[Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes
Abstract
1. Introduction
2. Experimental Section
3. Instruments
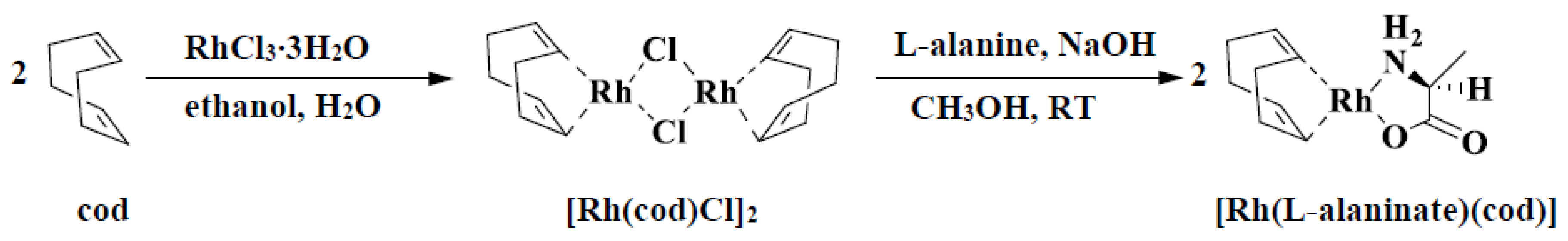
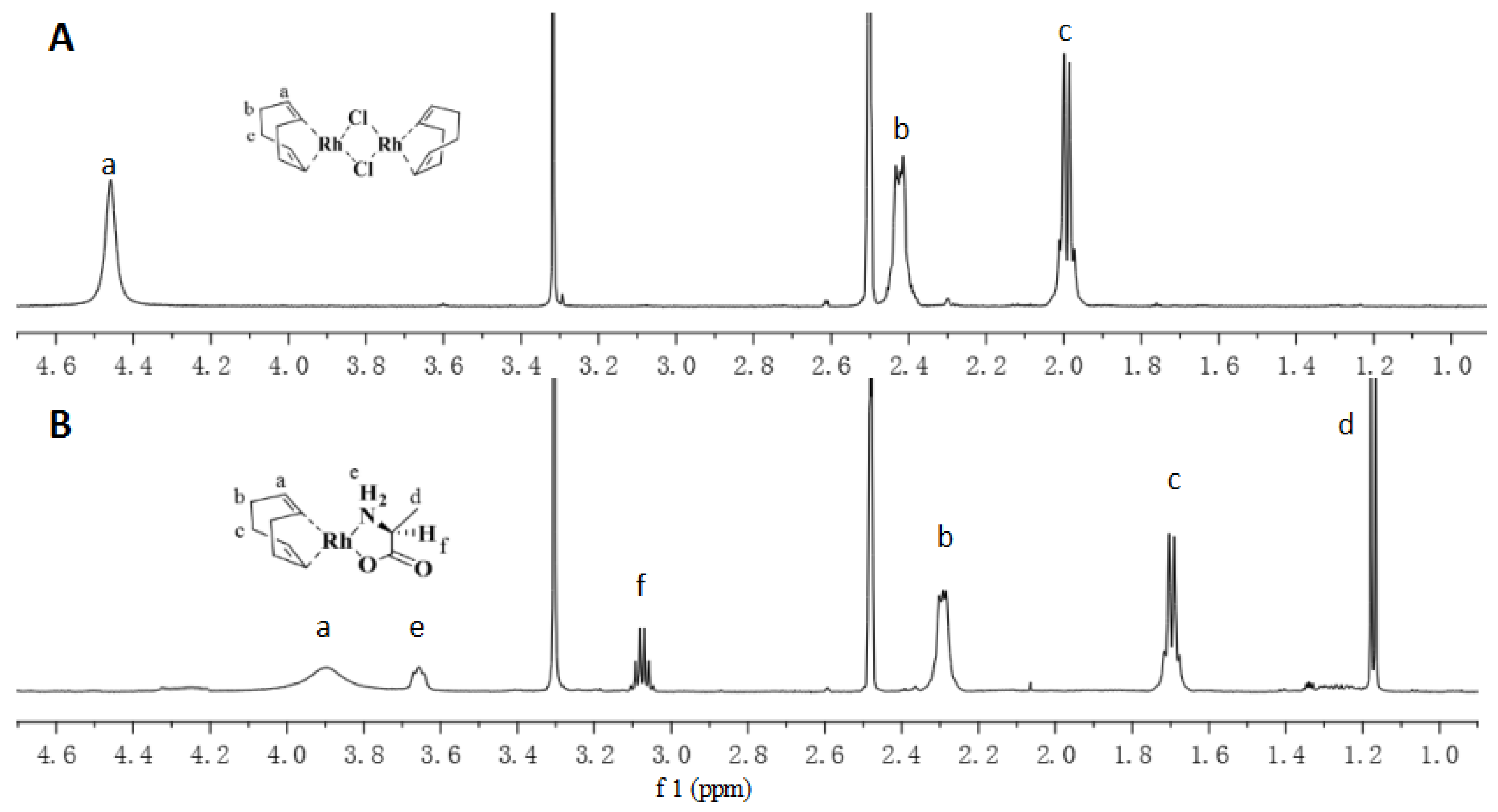
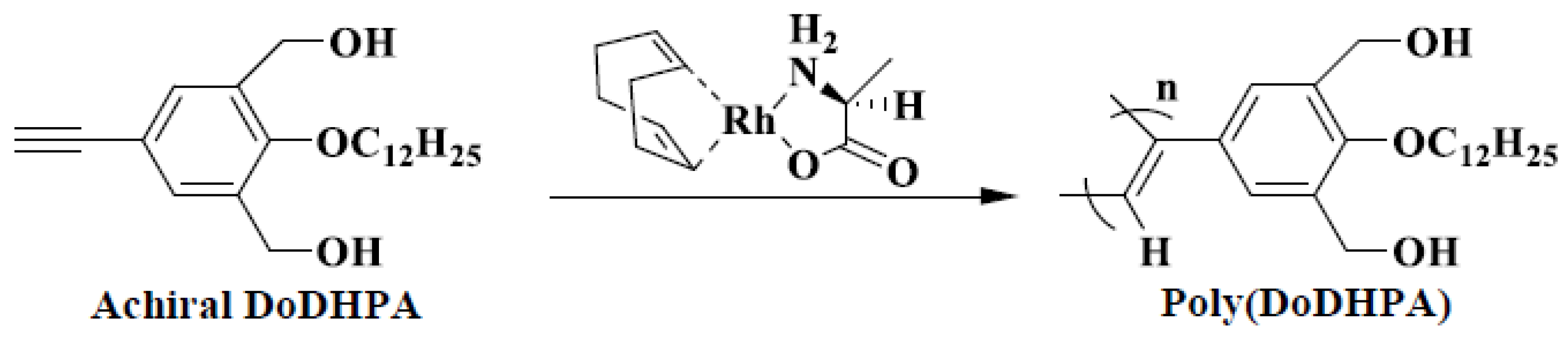
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ohta, T.; Nakahara, S.; Shigemura, Y.; Hattori, K.; Furukawa, I. α-Amino acid: An affective ligand for asymmetric catalysis of transfer hydrogenation of ketones. Appl. Organomet. Chem. 2001, 15, 699–709. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Lee, S.; Suzuki, N.; Fujiki, M.; Lee, C.L.; Kwak, G. Optically active conjugated polymer from solvent chirality transfer polymerization in monoterpenes. Macromol. Rapid Commun. 2013, 34, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.G.; Shi, Y.Q.; Ma, L.Q.; Wang, Y.Z.; Zang, Y.; Peng, J.J. Asymmetric polymerisation of substituted phenylacetylene using chiral Rh(2,5-norbornadiene)(L-proline) catalyst. Chem. Pap. 2014, 69, 756–760. [Google Scholar] [CrossRef]
- Saeed, I.; Shiotsuki, M.; Masuda, T. Effect of diene ligands in the rhodium-catalyzed polymerization of phenylacetylene. Macromolecules 2006, 39, 8977–8981. [Google Scholar] [CrossRef]
