Biosynthetic-Synthetic Polymer Conjugates. J. Macromol. Sci. Part C Polym. Rev. 2007, 47, 63–92. [Google Scholar] [CrossRef]
- Sun, J.; Zuckermann, R.N. Peptoid Polymers: A Highly Designable Bioinspired Material. ACS Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Murnen, H.K.; Kline, S.R.; Zuckermann, R.N.; Segalman, R.A. Determination of the persistence length of helical and non-helical polypeptoids in solution. Soft Matter 2012, 8, 3673–3680. [Google Scholar] [CrossRef]
- Sinaga, A.; Hatton, T.A.; Tam, K.C. Poly(acrylic acid)-block-poly(l-valine): Evaluation of β-Sheet Formation and Its Stability Using Circular Dichroism Technique. Biomacromolecules 2007, 8, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Bauri, K.; Ghosh Roy, S.; De, P. Side-Chain Amino-Acid-Derived Cationic Chiral Polymers by Controlled Radical Polymerization. Macromol. Chem. Phys. 2016, 217, 365–379. [Google Scholar] [CrossRef]
- Sanda, F.; Nakamura, M.; Endo, T. Syntheses and Radical Copolymerization Behavior of Optically Active Methacrylamides Having l- and d-Leucine Moieties. Interaction between l- and d-Forms. Macromolecules 1996, 29, 8064–8068. [Google Scholar] [CrossRef]
- Casolaro, M.; Casolaro, I. Stimuli-Responsive Hydrogels Bearing α-Amino Acid Residues: A Potential Platform for Future Therapies. J. Biomed. Eng. Med. Device 2016, 1, 111. [Google Scholar] [CrossRef]
- Mori, H.; Kato, I.; Endo, T. Dual-Stimuli-Responsive Block Copolymers Derived from Proline Derivatives. Macromolecules 2009, 42, 4985–4992. [Google Scholar] [CrossRef]
- Maji, T.; Banerjee, S.; Bose, A.; Mandal, T.K. A Stimuli-Responsive Methionine-Based Zwitterionic Methacryloyl Sulfonium Sulfonate Monomer and the Corresponding Antifouling Polymer with Tunable Thermosensitivity. Polym. Chem. 2017, 8, 3164–3176. [Google Scholar] [CrossRef]
- Maji, T.; Banerjee, S.; Biswas, Y.; Mandal, T.K. Dual-Stimuli-Responsive L-Serine-Based Zwitterionic UCST-type Polymer with Tunable Thermosensitivity. Macromolecules 2015, 48, 4957–4966. [Google Scholar] [CrossRef]
- Gao, G.; Sanda, F.; Masuda, T. Synthesis and Properties of Amino Acid-Based Polyacetylenes. Macromolecules 2003, 36, 3932–3937. [Google Scholar] [CrossRef]
- Cheuk, K.K.L.; Li, B.S.; Lam, J.W.Y.; Xie, Y.; Tang, B.Z. Synthesis, Chain Helicity, Assembling Structure, and Biological Compatibility of Poly(Phenylacetylene)s Containing l-Alanine Moieties. Macromolecules 2008, 41, 5997–6005. [Google Scholar] [CrossRef]
