Fabrication of Novel Ball-Like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
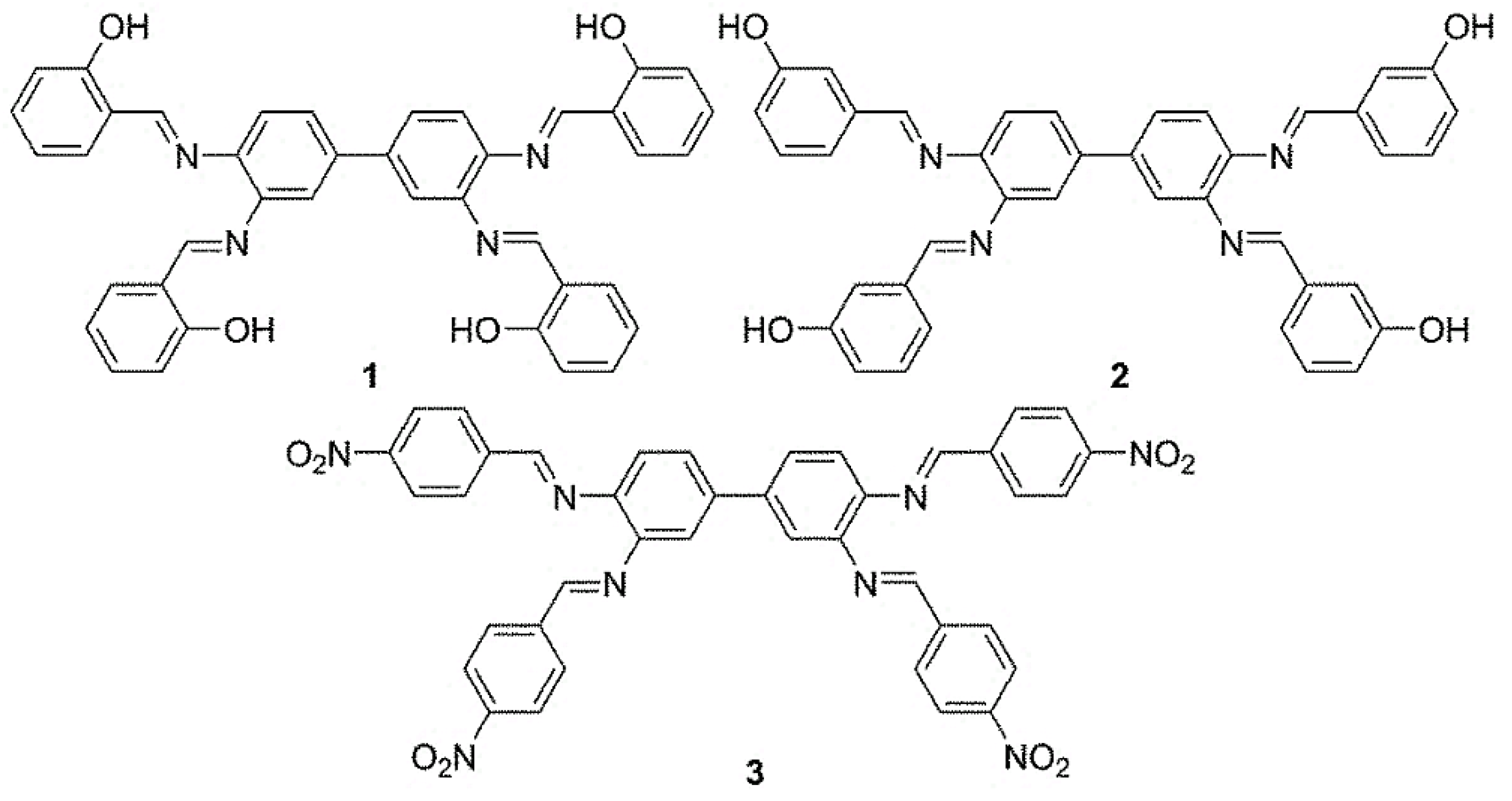
2.2. Schiff Bases 1–3
2.3. Preparation of PS Films
2.4. Fourier Transform Infrared Spectroscopy of PS Films
2.5. Weight Loss of PS Films
2.6. Viscometry of PS Films
3. Results and Discussion
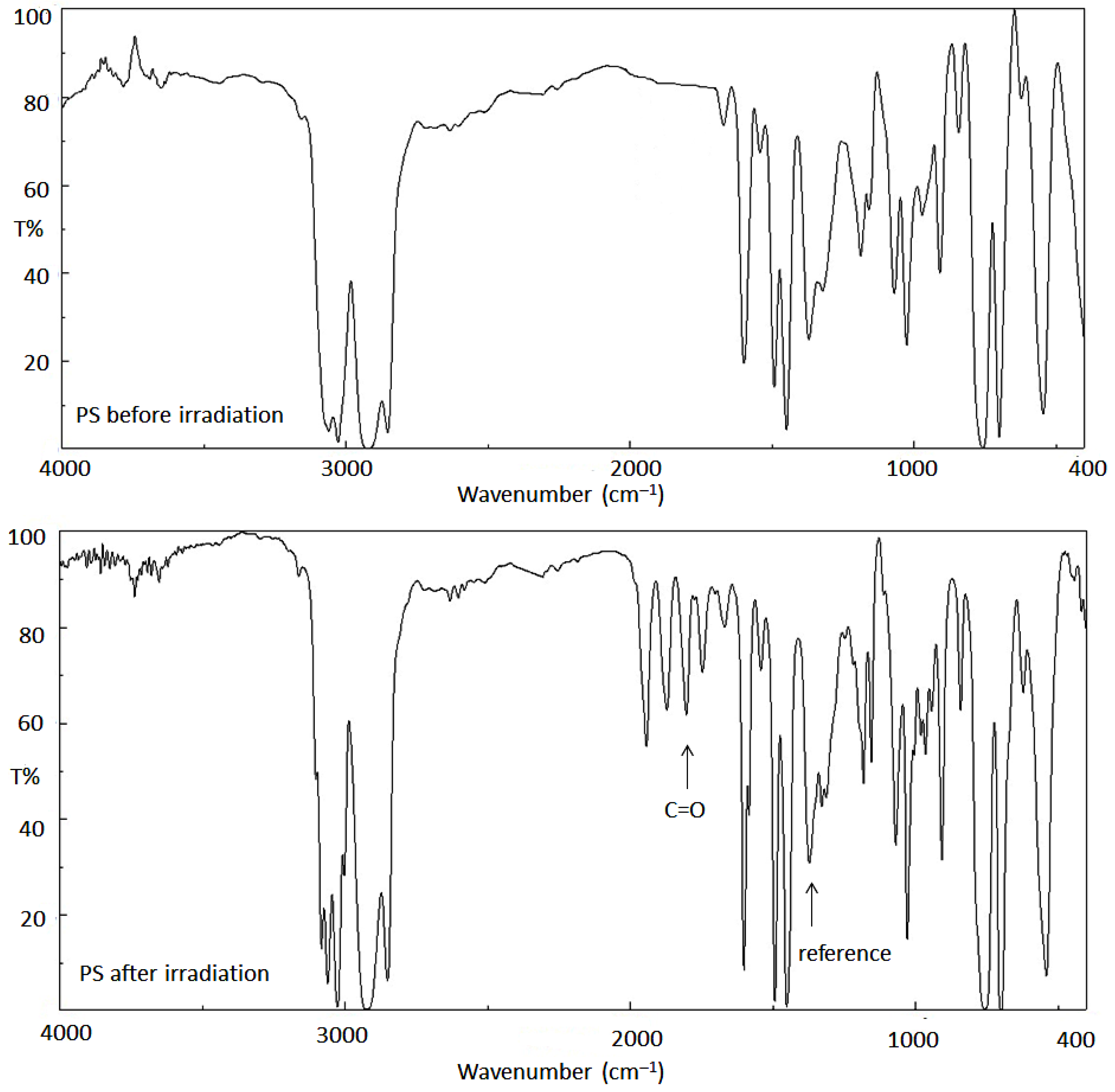
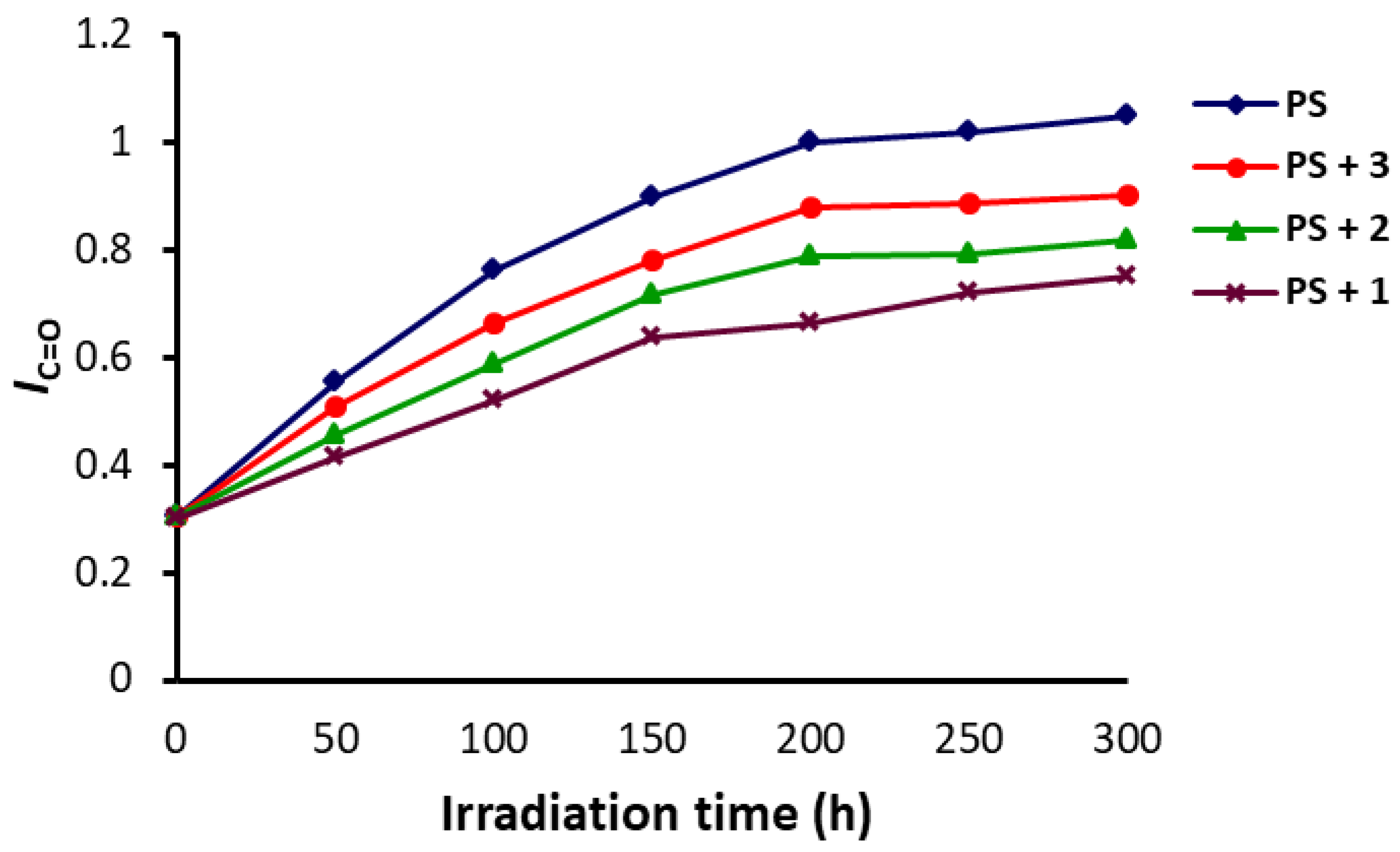
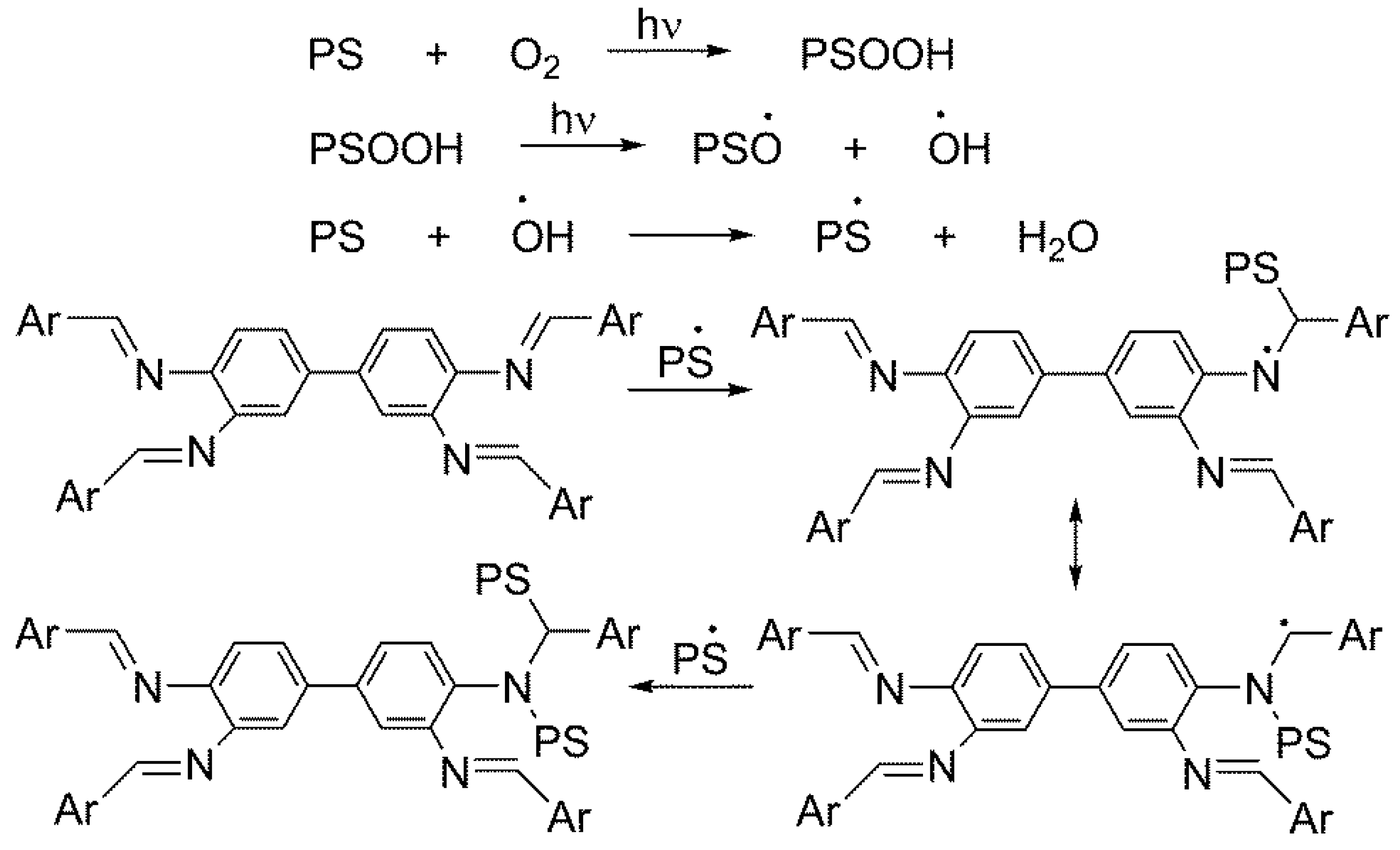
3.1. IR Spectroscopy of PS
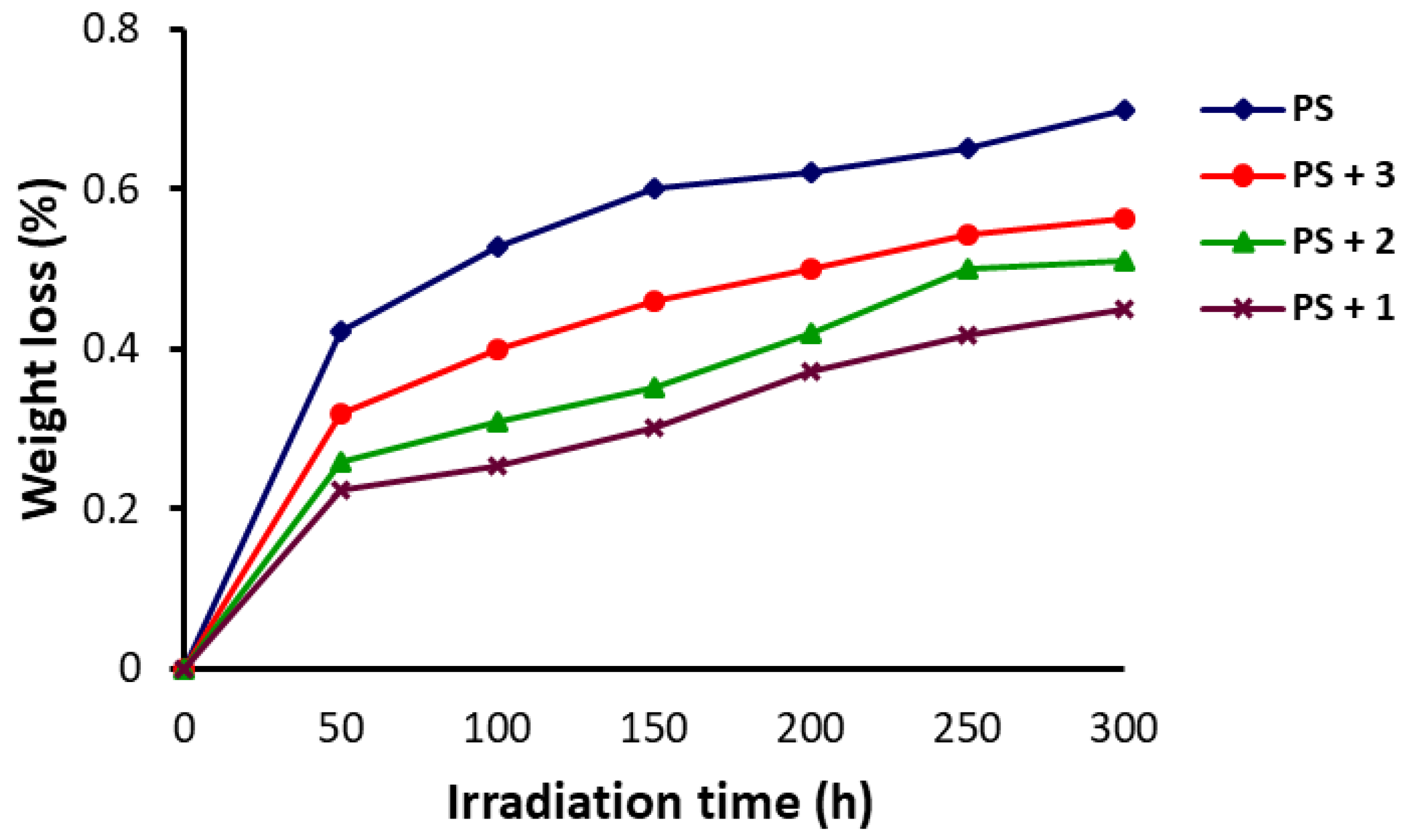
3.2. Weight Loss of PS
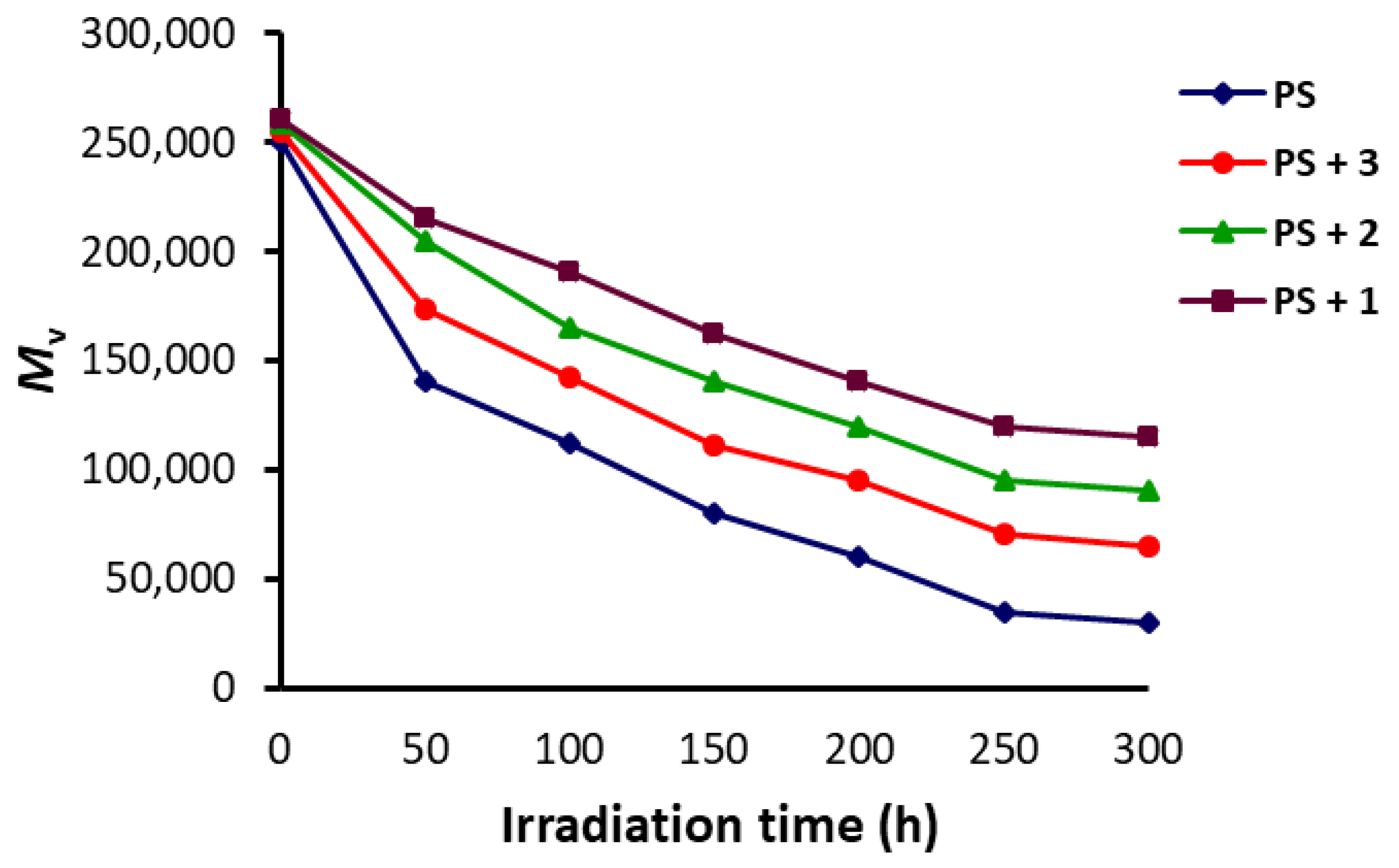
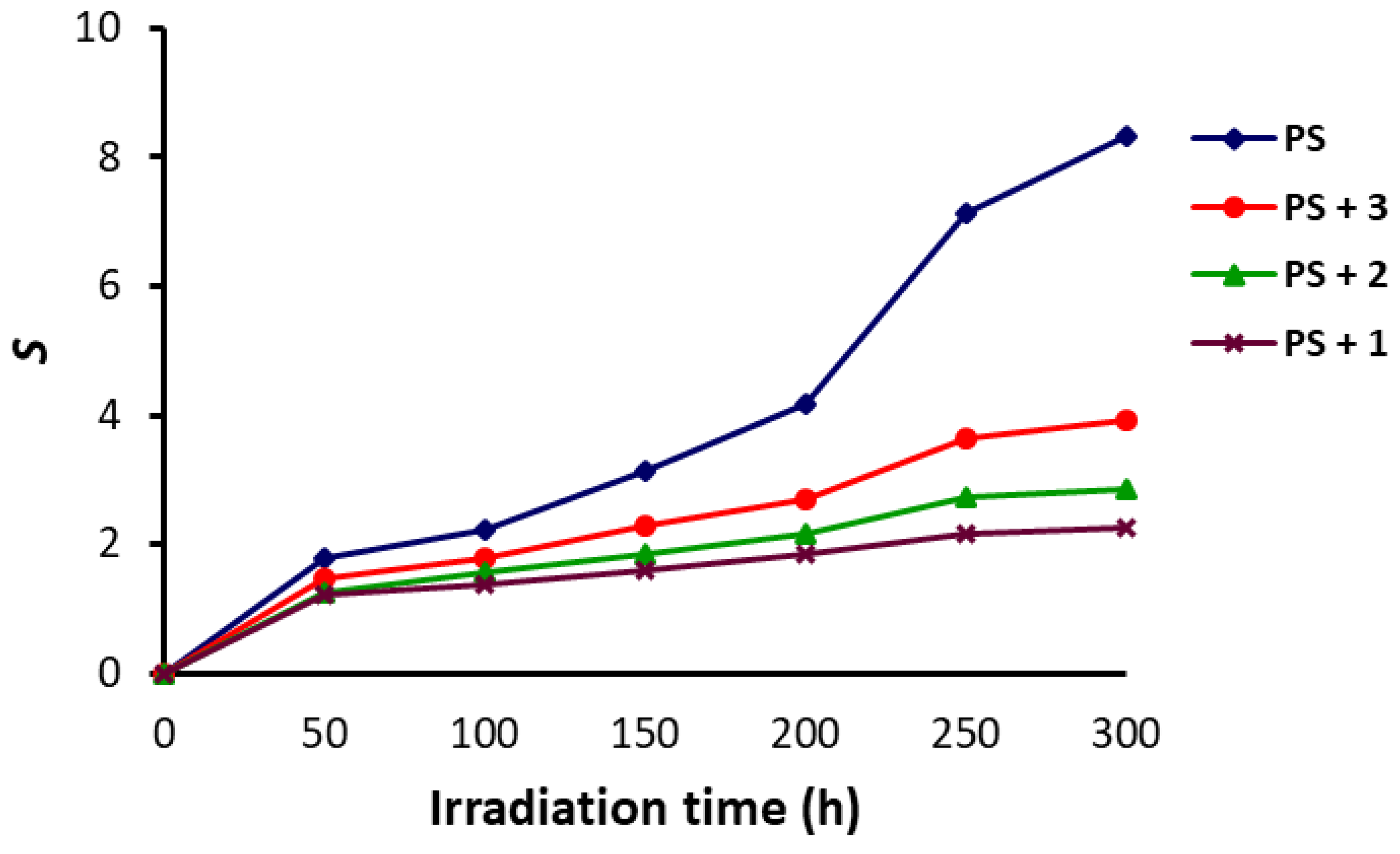
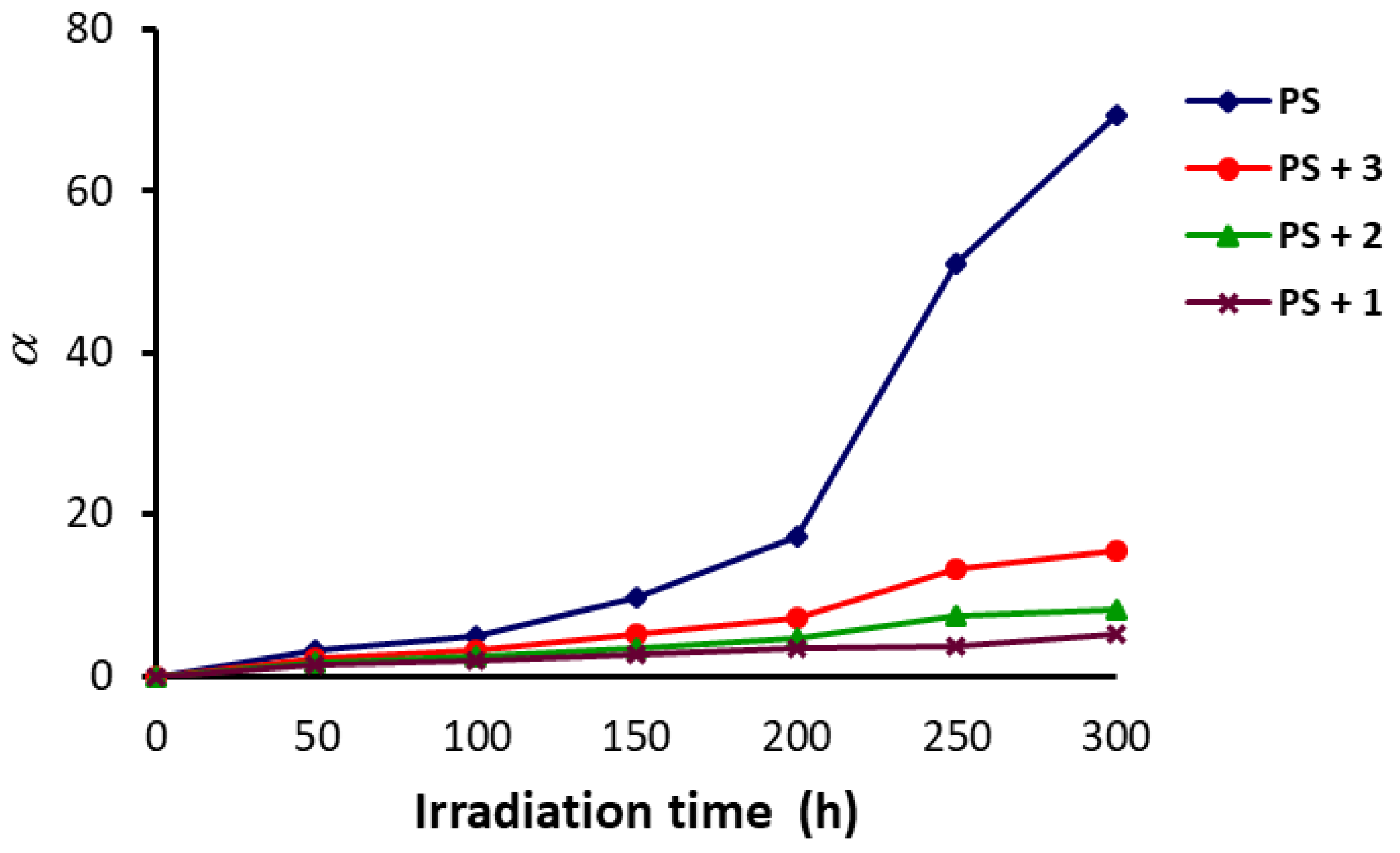
3.3. Molecular Weight of PS
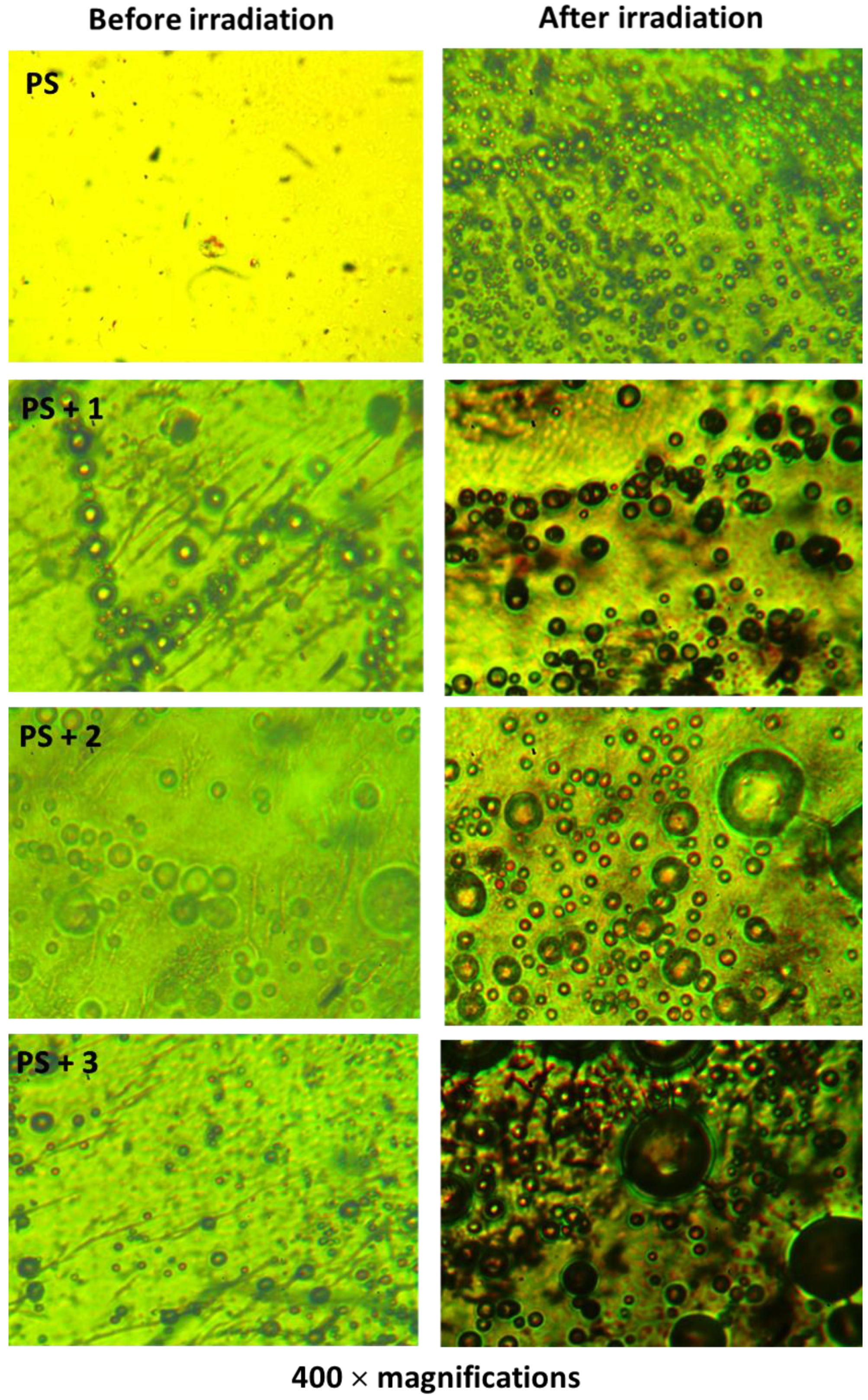
3.4. Microscopic Surface Morphology of PS
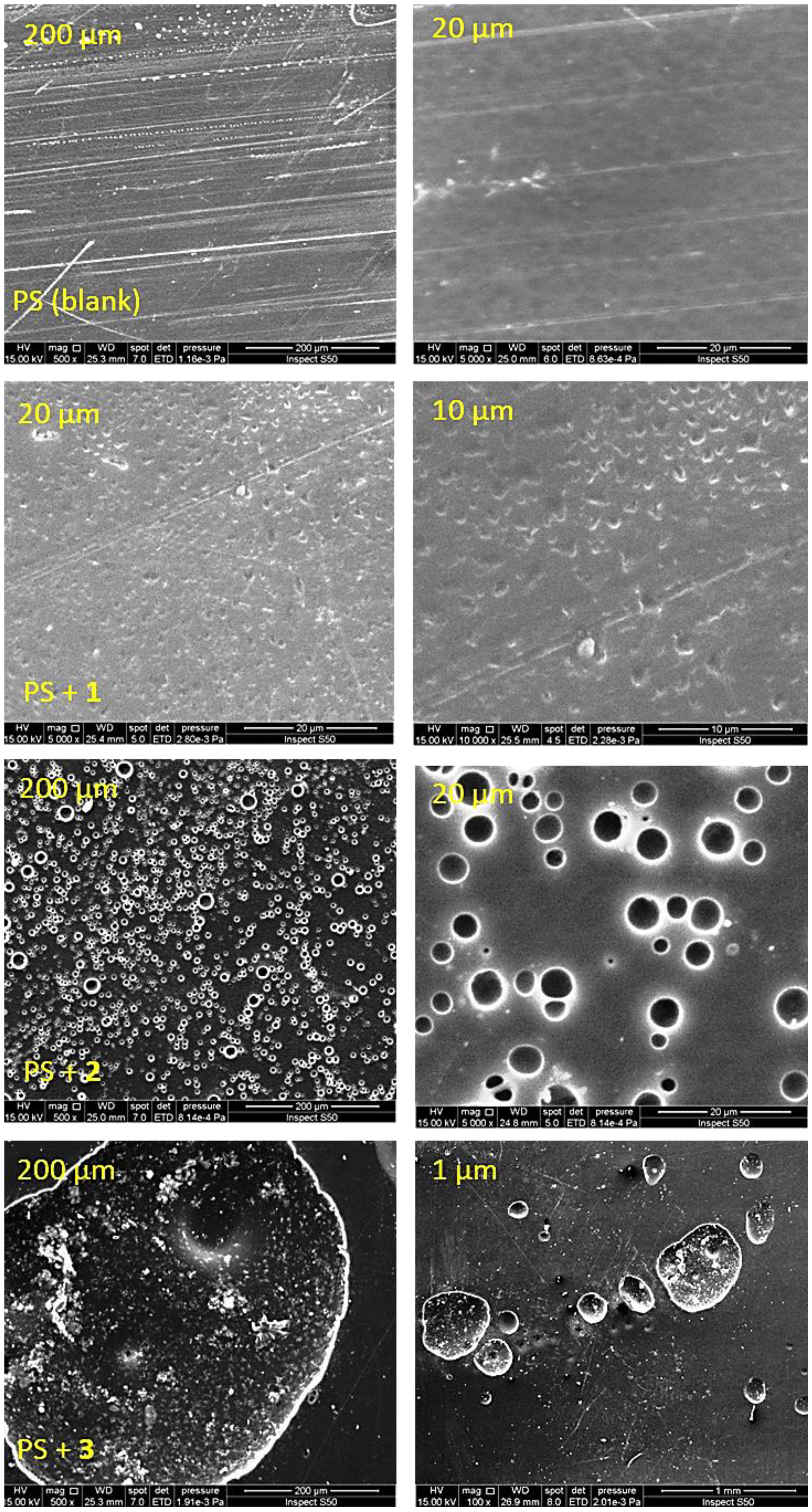
3.5. Scanning Electron Microscopy (SEM) of PS
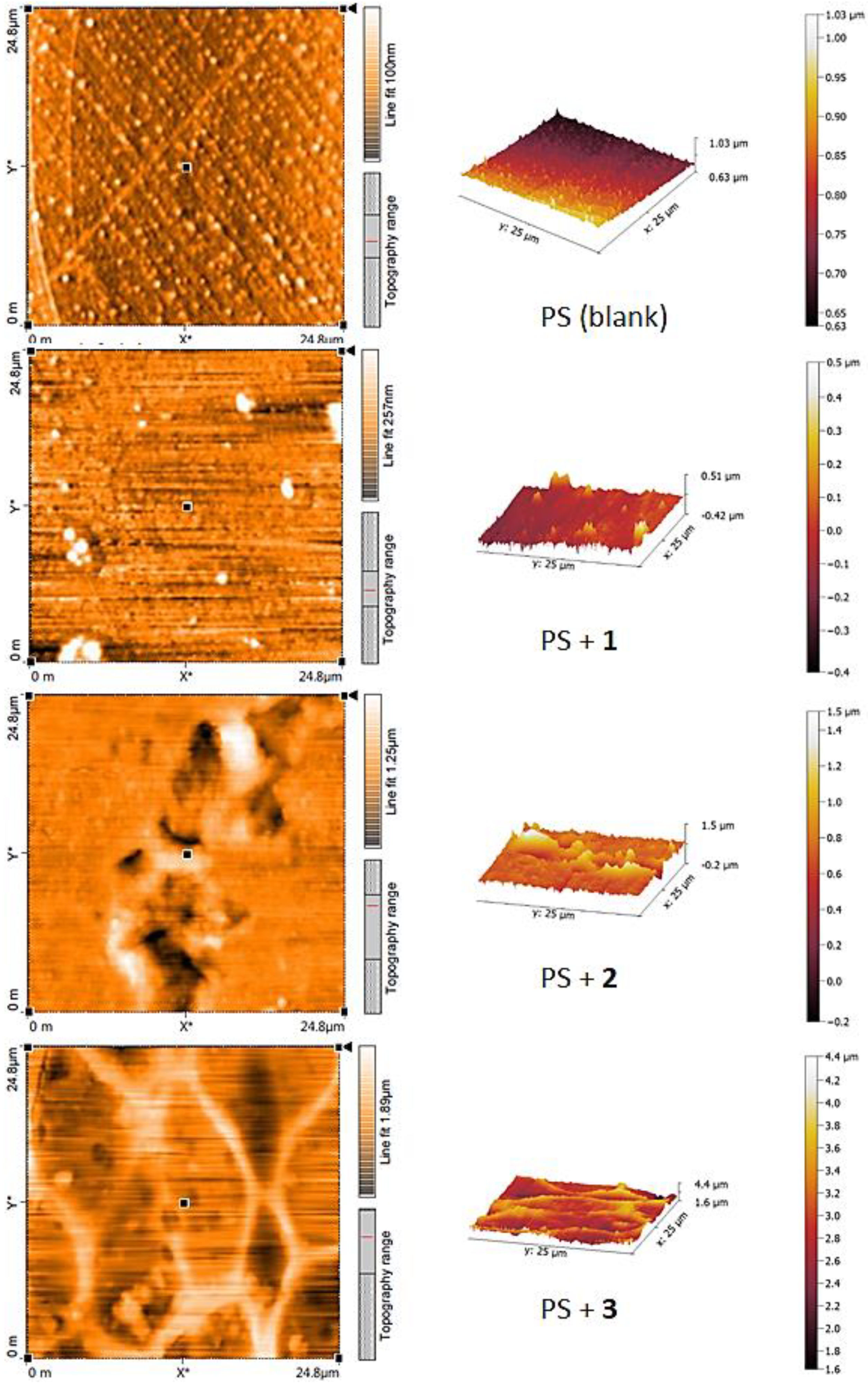
3.6. Atomic Force Microscopy (AFM) of PS