- Jia, H.G.; Shi, Y.Q.; Ma, L.Q.; Gao, X.; Wang, Y.Z.; Zang, Y.; Peng, J.J.; Aoki, T.; Teraguchi, M.; Kaneko, T.; Masuda, T. Novel isolated, L-amino acid-ligated rhodium catalysts that induce highly helix-sense-selective polymerization of an achiral 3,4,5-trisubstituted phenylacetylene. J. Polym. Sci. Part A 2016, 54, 2346–2351. [Google Scholar] [CrossRef]
- Ciammaichella, A.; Cardoni, V.; Leoni, A.; Tagliatesta, P. Rhodium porphyrin bound to a merrifield resin as heterogeneous catalyst for the cyclopropanation reaction of olefins. Molecules 2016, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Ichikawa, Y.; Hayashi, T.; Onishi, N.; Shiotsuki, M.; Masuda, T. Asymmetric polymerization of achiral arylacetylenes giving helical polyacetylenes in the presence of a rhodium catalyst with a C2-symmetric tetrafluorobenzobarrelene ligand. Organometallics 2009, 28, 4890–4893. [Google Scholar] [CrossRef]
- Riener, K.; Hogerl, M.P.; Gigler, P.; Kuhn, F.E. Rhodium-catalyzed hydrosilylation of ketones: Catalyst development and mechanistic insights. ACS Catal. 2012, 2, 613–621. [Google Scholar] [CrossRef]