- Hopkins, T.E.; Pawlow, J.H.; Koren, D.L.; Deters, K.S.; Solivan, S.M.; Davis, J.A.; Gómez, F.J.; Wagener, K.B. Chiral Polyolefins Bearing Amino Acids. Macromolecules 2001, 34, 7920–7922. [Google Scholar] [CrossRef]
- Sanda, S.; Endo, T. Synthesis and Cationic Polymerization of A Novel Optically Active Vinyl Ether with L-Proline Structure. Macromol. Chem. Phys. 1997, 198, 1209–1216. [Google Scholar] [CrossRef]
- Allcock, H.R.; Pucher, S.R.; Scopelianos, A.G. Poly[(Amino Acid Ester)Phosphazenes] as Substrates for the Controlled Release of Small Molecules. Biomaterials 1994, 15, 563–569. [Google Scholar] [CrossRef]
- Roy, S.G.; De, P. pH Responsive Polymers with Amino Acids in the Side Chains and their Potential Applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ladmiral, V.; Charlot, A.; Semsarilar, M.; Armes, S.P. Synthesis and Characterization of Poly(Amino Acid Methacrylate)-Stabilized Diblock Copolymer Nano-Objects. Polym. Chem. 2015, 6, 1805–1816. [Google Scholar] [CrossRef]
- Sugiyama, K.; Rikimaru, S.; Okada, Y.; Shiraishi, K. Preparation and Application of Chiral Recognizable Thermosensitive Polymers and Hydrogels Consisting of N-Methacryloyl-S-Phenylalanine Methyl Ester. J. Appl. Polym. Sci. 2001, 82, 228–236. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Sun, J.; He, G.; Li, Y.; Zhang, G. Preparation of Thermoresponsive Polymers Bearing Amino Acid Diamide Derivatives via RAFT Polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3573–3586. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, J.; Xie, P.; Wu, Y.; Deng, J. Chiral, pH-Sensitive Polyacrylamide Hydrogels: Preparation and Enantio-Differentiating Release Ability. Polymer 2015, 68, 246–252. [Google Scholar] [CrossRef]
- Wang, X.; Gan, H.; Sun, T.; Su, B.; Fuchs, H.; Vestweber, D.; Butz, S. Stereochemistry Triggered Differential Cell Behaviours on Chiral Polymer Surfaces. Soft Matter 2010, 6, 3851–3855. [Google Scholar] [CrossRef]
- Zuckermann, R.N. Peptoid Origins. Pept. Sci. 2011, 96, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Mauro, N.; Falciola, L.; Pifferi, V.; Bartoli, C.; Gazzarri, M.; Chiellini, F.; Ranucci, E. Amphoteric, Prevailingly Cationic l-Arginine Polymers of Poly(amidoamino acid) Structure: Synthesis, Acid/Base Properties and Preliminary Cytocompatibility and Cell-Permeating Characterizations. Macromol. Biosci. 2014, 14, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.; Mauro, N.; Terenzi, A.; Alongi, J.; Lazzari, F.; Ganazzoli, F.; Raffaini, G.; Ranucci, E.; Ferruti, P. Self-Ordering Secondary Structure of d- and l-Arginine-Derived Polyamidoamino Acids. ACS Macro Lett. 2017, 6, 987–991. [Google Scholar] [CrossRef]