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.-H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Gary, J.E. Polystyrene: Properties, Performance and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011. [Google Scholar]
- Lynwood, C. Polystyrene: Synthesis, Characteristics and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2014. [Google Scholar]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Technology Ltd.: Shropshire, UK, 2000. [Google Scholar]
- De Rosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Geuskens, G.; David, C. Degradation and Stabilization of Polymers; Halsted, Wiley: New York, NY, USA, 1975. [Google Scholar]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Smith, S.H.; Taylor, L.T. Extraction of various additives from polystyrene and their subsequent analysis. Chromatographia 2002, 56, 165–169. [Google Scholar] [CrossRef]
- Hernández, C.G.; González, R.; Soto, J.J.; Rosales, I. Photo-Oxidation of Polystyrene Film Irradiated with UV-B; Springer: Gewerbestasse, Switzerland, 2016. [Google Scholar]
- Pinto, L.F.A.; Goi, B.E.; Schmitt, C.C.; Neumann, M.G. Photodegradation of polystyrene films containing UV-visible sensitizers. J. Res. Updates Polym. Sci. 2013, 2, 39–47. [Google Scholar] [CrossRef]
- Torikai, A.; Kobatake, T.; Okisaki, F.; Shuyama, H. Photodegradation of polystyrene containing flame-retardants: Wavelength sensitivity and efficiency of degradation. Polym. Degrad. Stab. 1995, 50, 261–267. [Google Scholar] [CrossRef]
- Rabie, S.T.; Mahran, A.M.; Kamel, E.M.; Abdel Hamid, N.H. Photodegradation of polystyrene stabilized with uracil derivative. J. Appl. Sci. Res. 2008, 4, 2018–2026. [Google Scholar]
- Yousif, E.; Ayad Hameed, A.; Salih, N.; Salimon, J.; Abdullah, B.M. New photostabilizers for polystyrene based on 2,3-dihydro-(5-mercapto-1,3,4-oxadiazol-2-yl)-phenyl-2-(substituted)-1,3,4-oxazepine-4,7-dione compounds. Springerplus 2013, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Yousif, E.; Yusop, R.M. Ultra violet spectra studies of polystyrene films in presence of some transition metal complexes with 4-amino-5-pyridyl)-4H-1,2,4-triazole-3-thiol. Orient. J. Chem. 2015, 31, 591–596. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New stabilizers for polystyrene based on 2-N-salicylidene-5- (substituted)-1,3,4-thiadiazole compounds. J. Saudi Chem. Soc. 2012, 16, 299–306. [Google Scholar] [CrossRef]
- Sastre, R.; Catalina, F.; Mateo, J.L.; Claramunt, R.; Santa-Maria, M.D.; Catalán, J. Mechanism of photostabilization of polystyrene film by dihydroxyphenyl-pirazoles. J. Polym. Sci. A Polym. Chem. 1990, 28, 3661–3668. [Google Scholar] [CrossRef]
- Goldshtein, J.; Margel, S. Synthesis and characterization of polystyrene/2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethylethyl)-4-methyl-phenol composite microspheres of narrow size distribution for UV irradiation protection. Colloid Polym. Sci. 2011, 289, 1863–1874. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R.; El-Hiti, G.A.; Yusop, R.M. Spectroscopic and photochemical stability of polystyrene films in the presence of metal complexes. J. Taibah Univ. Sci. 2017, 11, 997–1007. [Google Scholar] [CrossRef]
- Haddad, R.; Yousif, E.; Yusop, R.M. Ultra violet spectra studies of polystyrene films in presence of some transition metal complexes with 4-amino-5-pyridyl)-4H-1,2,4-triazole-3-thiol. Orient. J. Chem. 2014, 30, 1565–1569. [Google Scholar] [CrossRef]
- Rabie, S.T.; Ahmed, A.E.; Sabaa, M.W.; Abd El-Ghaffar, M.A. Maleic diamides as photostabilizers for polystyrene. J. Ind. Eng. Chem. 2013, 19, 1869–1878. [Google Scholar] [CrossRef]
- Abd, M.A.; Zahra, A.A.I.A.; Shenta, A.A. Photostabilization of polystyrene films by anthraquinones derivatives and their complexes with copper(II), oxovanadium(IV) and nickel(II) ions. J. Basrah Res. 2009, 35, 81–97. [Google Scholar]
- Torikai, A.; Takeuchi, T.; Fueki, K. Photodegradation of polystyrene and polystyrene containing benzophenone. Polym. Photochem. 1983, 3, 307–320. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed]