- Onishi, N.; Shiotsuki, M.; Sanda, F.; Masuda, T. Polymerization of phenylacetylenes with rhodium zwitterionic complexes: Enhanced catalytic activity by π-acidic diene ligands. Macromolecules 2009, 42, 4071–4076. [Google Scholar] [CrossRef]
- Jaseer, E.A.; Casado, M.A.; Al-Saadi, A.A.; Oro, L.A. Intermolecular hydroamination versus stereoregular polymerization of phenylacetylene by rhodium catalysts based on N–O bidentate ligands. Inorg. Chem. Commun. 2014, 40, 78–81. [Google Scholar] [CrossRef]
- Sato, T.; Aoki, T.; Teraguchi, M.; Kaneko, T.; Kim, S.Y. Role of chiral amine cocatalysts in the helix-sense-selective polymerization of a phenylacetylene using a catalytic system. Polymer 2004, 45, 8109–8114. [Google Scholar] [CrossRef]
- Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J. Am. Chem. Soc. 2003, 125, 6346–6347. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Teraguchi, M.; Aoki, T.; Abe, Y.; Kaneko, T.; Hadano, S.; Namikoshi, T.; Ohishi, T. Three mechanisms of asymmetric polymerization of phenylacetylenes having an L-amino ether residue and two hydroxy groups. Macromolecules 2010, 43, 8353–8362. [Google Scholar] [CrossRef]
