Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
3. Results and Discussion
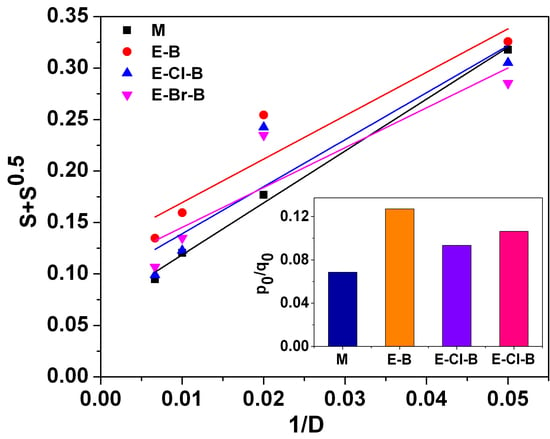
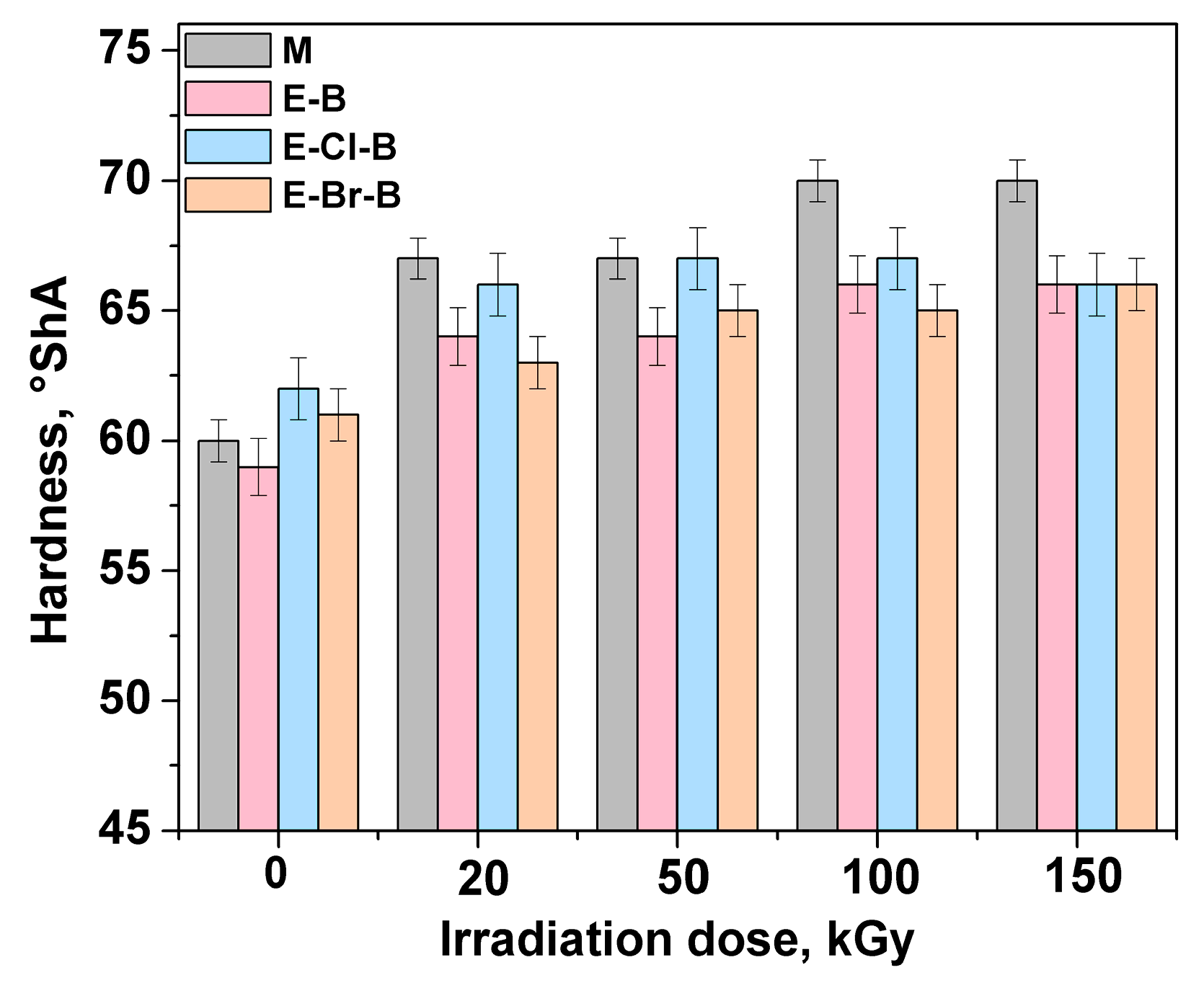
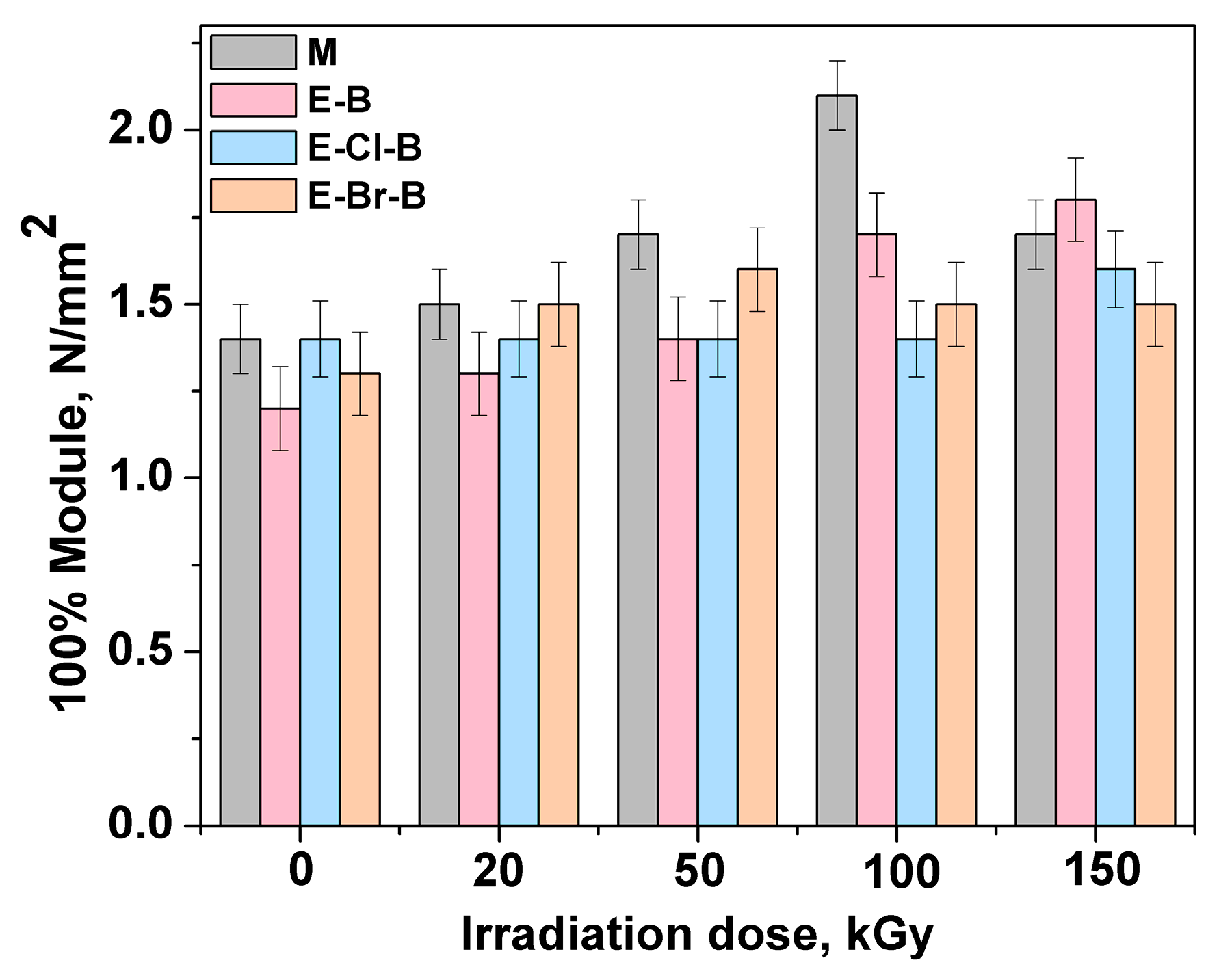
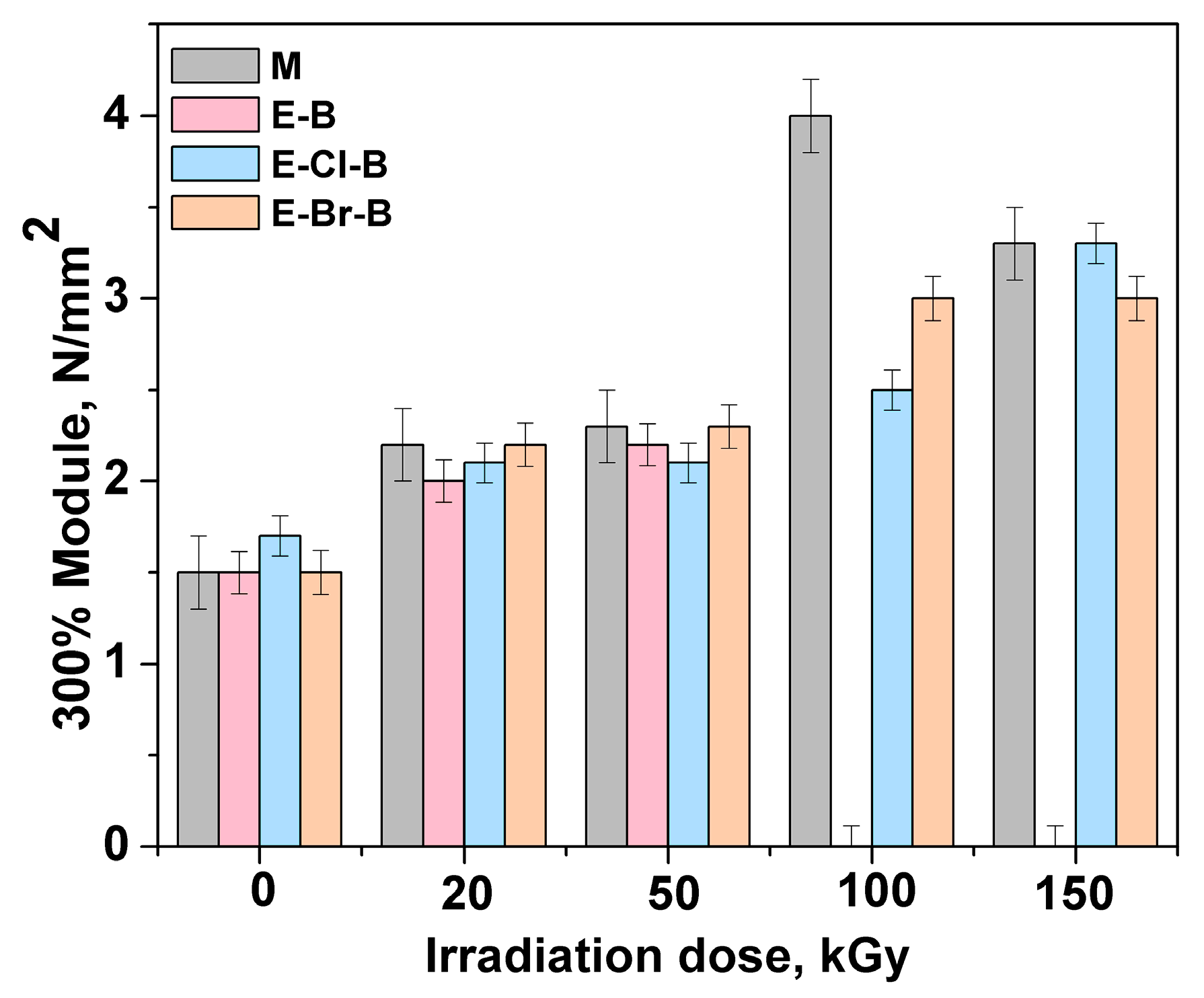
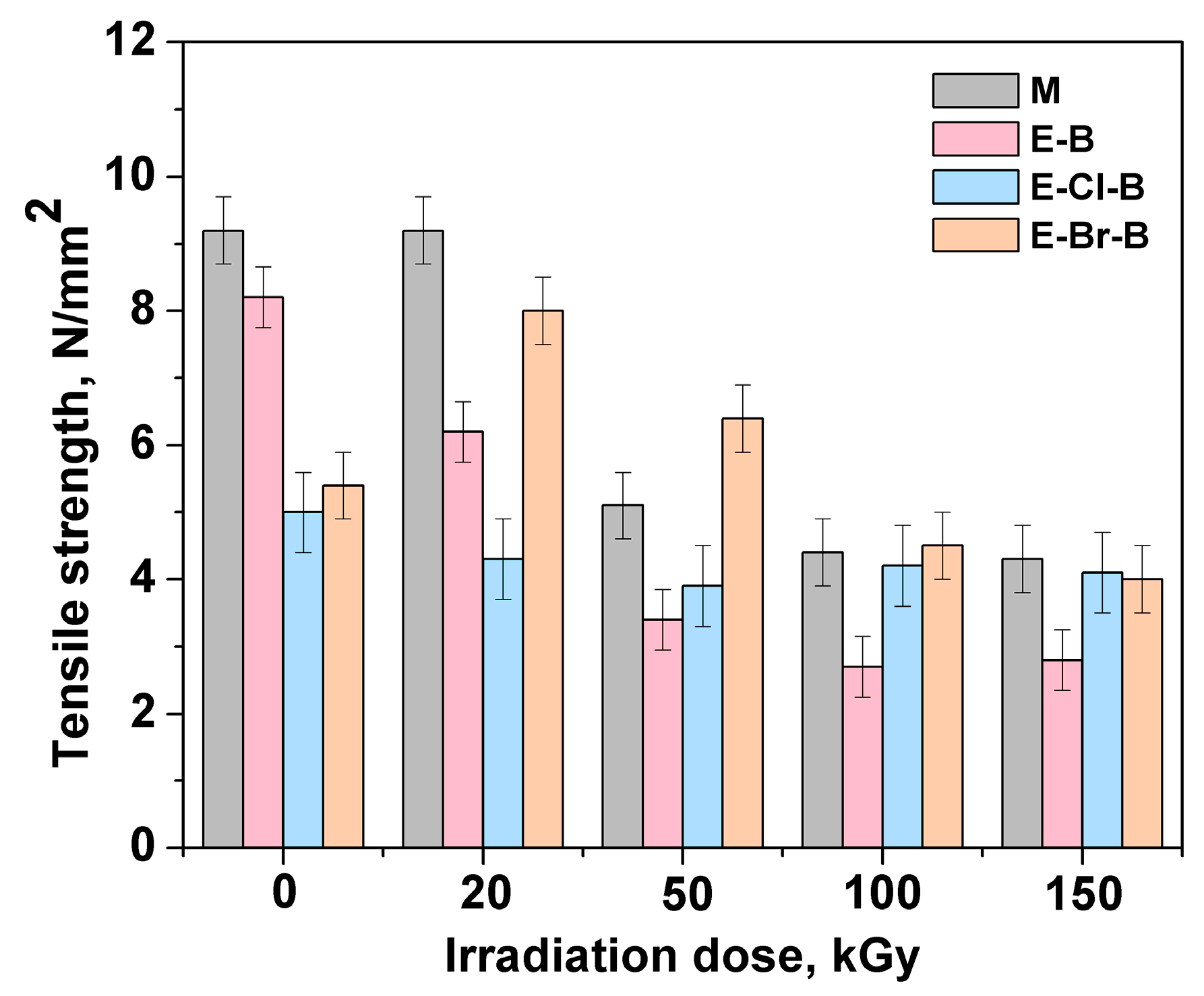
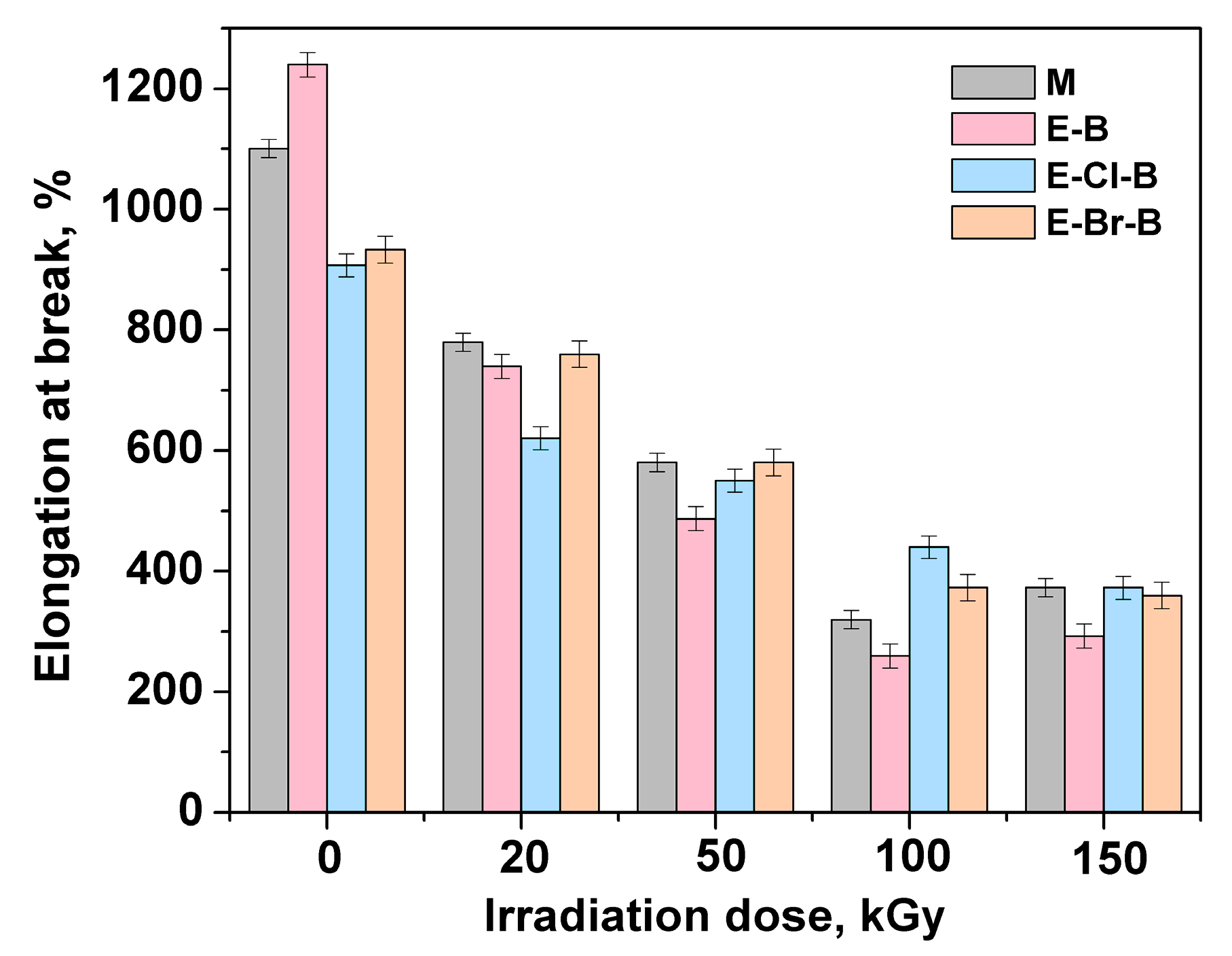
3.1. Mechanical Properties
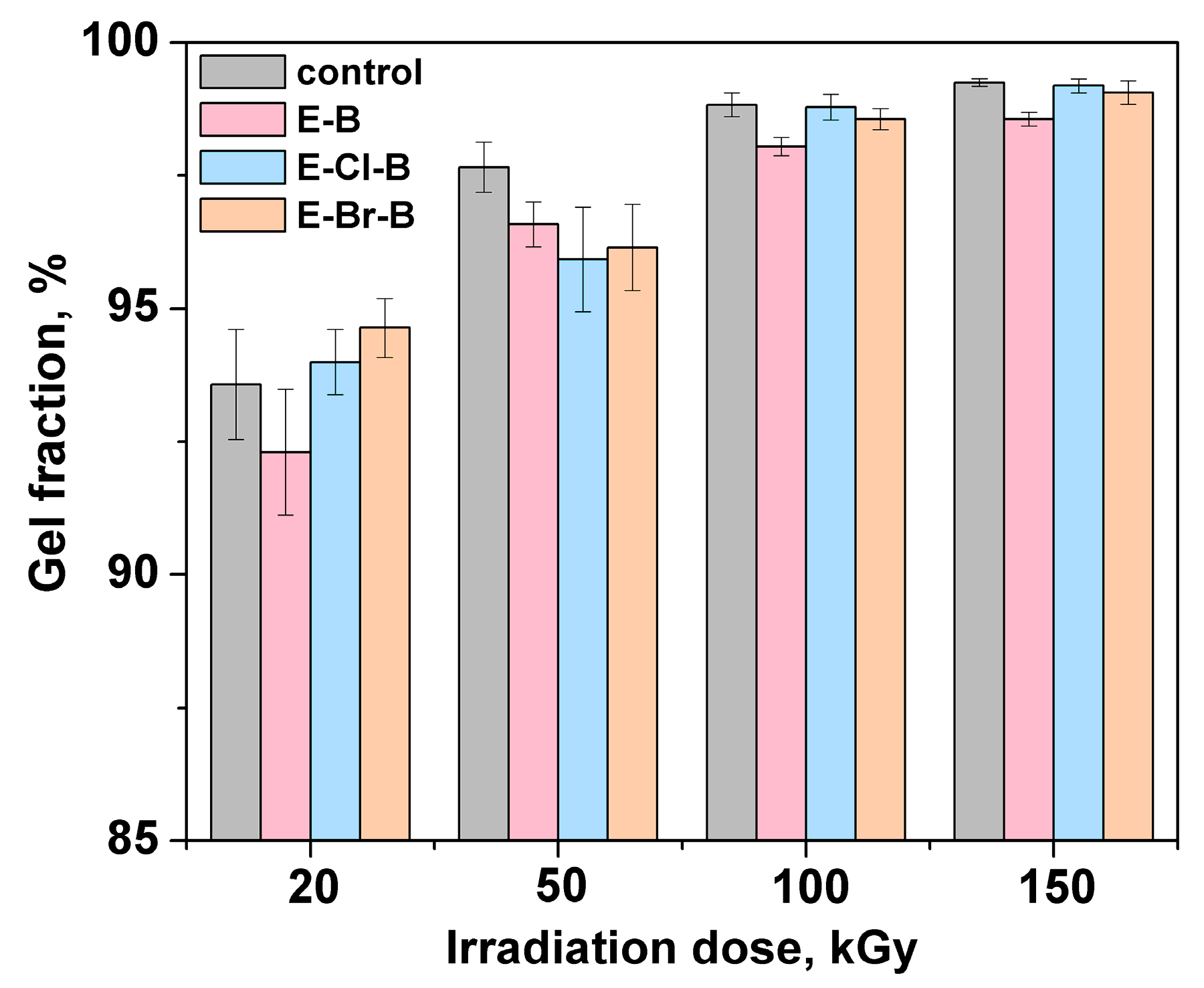
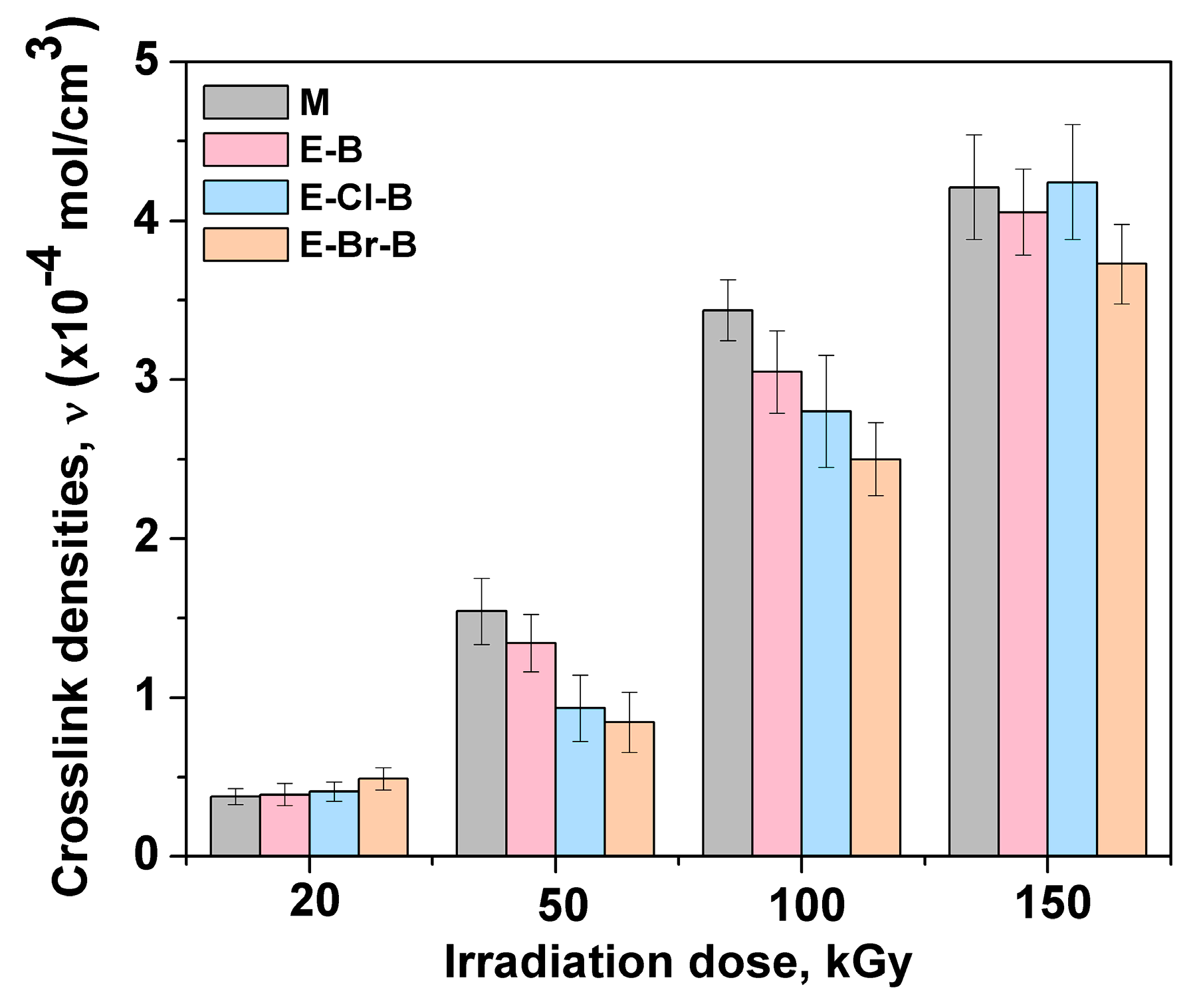
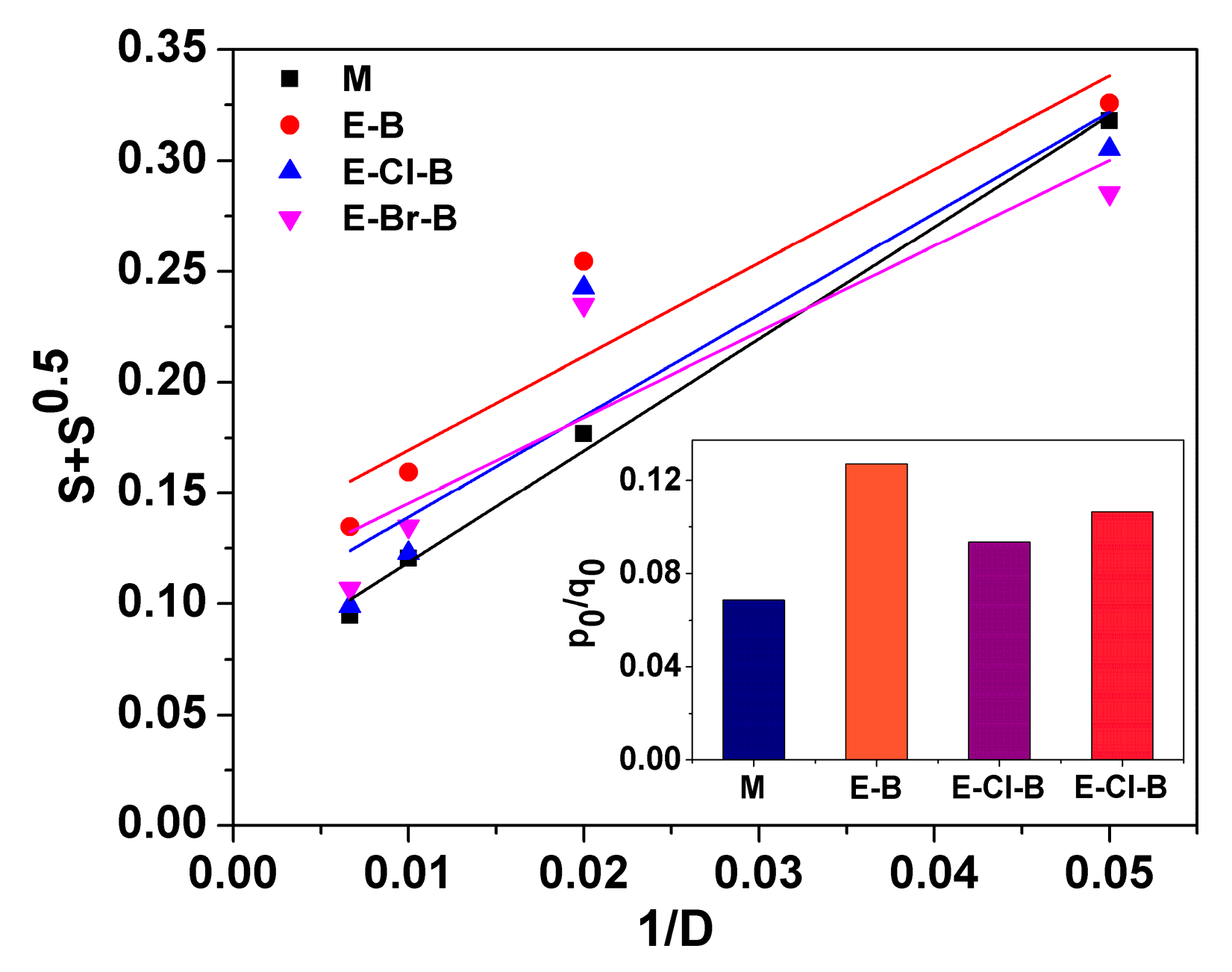