- Ferruti, P. Poly(amidoamine)s: Past, Present, and Perspectives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Bignotti, F.; Sozzani, P.; Ranucci, E.; Ferruti, P. NMR Studies, Molecular Characterization, and Degradation Behavior of Poly(amido amine)s. 1. Poly(amido amine) Deriving from the Polyaddition of 2-Methylpiperazine to 1,4-Bis(acryloyl)piperazine. Macromolecules 1994, 27, 7171–7178. [Google Scholar] [CrossRef]
- Danusso, F.; Ferruti, P. Synthesis of Tertiary Amine Polymers. Polymer 1970, 11, 88–113. [Google Scholar] [CrossRef]
- Wagner, H.L.; Long, F.A. Properties of an Amphoteric Polymer of Vinylpyridine and Acrylic Acid. J. Phys. Chem. 1951, 55, 1512–1526. [Google Scholar] [CrossRef]
- Lappan, U.; Geißler, U.; Oelmann, M.; Schwarz, S. Apparent Dissociation Constants of Polycarboxylic Acids in Presence of Polycations. Colloid Polym. Sci. 2012, 290, 1665–1670. [Google Scholar] [CrossRef]
- Katchalsky, A.; Spitnik, P. Potentiometric titrations of polymethacrylic acid. J. Polym. Sci. 1947, 2, 432–446. [Google Scholar] [CrossRef]
- Mele, A.; Raffaini, G.; Ganazzoli, F.; Juza, M.; Schurig, V. Macrocycle Conformation and Self-Inclusion Phenomena in Octakis-(3-O-butanoyl-2,6-di-O-pentyl)-γ-Cyclodextrin (Lipodex E) by NMR Spectroscopy and Molecular Dynamics. Carbohydr. Res. 2003, 338, 625–635. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Surface Hydration of Polymeric (Bio)Materials: A Molecular Dynamics Simulation Study. J. Biomed. Mater. Res. Part A 2010, 92, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Ganazzoli, F. Protein Adsorption on Biomaterial and Nanomaterial Surfaces: A Molecular Modeling Approach to Study Non-Covalent Interactions. J. Appl. Biomater. Biomech. 2010, 8, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Ganazzoli, F.; Malpezzi, L.; Fuganti, C.; Fronza, G.; Panzeri, W.; Mele, A. Validating a Strategy for Molecular Dynamics Simulations of Cyclodextrin Inclusion Complexes Through Single-Crystal X-ray and NMR Experimental Data: A Case Study. J. Phys. Chem. B 2009, 113, 9110–9122. [Google Scholar] [CrossRef] [PubMed]