- Zang, Y.; Ohishi, T.; Aoki, T.; Teraguchi, M.; Kaneko, T. Helix-sense-selective polymerization of substituted acetylenes by using an isolated Rh chiral initiator with an amino acid ligand. Chem. Lett. 2013, 42, 430–432. [Google Scholar] [CrossRef]
- Teraguchi, M.; Tanioka, D.; Kaneko, T.; Aoki, T. Helix-sense-selective polymerization of achiral phenylacetylenes with two N-alkylamide groups to generate the one-handed helical polymers stabilized by intramolecular hydrogen bonds. ACS Macro Lett. 2012, 1, 1258–1261. [Google Scholar] [CrossRef]
- Paiaro, G.; Musco, A.; Diana, G. Chemical and structural characterization of some π-allylicderivatives of rhodium(III). J. Organomet. Chem. 1965, 4, 466–474. [Google Scholar] [CrossRef]
- Hetterscheid, D.G.H.; Hendriksen, C.; Dzik, W.I.; Smits, J.M.M.; Eck, E.R.H.; Rowan, A.E.; Busico, V.; Vacatello, M.; Castell, V.V.A.; Segre, A.; Jellema, E.; Bloemberg, T.G.; Bruin, B. Rhodium-mediated stereoselective polymerization of “carbenes”. J. Am. Chem. Soc. 2006, 128, 9746–9752. [Google Scholar] [CrossRef] [PubMed]