3.2. Gel Fraction and Crosslink Density
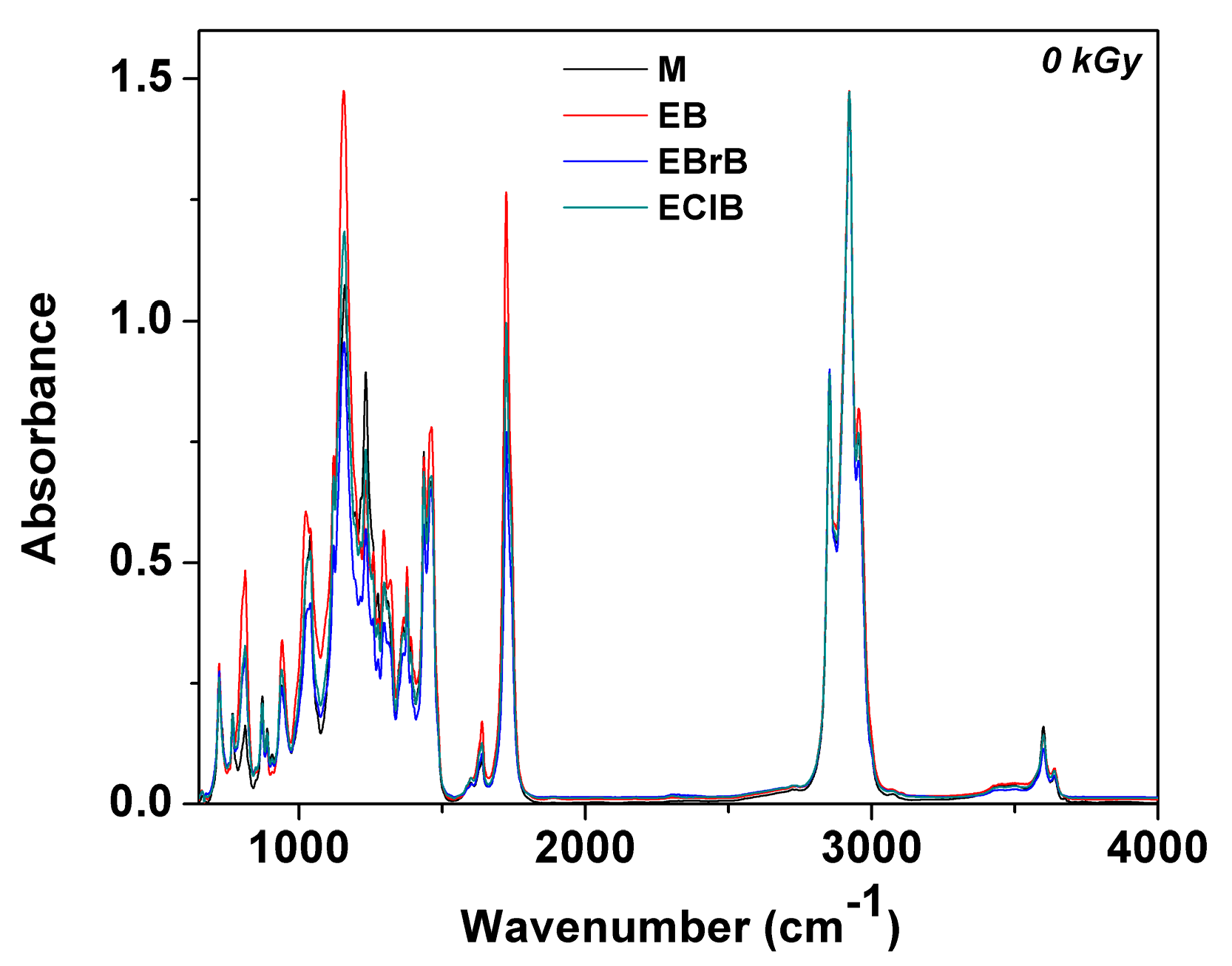
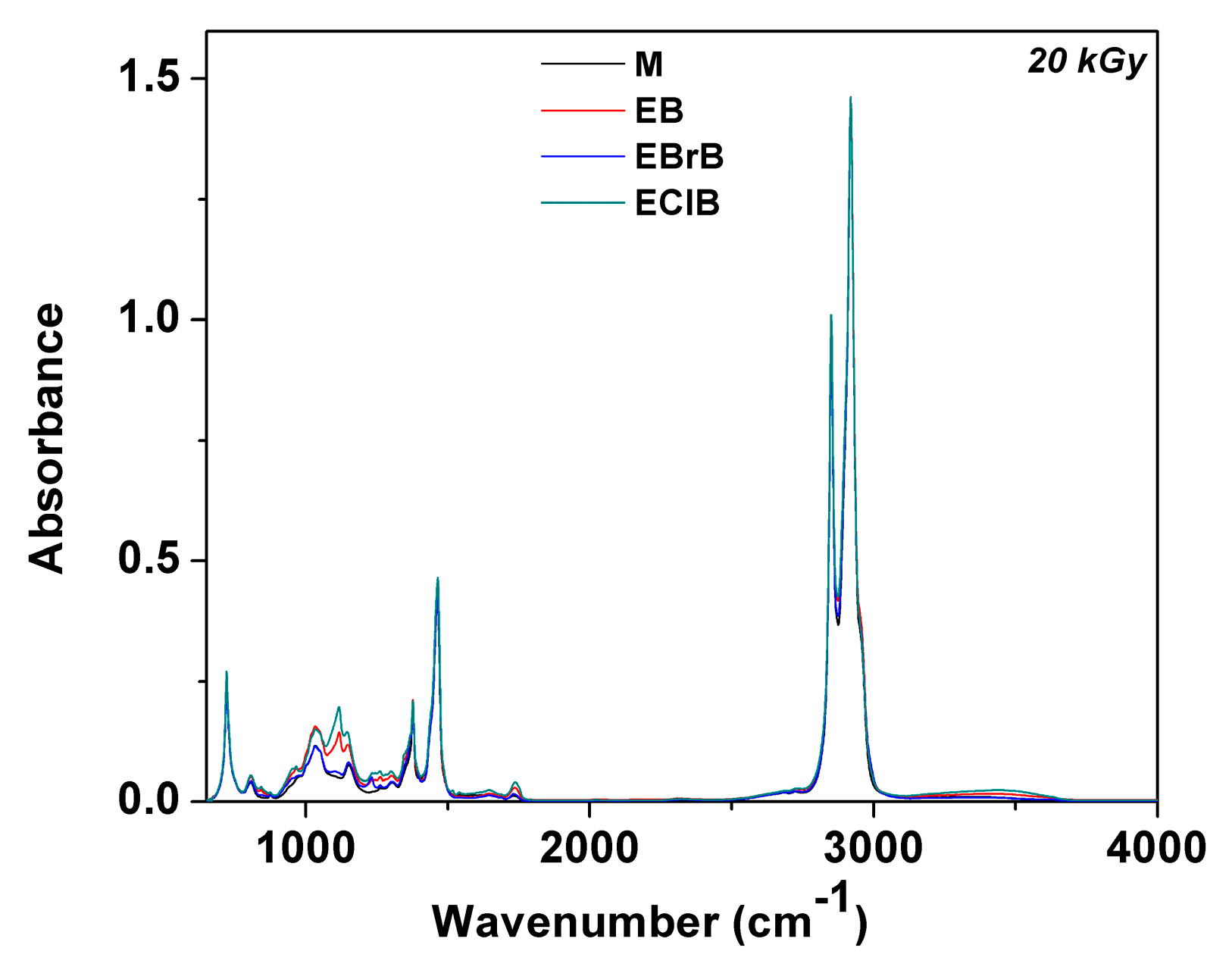
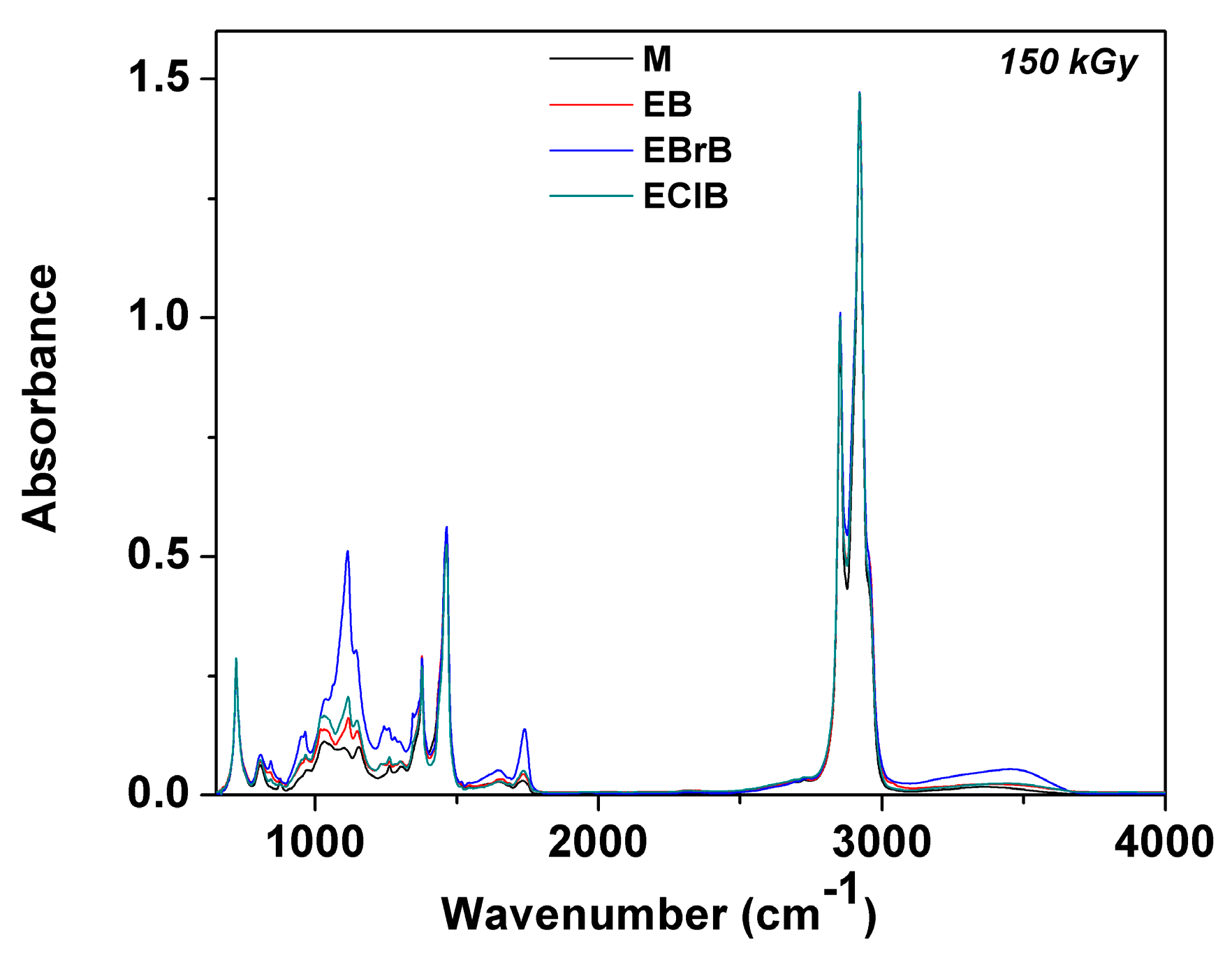
3.3. FTIR Analysis
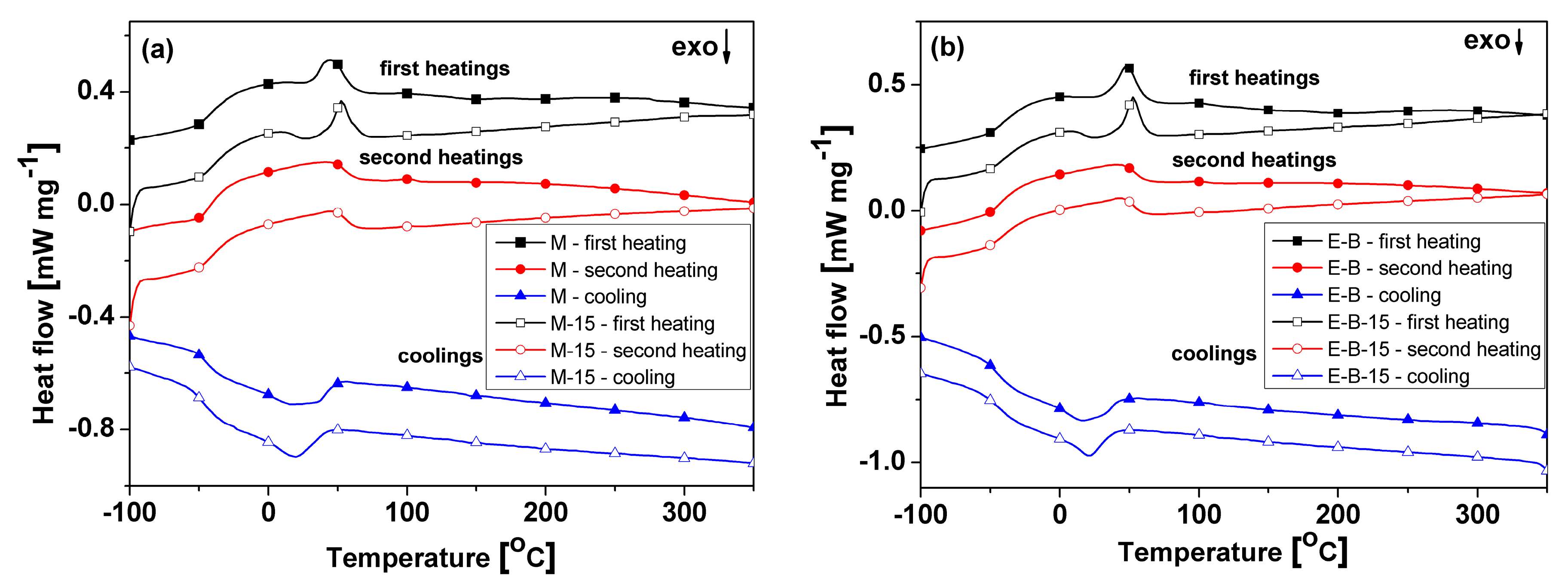
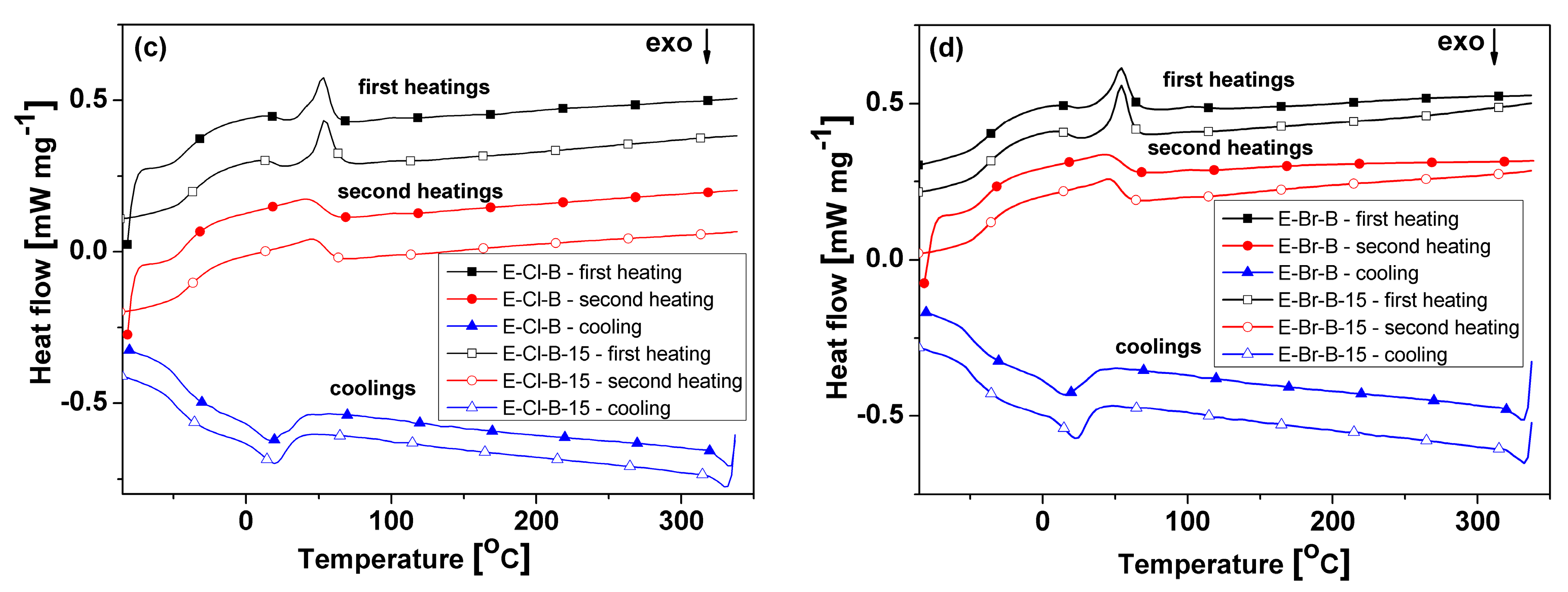
3.4. Thermal Behavior
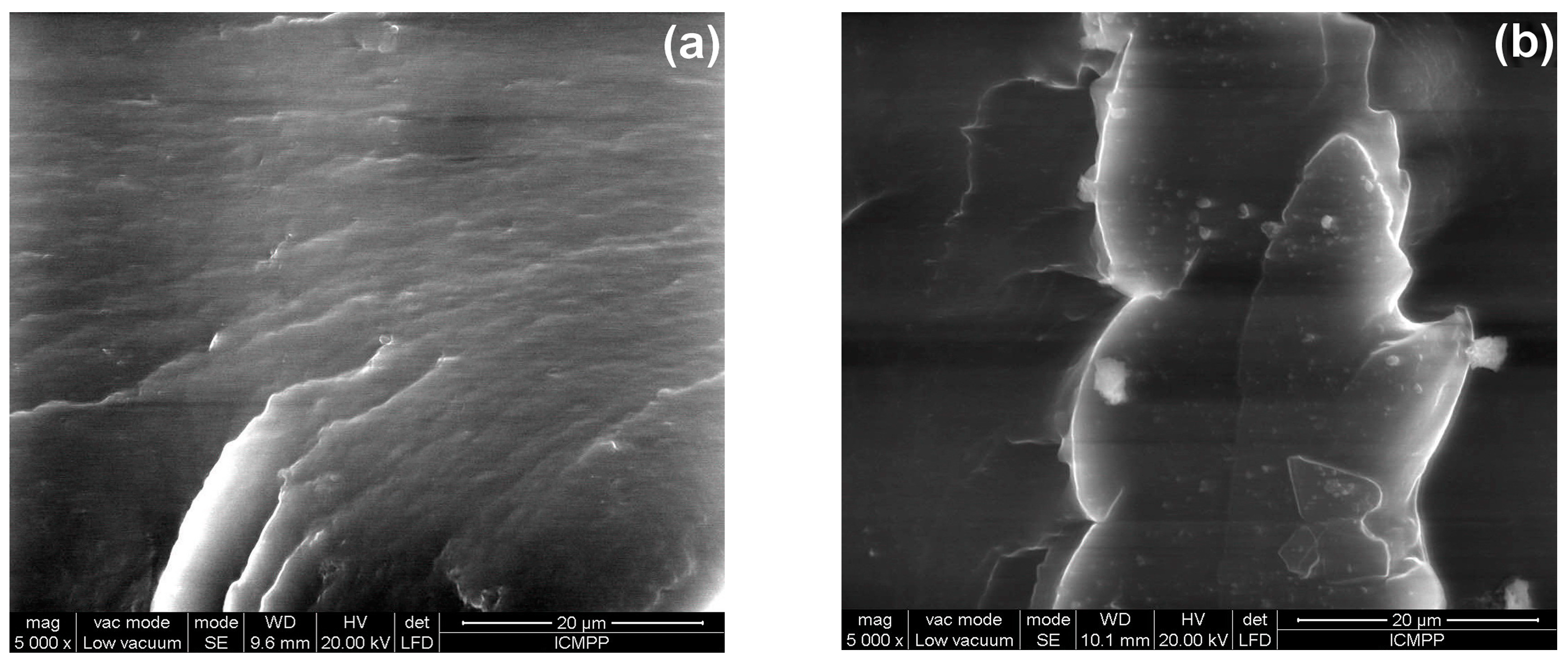
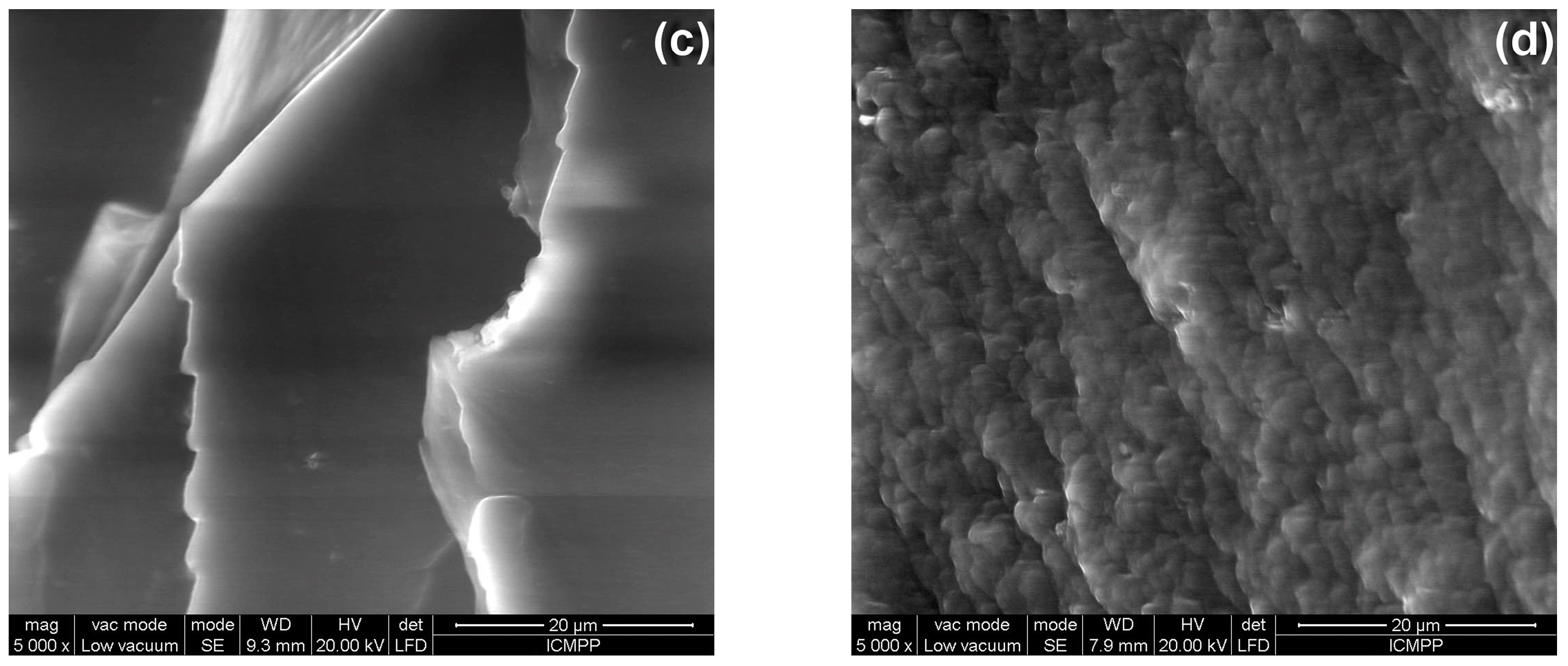
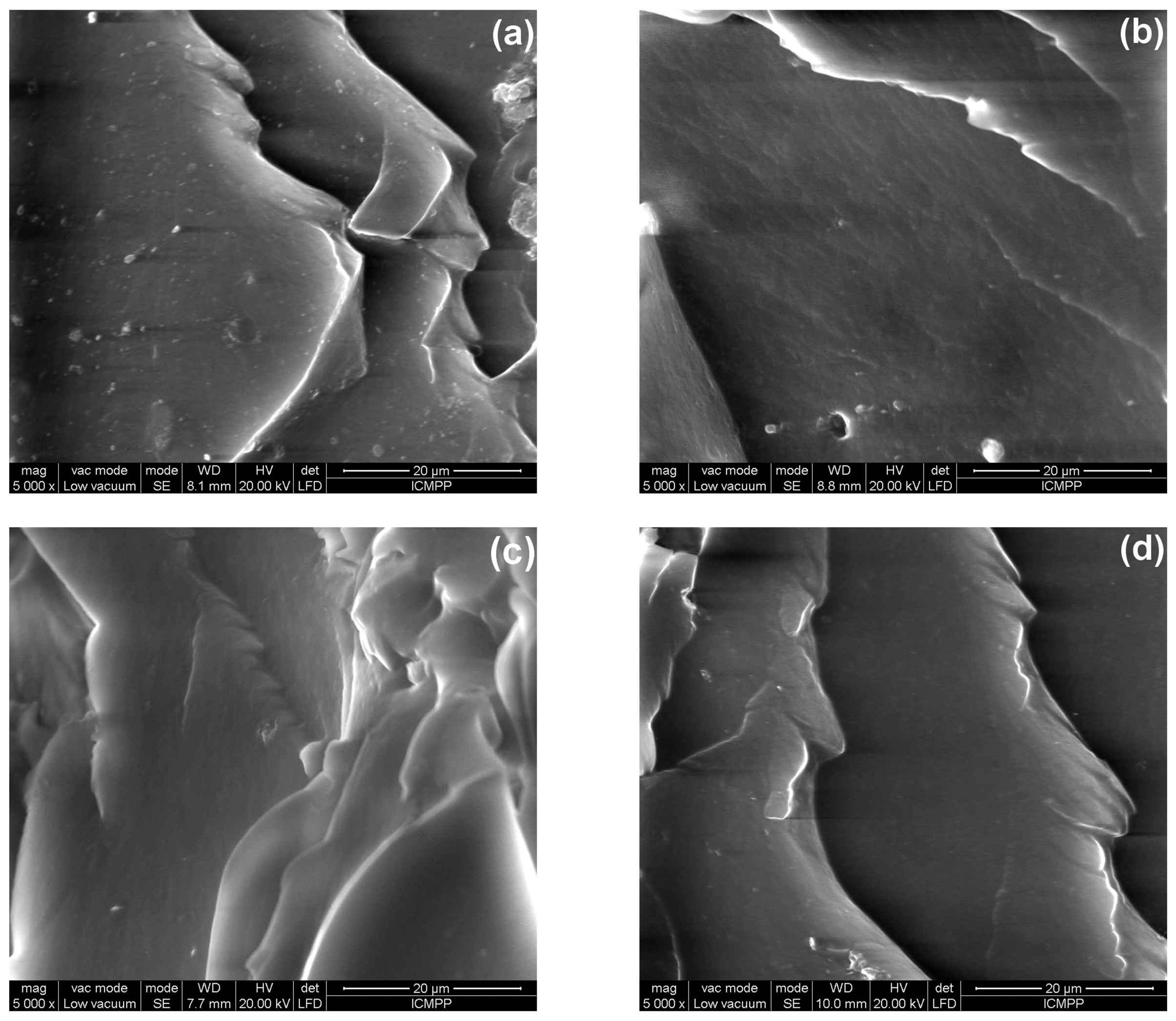
3.5. Morphological Characterization