- Shaalam, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Rabek, J.; Ranby, B. Photodegradation, Photooxidation and Photostabilization of Polymer; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Mark, J. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Biazar, E.; Zeinali, R.; Montazeri, N.; Pourshamsian, K.; Behrouz, M.; Asefnejad, A.; Khoshzaban, A.; Shahhosseini, G.; Najafabadi, M.S.; Abyani, R.; et al. Cell engineering: Nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiation. Int. J. Nanomed. 2010, 5, 549–556. [Google Scholar] [CrossRef]
- Sharma, T.; Aggarwal, S.; Kumar, S.; Mittal, V.K.; Kalsi, P.C.; Manchanda, V.K. Effect of gamma irradiation on the optical properties of CR-39 polymer. J. Mater. Sci. 2007, 42, 1127–1130. [Google Scholar] [CrossRef]
- Lucki, J.; Rånby, B. Photo-oxidation of polystyrene–Part 2: Formation of carbonyl groups in photo-oxidised polystyrene. Polym. Degrad. Stab. 1979, 1, 165–179. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Erlandsson, B.; Albertsson, A.-C.; Karlsson, S. Molecular weight determination in degraded oxidizable and hydrolyzable polymers giving deviation from accurate using calibration and the Mark-Houwink-Sakaruda (MHS) equation. Polym. Degrad. Stab. 1997, 57, 15–23. [Google Scholar] [CrossRef]
- Rabek, J.F. Mechanism of Photophysical Process and Photochemical Reaction in Polymers; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, T.; Chen, J.; Gao, H.; Cai, W. Novel synthesis of CuO nanofiber balls and films and their UV-visible light filteration property. Ceram. Int. 2016, 42, 8505–8512. [Google Scholar] [CrossRef]
- Goudy, A.; Gee, M.L.; Biggs, S.; Underwood, S. Atomic force microscopy study of polystyrene latex film morphology: Effects of aging and annealing. Langmuir 1995, 11, 4454–4459. [Google Scholar] [CrossRef]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. Appl. Polym. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Reginald, R.J.; Carson Meredith, J.C. Measurement of polyamide and polystyrene adhesion with coated-tip atomic force microscopy. J. Colloid Interface Sci. 2007, 314, 52–62. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of Novel Ball-Like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers. Polymers 2018, 10, 1185. https://doi.org/10.3390/polym10111185
Yousif E, Ahmed DS, El-Hiti GA, Alotaibi MH, Hashim H, Hameed AS, Ahmed A. Fabrication of Novel Ball-Like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers. Polymers. 2018; 10(11):1185. https://doi.org/10.3390/polym10111185
Chicago/Turabian StyleYousif, Emad, Dina S. Ahmed, Gamal A. El-Hiti, Mohammad Hayal Alotaibi, Hassan Hashim, Ayad S. Hameed, and Ahmed Ahmed. 2018. "Fabrication of Novel Ball-Like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers" Polymers 10, no. 11: 1185. https://doi.org/10.3390/polym10111185
APA StyleYousif, E., Ahmed, D. S., El-Hiti, G. A., Alotaibi, M. H., Hashim, H., Hameed, A. S., & Ahmed, A. (2018). Fabrication of Novel Ball-Like Polystyrene Films Containing Schiff Base Microspheres as Photostabilizers. Polymers, 10(11), 1185. https://doi.org/10.3390/polym10111185