- Enamullah, M.; Sharmin, A.; Hasegawa, M.; Hoshi, T.; Chamayou, A.C.; Janiak, C. Syntheses, spectroscopic studies, and crystal structures of chiral [Rh(aminocarboxylato)(η4-cod)] and chiral [Rh(amino alcohol)(η4-cod)]-(acetate) complexes with an example of a spontaneous resolution of a racemic mixture into homochiral helix-enantiomers. Eur. J. Inorg. Chem. 2006, 2146–2154. [Google Scholar] [CrossRef]
- Janiak, C.; Chamayou, A.C.; Uddin, A.K.M.; Uddin, M.; Hagen, K.S.; Enamullah, M. Polymorphs, enantiomorphs, chirality and helicity in [Rh{N,O}(η4-cod)] complexes with {N,O} = salicylaldiminato Schiff base or aminocarboxylato ligands. Dalton Trans. 2009, 3698–3709. [Google Scholar] [CrossRef] [PubMed]
- Enamullah, M.; Hasegawa, M.; Hoshi, T.; Okubo, J. Synthetic and spectroscopic studies of [(η4-cod)Rh(I)-amino-acidato] complexes. J. Bangladesh Chem. Soc. 2005, 18, 165–173. [Google Scholar]
- Jimenez, M.V.; Perez-Torrente, J.J.; Bartolome, M.I.; Vispe, E.; Lahoz, F.J.; Oro, L.A. Cationic rhodium complexes with hemilabile phosphine ligands as polymerization catalyst for high molecular weight stereoregular poly(phenylacetylene). Macromolecules 2009, 42, 8146–8156. [Google Scholar] [CrossRef]