3.6. Surface Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Duin, M.; Orza, R.; Peters, R.; Chechik, V. Mechanism of peroxide crosslinking of EPDM rubber. Macromol. Symp. 2010, 291–292, 66–74. [Google Scholar] [CrossRef]
- Rajkuman, K.; Dwivedi, C.; Thavamani, P.; Jeyanthi, P.; Pazhanisamy, P. Effect of nanosilica on ethylene propylene diene monomer rubber nanocomposites. Int. J. Innov. Res. Dev. 2013, 2, 831–841. [Google Scholar]
- Airinei, A.; Stelescu, D.M.; Timpu, D.; Ioanid, A. Morphological structure and surface properties maleate ethylene propylene diene organoclay nanocomposite. Polym. Compos. 2012, 33, 379–387. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Craciun, G. Vulcanization of ethylene-propylene-terpolimer-based in rubber mixtures by radiation processing. J. Appl. Polym. Sci. 2012, 128, 2325–2336. [Google Scholar] [CrossRef]
- Ashok, N.; Balachandran, M.; Lawrence, F.; Sebastian, N. EPDM–chlorobutyl rubber blends in γγ-radiation and hydrocarbon environment: Mechanical transport and ageing behavior. J. Appl. Polym. Sci. 2017, 134, 45195. [Google Scholar] [CrossRef]
- Celette, N.; Stevenson, I.; David, L.; Davemas, J.; Vigier, G.; Seytre, G. Irradiation effects on the relaxation behaviour of EPDM elastomers. Polym. Int. 2004, 53, 495–505. [Google Scholar] [CrossRef]
- Karaagac, B.; Kaner, D.; Deniz, V. The effects of compatibility of the mechanical properties and fatigue resistance of butyl/EPDM rubber blends. Polym. Compos. 2010, 31, 1869–1873. [Google Scholar] [CrossRef]
- Jose, S.T.; Anand, A.K.; Joseph, R. EPDM/CIIR blends improved mechanical properties through procuring. Polym. Bull. 2009, 63, 135–146. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bbandyopadhyay, D. In Proceedings of the International Conference on Rubber and Rubber-Like Materials, Jamshedpur, India, 6–8 November 1986; pp. 113–150.