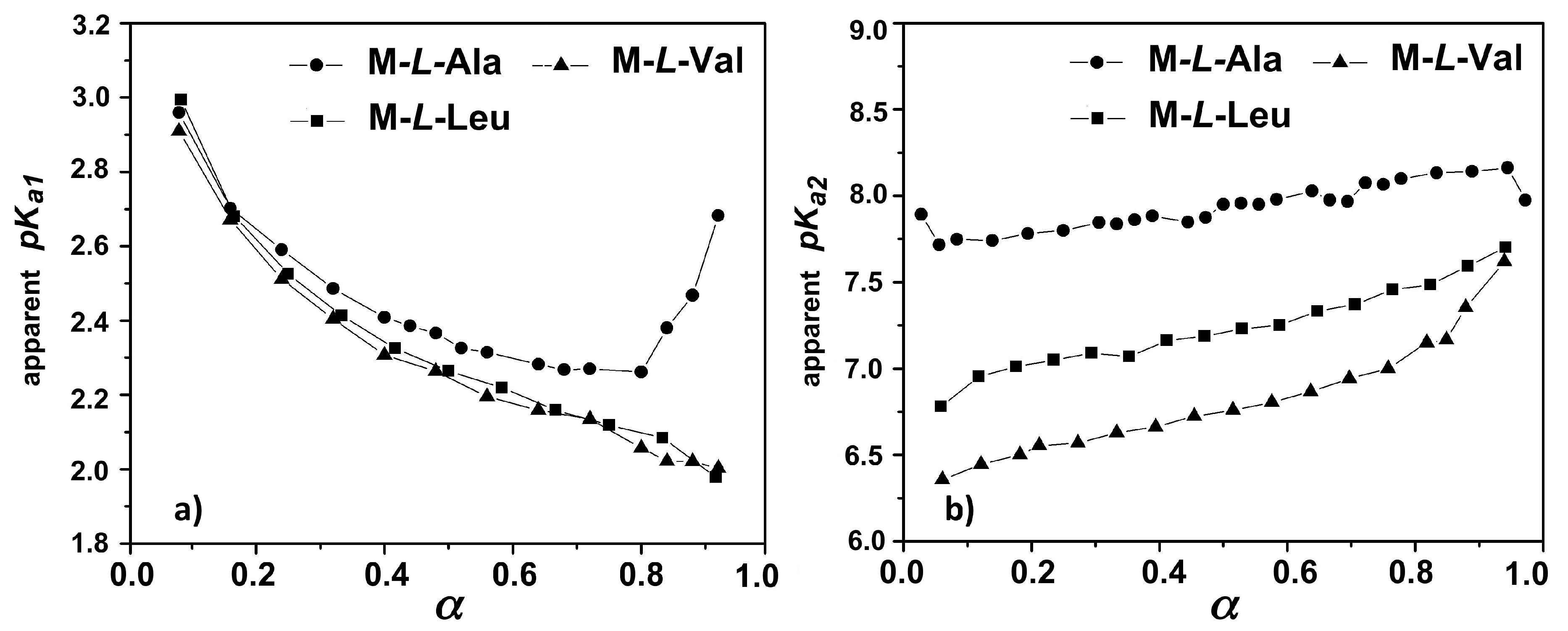
| Sample | pKa1a,b | β1a | pKa2b,c | β2c | IP d |
|---|---|---|---|---|---|
| M-l-Ala | 2.12 ± 0.04 | 0.76 ± 0.05 | 8.13 ± 0.17 | 1.32 ± 0.04 | 5.1 |
| M-l-Val | 2.08 ± 0.06 | 0.68 ± 0.09 | 6.78 ± 0.03 | 1.45 ± 0.08 | 4.4 |
| M-l-Leu | 2.11 ± 0.02 | 0.61 ± 0.07 | 7.37 ± 0.16 | 1.38 ± 0.07 | 4.7 |
| Polymer | pH | Charge (e) | Rg a (nm) | R b (nm) | S c (nm2) | Volume d (nm3) | μ e (D) |
|---|---|---|---|---|---|---|---|
| 1 | +10 | 1.07 | 1.57 | 21.1 | 2.35 | 21.8 | |
| M-l-Ala | 5 | 0 | 0.74 | 1.09 | 12.5 | 2.61 | 66.2 |
| 11 | −10 | 0.80 | 1.23 | 16.6 | 2.65 | 40.2 | |
| 1 | +10 | 1.04 | 1.41 | 22.1 | 2.55 | 19.6 | |
| M-l-Val | 5 | 0 | 0.73 | 1.57 | 13.2 | 2.80 | 20.6 |
| 11 | −10 | 0.85 | 1.46 | 19.9 | 2.66 | 38.1 | |
| 1 | +10 | 1.11 | 1.73 | 24.1 | 2.70 | 24.4 | |
| M-l-Leu | 5 | 0 | 0.85 | 0.84 | 19.9 | 2.95 | 43.0 |
| 11 | −10 | 0.80 | 1.14 | 16.9 | 3.11 | 28.0 |
| Polymer | Rh (nm) pH 1.5 | Rh (nm) pH 5.0 | Rh (nm) pH 9.0 |
|---|---|---|---|
| M-l-Ala | 1.56 ± 0.52 | 1.54 ± 0.43 | 0.59 ± 0.17 |
| M-l-Val | 1.13 ± 0.33 | 0.90 ± 0.21 | 0.45 ± 0.17 |
| M-l-Leu | 1.40 ± 0.41 | 1.61 ± 0.49 | 0.67 ± 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzari, F.; Manfredi, A.; Alongi, J.; Mendichi, R.; Ganazzoli, F.; Raffaini, G.; Ferruti, P.; Ranucci, E. Self-Structuring in Water of Polyamidoamino Acids with Hydrophobic Side Chains Deriving from Natural α-Amino Acids. Polymers 2018, 10, 1261. https://doi.org/10.3390/polym10111261
Lazzari F, Manfredi A, Alongi J, Mendichi R, Ganazzoli F, Raffaini G, Ferruti P, Ranucci E. Self-Structuring in Water of Polyamidoamino Acids with Hydrophobic Side Chains Deriving from Natural α-Amino Acids. Polymers. 2018; 10(11):1261. https://doi.org/10.3390/polym10111261
Chicago/Turabian StyleLazzari, Federica, Amedea Manfredi, Jenny Alongi, Raniero Mendichi, Fabio Ganazzoli, Giuseppina Raffaini, Paolo Ferruti, and Elisabetta Ranucci. 2018. "Self-Structuring in Water of Polyamidoamino Acids with Hydrophobic Side Chains Deriving from Natural α-Amino Acids" Polymers 10, no. 11: 1261. https://doi.org/10.3390/polym10111261
APA StyleLazzari, F., Manfredi, A., Alongi, J., Mendichi, R., Ganazzoli, F., Raffaini, G., Ferruti, P., & Ranucci, E. (2018). Self-Structuring in Water of Polyamidoamino Acids with Hydrophobic Side Chains Deriving from Natural α-Amino Acids. Polymers, 10(11), 1261. https://doi.org/10.3390/polym10111261