- Trhlikova, O.; Zednik, J.; Balcar, H.; Brus, J.; Sedlacek, J. [Rh(cycloolefin)(acac)] complexes as catalysts of polymerization of aryl- and alkylacetylenes: Influence of cycloolefin ligand and reaction conditions. J. Mol. Catal. A 2013, 378, 57–66. [Google Scholar] [CrossRef]
- Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Motoshige, R.; Sasaki, T.; Tabata, M. Structural determination of stretched helix and contracted helix having yellow and red colors of poly(2-ethynylnaphthalene) prepared with a [Rh(norbornadiene)Cl]2-triethylamine catalyst. Polymer 2014, 55, 2356–2361. [Google Scholar] [CrossRef]
- Jellema, E.; Budzelaar, P.H.M.; Reek, J.N.H.; Bruin, B. Rh-Mediated Polymerization of carbenes: Mechanism and stereoregulation. J. Am. Chem. Soc. 2007, 129, 11631–11641. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Eckerle, P.; Miyatake, T.; Kainosho, M.; Ono, A.; Ikariya, T.; Noyori, R. Well-controlled polymerization of phenylacetylenes with organorhodium(I) complexes: Mechanism and structure of the polyenes. J. Am. Chem. Soc. 1999, 121, 12035–12044. [Google Scholar] [CrossRef]
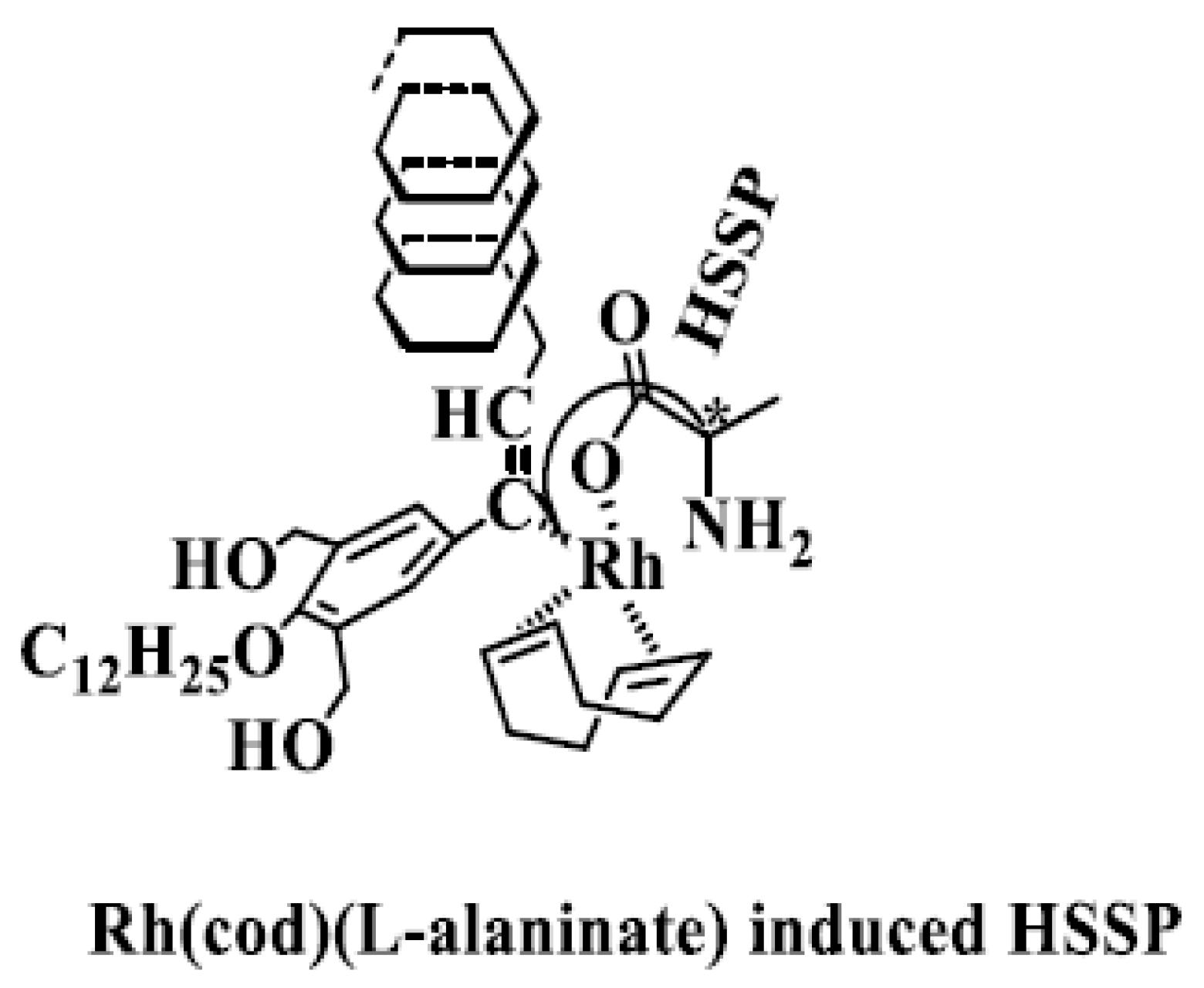
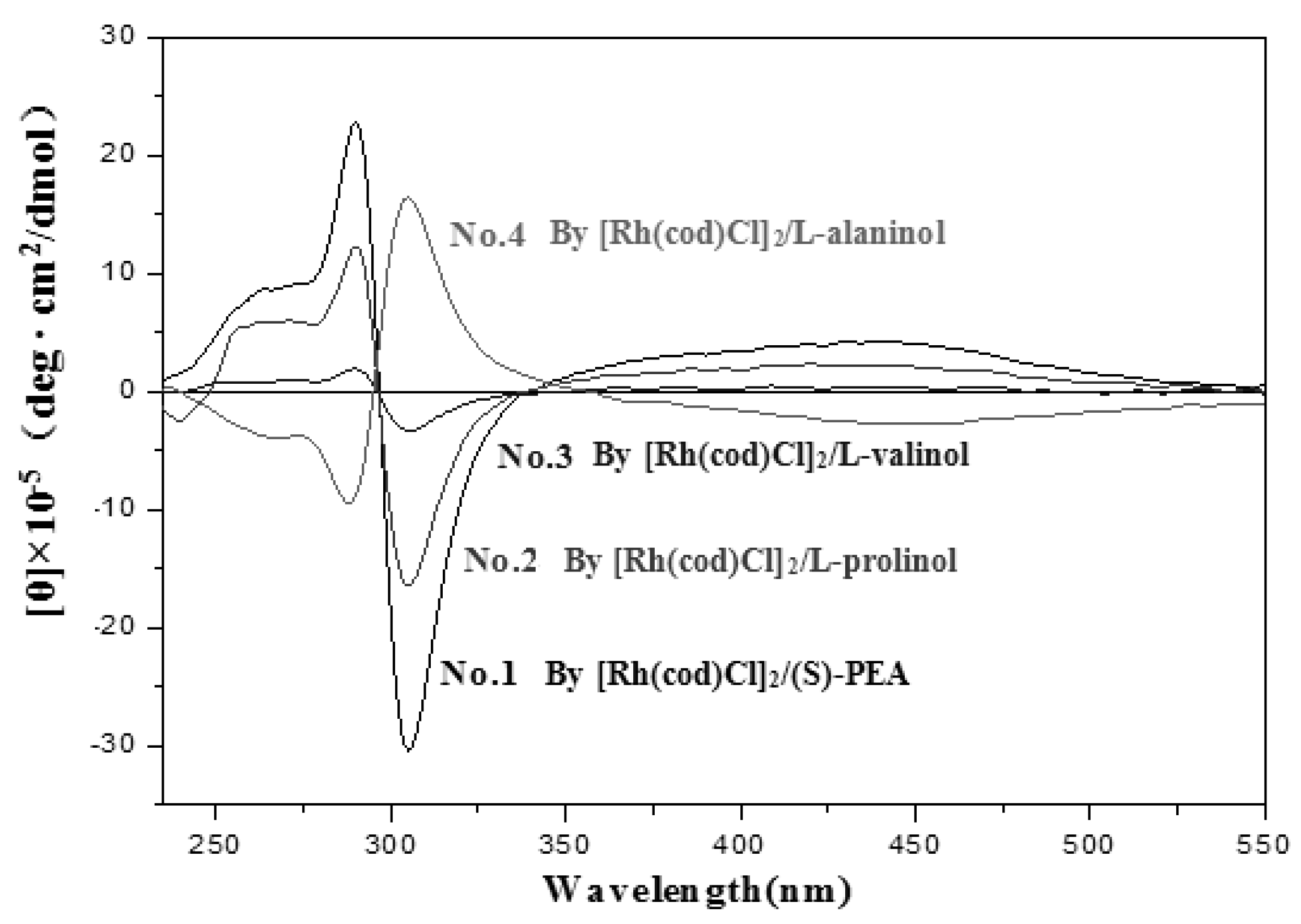