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Dumitrescu, M. New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci. World J. 2014, 2014, 684047. [Google Scholar] [CrossRef] [PubMed]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Fifere, N.; Craciun, G.; Varganici, C.; Doroftei, F. Exploring the effect of electron beam/irradiation on the properties of some EPDM-flax fiber composites. Polym. Compos. 2017. [Google Scholar] [CrossRef]
- Scagliusi, S.R.; Cardoso, E.C.; Lungao, A.B. Radiation–induced degradation of butyl rubber vulcanized by three different crosslinking systems. Radiat. Phys. Chem. 2012, 81, 991–994. [Google Scholar] [CrossRef]
- Hill, D.J.; Odonnell, J.H.; Perera, M.C.; Pomery, P.J. High-energy radiation effects on halogenated butyl rubbers. Polymer 1995, 36, 4185–4192. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Zuga, N. The use of polyfunctional monomers in the radical cure of chlorinated polyethylene. Polym. J. 2011, 43, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Stelescu, M.D. Influence of the curing system on the properties of thermoplastic vulcanized EPDM/plasticized PVC. Mater. Plast. 2011, 48, 240–244. [Google Scholar]
- Flory, P.J.; Rehner, J. Statistical mechanism of crosslinked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Planes, E.; Chazeau, L.; Vigier, G.; Fournier, J. Evolution of EPDM networks aged by gamma irradiation–consequence on the mechanical properties. Polymer 2009, 50, 4028–4038. [Google Scholar] [CrossRef]
- O’Donell, J.; Whittaker, A.K. The radiation crosslinking and scission of ethylene-propylene copolymer studied by solid-state nuclear magnetic resonance. Br. Polym. J. 1985, 17, 51–55. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Aspects Regarding Radiation Crosslinking of Elestomers. In Advanced Elastomers–Technology, Properties and Applications; Boczkowska, A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–34. ISBN 978-9535107392. [Google Scholar]
- Majumder, P.S.; Bhowmick, A.K. Structure-property relationship of electron-beam-modified EPDM rubber. J. Appl. Polym. Sci. 2000, 77, 323–337. [Google Scholar] [CrossRef]
- Volintiru, T.; Ivan, G.I. Technological Fundamentals of Elastomer Processing; Tehnica: Bucuresti, Romania, 1974. [Google Scholar]
- Ramachandran, P.; Naskar, K.; Nando, G.B. Exploring the effect of radiation crosslinking on the physico-mechanical, dynamic mechanical and dielectric properties of EOC-PDMS blends for cable insulation applications. Polym. Adv. Technol. 2017, 28, 80–93. [Google Scholar] [CrossRef]
- Blanks, R.F.; Prausnitz, J.M. Thermodynamics of polymer solubility in polar and nonpolar systems. Ind. Eng. Chem. Fundam. 1969, 3. [Google Scholar] [CrossRef]
- Fried, J.R. (Ed.) Polymer Science and Technology, 3rd ed.; Prentice Hall: New York, NY, USA, 2014; pp. 101–152. ISBN 978-0137039555. [Google Scholar]
- Small, P.A. Some factors affecting the solubility of polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 978-0444596123. [Google Scholar]
- Carswell-Pomerantz, T.; Babanalbandi, A.; Dong, L.; Hill, D.J.T.; Perera, M.C.S.; Pomery, P.J.; Saadat, G.; Wittaker, A.K. Changes in molecular structure and properties of irradiated polymers of different compositions. In Stability and Stabilization of Polymers under Irradiation; IAEA: Viena, Austria, 1999; pp. 111–128. [Google Scholar]
- Anelli, F.; Baccaro, S.; Carenza, M.; Palma, G. Radiation grafting of hydrophilic monomers onto ethylenepropylene rubber. Radiat. Phys. Chem. 1995, 46, 1031–1035. [Google Scholar] [CrossRef]
- Webb, R.N.; Shaffer, D.S.; Tsu, A.H. Butyl rubber. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2014; Volume 5, pp. 356–381. ISBN 978-1118633892. [Google Scholar]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Ighineanu, D. Electron beam processing of ethylene-propylene-terpolymer-based rubber mixtures. Int. J. Chem. Mater. Eng. 2018, 12, 258–262. [Google Scholar]
- Charlesby, A.; Pinner, S.H. Analysis of the solubility behavior of irradiated polyethylene and other polymers. Proc. R. Soc. Lond. A 1959, 249, 367–386. [Google Scholar] [CrossRef]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Kumar, V.; Goel, N.K.; Sabharwal, S. Radiation processed ethylene vinyl acetate–multiple walled carbon nanotube nanocomposites. Effect of MWNT addition on the gel content and crosslinking density. Express Polym. Lett. 2009, 3, 492–500. [Google Scholar] [CrossRef]
- Dubey, K.A.; Chaudhari, C.V.; Rao, R.; Bhardwaj, Y.K.; Goel, N.K.; Sabharwal, S. Radiation processing and characterization of poly(vinyl alcohol) nanocomposites. 1. Nano-particulate filler tuned crosslinking behavior. J. Appl. Polym. Sci. 2010, 118, 3490–3498. [Google Scholar] [CrossRef]
- Bremner, T.; Hill, D.J.T.; O’Donnell, J.H.; Perera, M.C.S.; Pomery, P.J. Mechanism of radiation degradation of polyisobutylene. J. Polym. Sci. Polym. Chem. 1996, 34, 971–984. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Kalu, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.S.; Bhowmick, A.K. Surface and bulk-properties of EPDM rubber modified by electron beam irradiation. Radiat. Phys. Chem. 1998, 53, 63–78. [Google Scholar] [CrossRef]
- Van Gisbergen, J.G.M.; Meijer, H.E.M.; Lemstra, P.J. Structural polymer blends: 2. Processing of polypropylene, EPDM blends: Controlled rheology and morphology fixation via electron beam irradiation. Polymer 1989, 30, 2153–2157. [Google Scholar] [CrossRef]
- Grochowicz, M. Investigation of the thermal behavior of 4-vinylpyridine-trimethylpropane trimethacrylate copolymeric microspheres. J. Therm. Anal. Calorim. 2014, 118, 1602–1611. [Google Scholar] [CrossRef]
- Fujimoto, K.; Nakade, S. Effects of termonomers on crosslinking rate and crosslinking structure of ethylene propylene terpolymers. J. Appl. Polym. Sci. 1969, 13, 1509–1522. [Google Scholar] [CrossRef]
- Fujimoto, K.; Wataya, K. The study of polymers by high-temperature ATR spectroscopy. J. Appl. Polym. Sci. 1969, 13, 2513–2526. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C. Property correlations for composites based on ethylene propylene diene rubber reinforced with flax fibers. Polym. Test. 2017, 59, 75–83. [Google Scholar] [CrossRef]