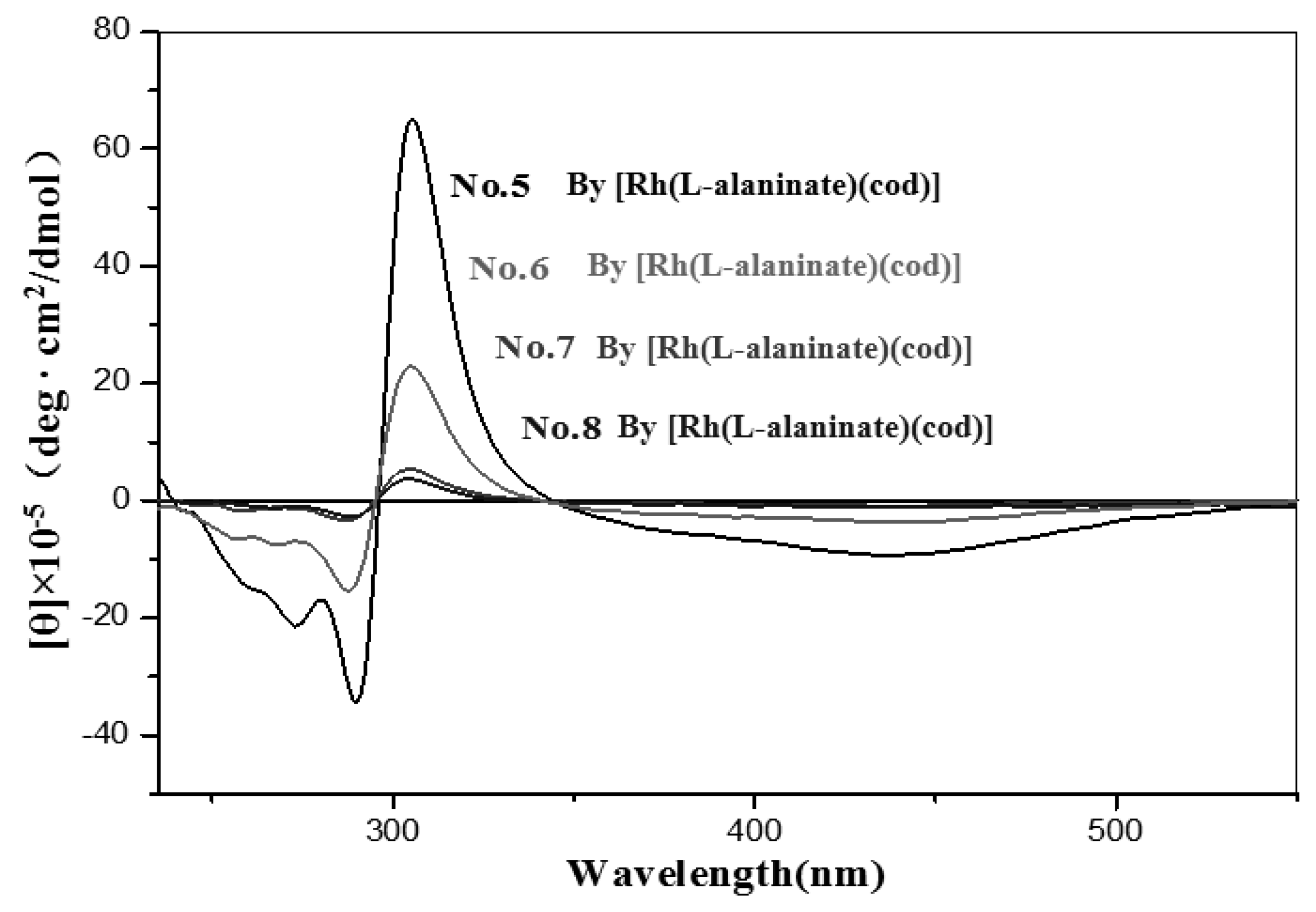
| No. | Catalyst | Cocatalyst | Solvent | Yield | Mwb (× 104) | Mw/Mnb | [θ]310 c (×10−5) |
|---|---|---|---|---|---|---|---|
| 1 | [Rh(cod)Cl]2 | (S)-PEA | Toluene | 23 | 9.82 | 2.35 | −24.70 |
| 2 | L-prolinol | Toluene | 10 | 9.83 | 3.88 | −13.16 | |
| 3 | L-valinol | Toluene | 8 | 2.93 | 1.15 | −2.77 | |
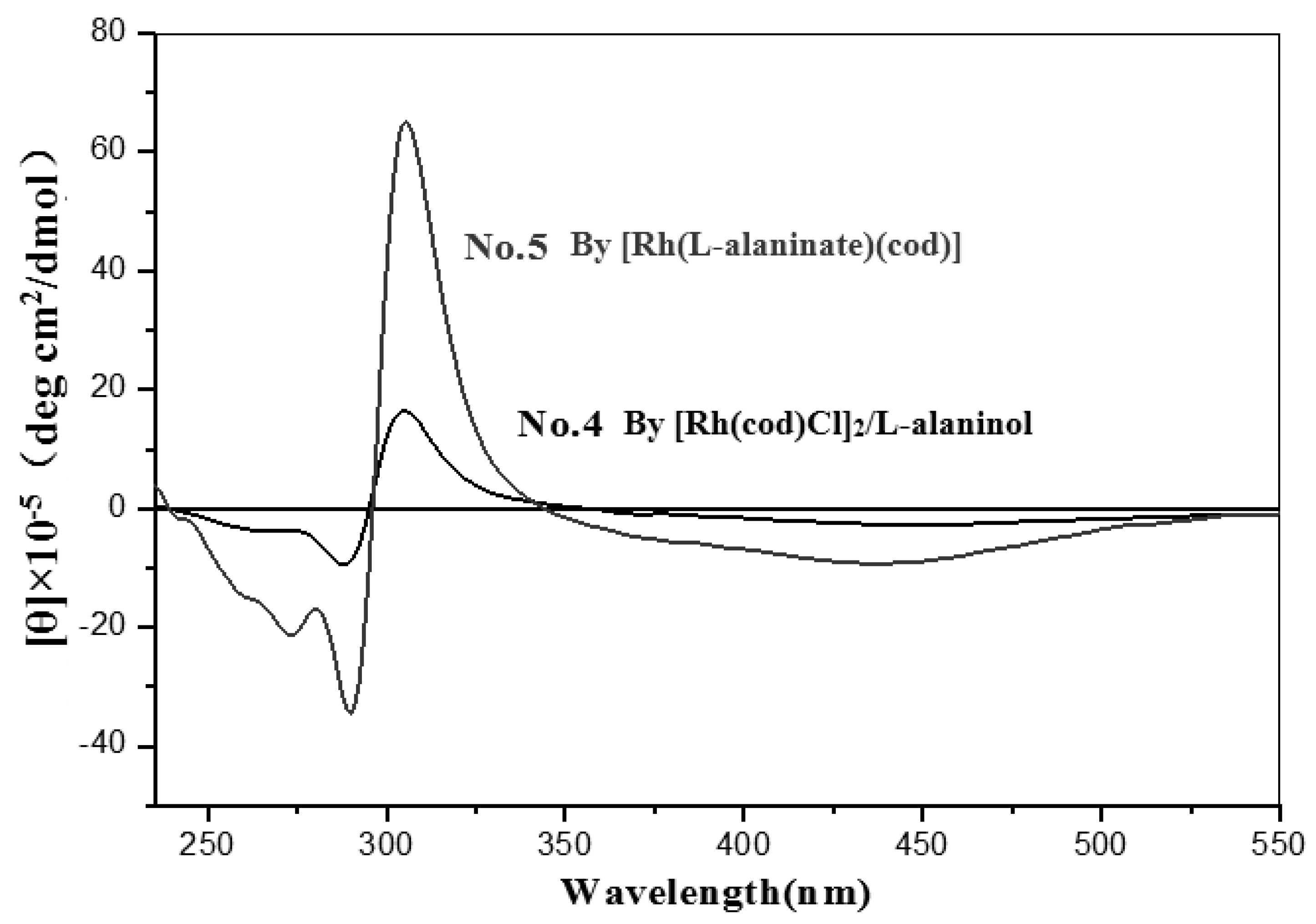
| 4 | L-alaninol | Toluene | 31 | 28.50 | 5.36 | 13.57 | |
| 5 | [Rh(L-alaninate)(cod)] | none | Toluene | 72 | 55.43 | 2.14 | 55.00 |
| 6 | none | CH2Cl2 | 25 | 4.57 | 2.30 | 19.10 | |
| 7 | none | Benzene | 39 | 15.14 | 2.64 | 4.26 | |
| 8 | none | Acetone | 17 | 20.64 | 3.95 | 2.83 | |
| 9 [12] | [Rh(nbd)Cl]2 | (R)-PEA | Toluene | 36 | 517.00 | - | 31.10 |
| 10 | L-alaninol | Toluene | 64 | 43.39 | 2.78 | 29.00 | |
| 11 | L-alaninol | CH2Cl2 | 35 | 20.65 | 4.93 | 17.00 | |
| 12 | [Rh(L-alaninate)(nbd)] | none | Toluene | 33 | 73.08 | 2.83 | 6.27 |
| 13 | none | CH2Cl2 | 83 | 245.26 | 5.25 | 79.11 |
| No. | Solvent | Yield(%) | Mnb(×104) | Mwb (×104) | Mw/Mnb |
|---|---|---|---|---|---|
| 1 | Toluene | 78.6 | 6.80 | 10.54 | 1.55 |
| 2 | CHCl3 | 82.5 | 9.58 | 12.74 | 1.33 |
| 3 | CH2Cl2 | 82.0 | 10.32 | 21.57 | 2.09 |
| 4 | THF | 87.8 | 12.78 | 28.49 | 2.23 |
| 5 | CH3OH | 92.5 | 17.26 | 30.21 | 1.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jia, H.; Shi, Y.; Ma, L.; Yang, G.; Wang, Y.; Xu, S.; Wang, J.; Zang, Y.; Aoki, T. [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes. Polymers 2018, 10, 1223. https://doi.org/10.3390/polym10111223
Wang Q, Jia H, Shi Y, Ma L, Yang G, Wang Y, Xu S, Wang J, Zang Y, Aoki T. [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes. Polymers. 2018; 10(11):1223. https://doi.org/10.3390/polym10111223
Chicago/Turabian StyleWang, Qingyu, Hongge Jia, Yongqiang Shi, Liqun Ma, Guoxing Yang, Yazhen Wang, Shuangping Xu, Jianjun Wang, Yu Zang, and Toshiki Aoki. 2018. "[Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes" Polymers 10, no. 11: 1223. https://doi.org/10.3390/polym10111223
APA StyleWang, Q., Jia, H., Shi, Y., Ma, L., Yang, G., Wang, Y., Xu, S., Wang, J., Zang, Y., & Aoki, T. (2018). [Rh(L-alaninate)(1,5-Cyclooctadiene)] Catalyzed Helix-Sense-Selective Polymerizations of Achiral Phenylacetylenes. Polymers, 10(11), 1223. https://doi.org/10.3390/polym10111223