- Varganici, C.D.; Ursache, O.; Gaina, C.; Gaina, V.; Simionescu, B.C. Studies of new hybrid materials prepared by both Diels-Alder and Michael addition reactions. J. Therm. Anal. Calorim. 2013, 111, 1561–1570. [Google Scholar] [CrossRef]
- Varganici, C.D.; Marangoci, N.; Rosu, L.; Barbu-Mic, C.; Rosu, D.; Pinteala, M.; Simionescu, B.C. TGA/DFA-FTIR-MS coupling as analytical tool for confirming inclusion complex occurrence in supramolecular host-guest architectures. J. Anal. Appl. Pyrolysis 2015, 115, 132–142. [Google Scholar] [CrossRef]
- Zaharescu, T.; Jipa, S.; Mantsch, A.; Borbath, I. Qualification of ethylene-propylene elastomers for nuclear applications. J. Adv. Res. Phys. 2010, 1, 011012. [Google Scholar]
- Rieke, P.C. Application of van Oss-Good theory of wettability to interpretation of interfacial free energies of heterogeneous nucleation. J. Cryst. Growth 1997, 182, 472–484. [Google Scholar] [CrossRef]
- Della Volpe, C.; Siboni, S. Acid-base surface free energies of solids and the definition of scales in the Good-van Oss-Chandhury theory. J. Adhes. Sci. Technol. 2000, 14, 235–272. [Google Scholar] [CrossRef]
- Grythe, K.F.; Hansen, F.K. Surface modification of EPDM rubber by plasma treatment. Langmuir 2006, 22, 6109–6124. [Google Scholar] [CrossRef] [PubMed]
- Budziak, C.J.; Vargha-Butler, E.I.; Neumann, A.W. Temperature dependence of contact angles on elastomers. J. Appl. Polym. Sci. 1991, 42, 1959–1964. [Google Scholar] [CrossRef]



| Ingredients (Parts Per Hundred Rubber (phr)) | Sample Code | |||
|---|---|---|---|---|
| M | E-B | E-Cl-B | E-Br-B | |
| EPDM (E) | 100 | 95 | 95 | 95 |
| Butyl rubber (IIR) | - | 5 | - | - |
| Cl-butyl rubber (Cl–IIR) | - | - | 5 | - |
| Br-butyl rubber (Br–IIR) | - | - | - | 5 |
| Trimethylolpropane trimethacrylate (TMPT) | 3 | 3 | 3 | 3 |
| Antioxidant | 0.5 | 0.5 | 0.5 | 0.5 |
| Total (phr) | 103.5 | 103.5 | 103.5 | 103.5 |
| Group | Fi (MPa)1/2 | ||
|---|---|---|---|
| Small | Hoy | Van Krevelen | |
| –CH3 | 438 | 303 | 420 |
| –CH2– | 272 | 269 | 280 |
| –CH– | 57 | 176 | 140 |
| >CH–CH< | 266 | 422 | 304 |
| >C–C< | 190 | 655 | 0 |
| –Cl | 552 | 420 | 471 |
| –Br | 696 | 528 | 614 |
| Sample/Method | ΣFi | ||
|---|---|---|---|
| Small | Hoy | Van Krevelen | |
| EPDM | 4370 | 4809.0 | 4924 |
| EPDM/IIR | 4576 | 6591.5 | 7328 |
| EPDM/Cl–IIR | 6747 | 6884.5 | 7519 |
| EPDM/Br–IIR | 6891 | 6992.5 | 7662 |
| Sample/Method | Small | Hoy | Van Krevelen | |||
|---|---|---|---|---|---|---|
| δp | χ | δp | χ | δp | χ | |
| EPDM | 14.4874 | 0.9325 | 15.9428 | 0.5590 | 16.3240 | 0.4913 |
| EPDM/IIR | 15.0084 | 0.7779 | 15.0437 | 0.7682 | 16.1246 | 0.4336 |
| EPDM/Cl–IIR | 14.1741 | 1.0367 | 14.4630 | 0.9403 | 15.7960 | 0.5884 |
| EPDM/Br–IIR | 13.1293 | 1.4452 | 13.3227 | 1.3625 | 14.5983 | 0.8976 |
| Sample | Tg1 (°C) | Tm1 (°C) | ΔHm1 (J g−1) | Tg2 (°C) | Tm2 (°C) | ΔHm2 (J g−1) | Tcr (°C) | ΔHcr (J g−1) | Tcc (°C) | ΔHcc (J g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| M | −35 | 45 | 11.4 | −40 | 49 | 22.08 | 13 | −20.66 | – | – |
| M–15 | −34 | 53 | 7.66 | −39 | 45 | 22.47 | 19 | −20 | 27 (h1) | −3.15 (h1) |
| E–B | −37 | 48 | 13.42 | −38 | 44 | 18.21 | 16/33 | −21.68 | – | – |
| E–B–15 | −32 | 53 | 8.96 | −39 | 43 | 16.42 | 21 | −19.35 | 25 (h1) | −2.97 (h1) |
| E–Cl–B | −35 | 40/53 | 9.56 | −39 | 41 | 12.56 | −19/18/31 | −19 | 29 (h1) | −1.03 (h1) |
| E–Cl–B–15 | −33 | 54 | 8.95 | −35 | 45 | 13.3 | 20 | −16.35 | 27 (h1) | −2.49 (h1) |
| E–Br–B | −36 | 54 | 8.84 | −39 | 43 | 12.4 | 16 | −14.1 | 25 (h1) | −1.09 (h1) |
| E–Br–B–15 | −37 | 54 | 10.24 | −41 | 45 | 13.51 | 23 | −15.1 | 26 (h1) | −2.05(h1) |
| Sample | T5% (°C) | Tmax (°C) | W (%) | T10% (°C) | T30% (°C) | T50% (°C) | R (%) |
|---|---|---|---|---|---|---|---|
| M | 433 | 472 | 98.30 | 445 | 462 | 469 | 1.21 |
| M–15 | 445 | 474 | 98.10 | 450 | 466 | 472 | 1.40 |
| E–B | 418 | 470 | 98.33 | 432 | 455 | 467 | 1.61 |
| E–B–15 | 422 | 473 | 97.02 | 434 | 458 | 469 | 2.95 |
| E–Cl–B | 410 | 471 | 98.81 | 430 | 455 | 466 | 1.13 |
| E–Cl–B–15 | 415 | 474 | 97.61 | 435 | 459 | 469 | 1.96 |
| E–Br–B | 411 | 472 | 96.45 | 429 | 455 | 467 | 3.50 |
| E–Br–B–15 | 418 | 474 | 95.51 | 432 | 457 | 470 | 4.30 |
| Samples | Contact Angle Values (θ) | |||
|---|---|---|---|---|
| Water | Formamide | Diiodomethane | ||
| Initial | M | 92.3 | 94.3 | 41.5 |
| E–B | 87.2 | 79.5 | 48.8 | |
| E–Cl–B | 99.5 | 86.0 | 47.1 | |
| E–Br–B | 100.1 | 91.7 | 66.4 | |
| 20 kGy | M | 108.6 | 95.5 | 67.2 |
| E–B | 104.5 | 93.5 | 75.0 | |
| E–Cl–B | 107.8 | 98.9 | 62.8 | |
| E–Br–B | 109.5 | 99.4 | 64.4 | |
| 150 kGy | M | 112.5 | 96.9 | 76.4 |
| E–B | 112.2 | 104.3 | 92.4 | |
| E–Cl–B | 112.8 | 106.7 | 72.6 | |
| E–Br–B | 110.8 | 105.6 | 61.5 | |
| Liquid | |||||
|---|---|---|---|---|---|
| Water (bidistilled) | 72.8 | 21.8 | 51 | 25.5 | 25.5 |
| Formamide | 58.0 | 39.0 | 19 | 2.28 | 39.6 |
| Diiodomethane | 50.8 | 50.8 | 0 | 0 | 0 |
| Samples | ||||||
|---|---|---|---|---|---|---|
| Initial | M | 38.85 | 8.3802 | 16.42 | 23.46 | 62.31 |
| E–B | 34.94 | 1.4680 | 10.94 | 8.01 | 42.96 | |
| E–Cl–B | 35.88 | 2.2094 | 3.87 | 5.85 | 41.73 | |
| E–Br–B | 24.91 | 1.1083 | 5.68 | 5.02 | 29.93 | |
| 20 kGy | M | 24.45 | 1.1683 | 2.01 | 3.07 | 27.52 |
| E–B | 20.13 | 0.3147 | 3.30 | 2.04 | 22.17 | |
| E–Cl–B | 26.97 | 2.9563 | 3.70 | 6.61 | 33.58 | |
| E–Br–B | 26.05 | 2.6156 | 2.89 | 5.50 | 31.55 | |
| 150 kGy | M | 19.38 | 0.2836 | 0.83 | 0.97 | 20.35 |
| E–B | 11.66 | 0.0973 | 2.69 | 1.02 | 12.68 | |
| E–Cl–B | 21.43 | 3.1203 | 3.62 | 6.72 | 28.15 | |
| E–Br–B | 27.71 | 5.6260 | 4.64 | 10.22 | 37.93 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers 2018, 10, 1206. https://doi.org/10.3390/polym10111206
Stelescu MD, Airinei A, Manaila E, Craciun G, Fifere N, Varganici C, Pamfil D, Doroftei F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers. 2018; 10(11):1206. https://doi.org/10.3390/polym10111206
Chicago/Turabian StyleStelescu, Maria Daniela, Anton Airinei, Elena Manaila, Gabriela Craciun, Nicusor Fifere, Cristian Varganici, Daniela Pamfil, and Florica Doroftei. 2018. "Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites" Polymers 10, no. 11: 1206. https://doi.org/10.3390/polym10111206
APA StyleStelescu, M. D., Airinei, A., Manaila, E., Craciun, G., Fifere, N., Varganici, C., Pamfil, D., & Doroftei, F. (2018). Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers, 10(11), 1206. https://doi.org/10.3390/polym10111206