Selective Polymer Distributed Bragg Reflector Vapor Sensors
Abstract
:1. Introduction
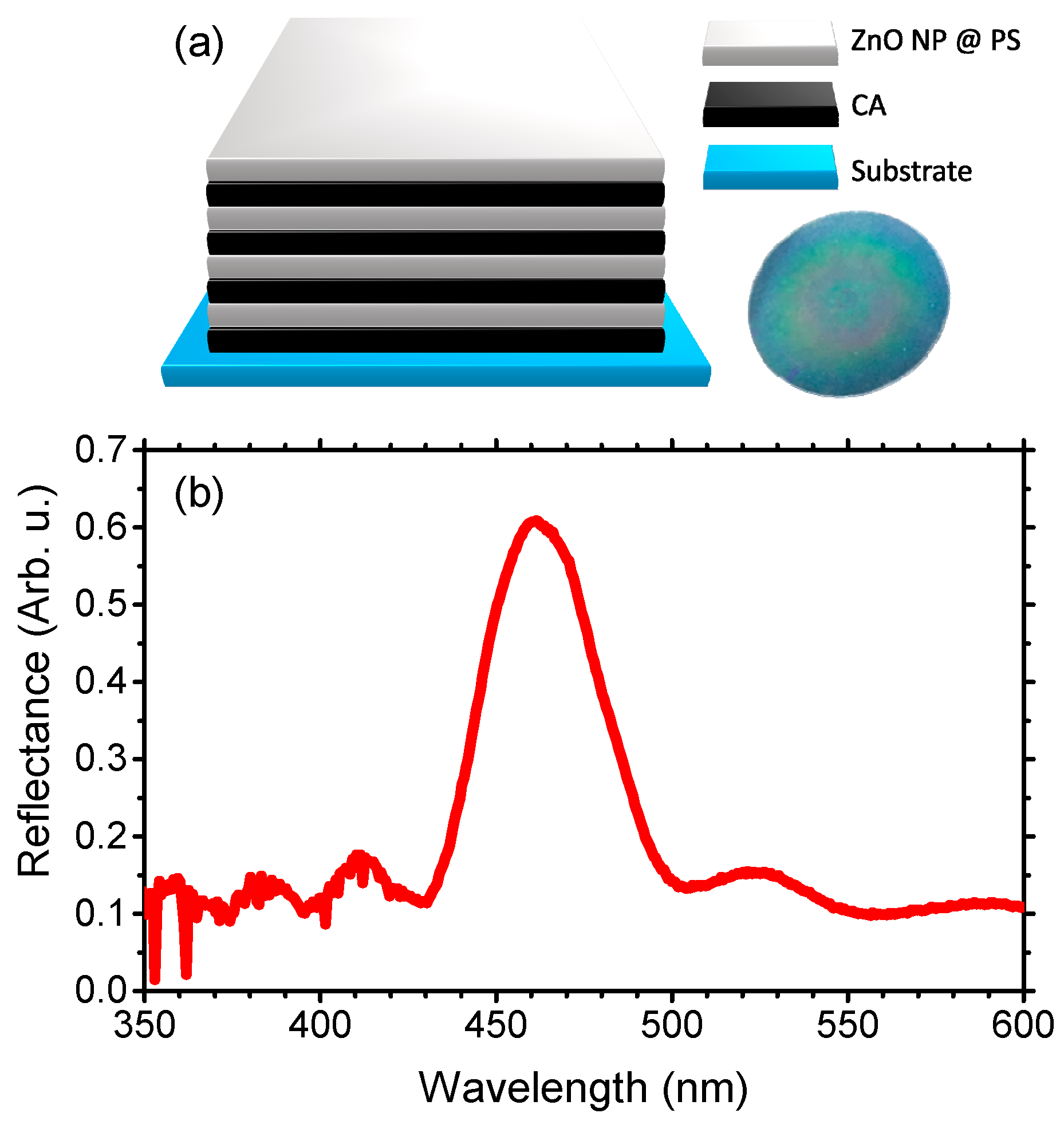
2. Materials and Methods
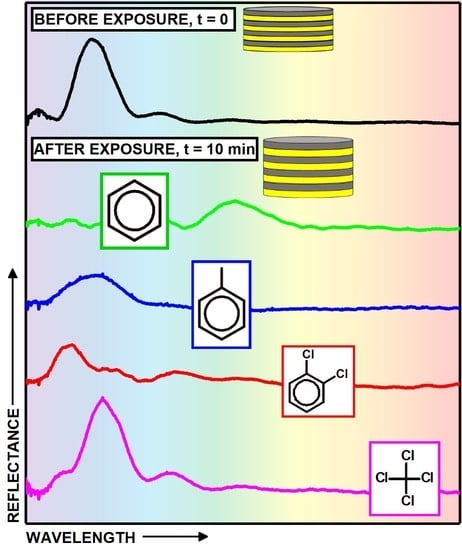
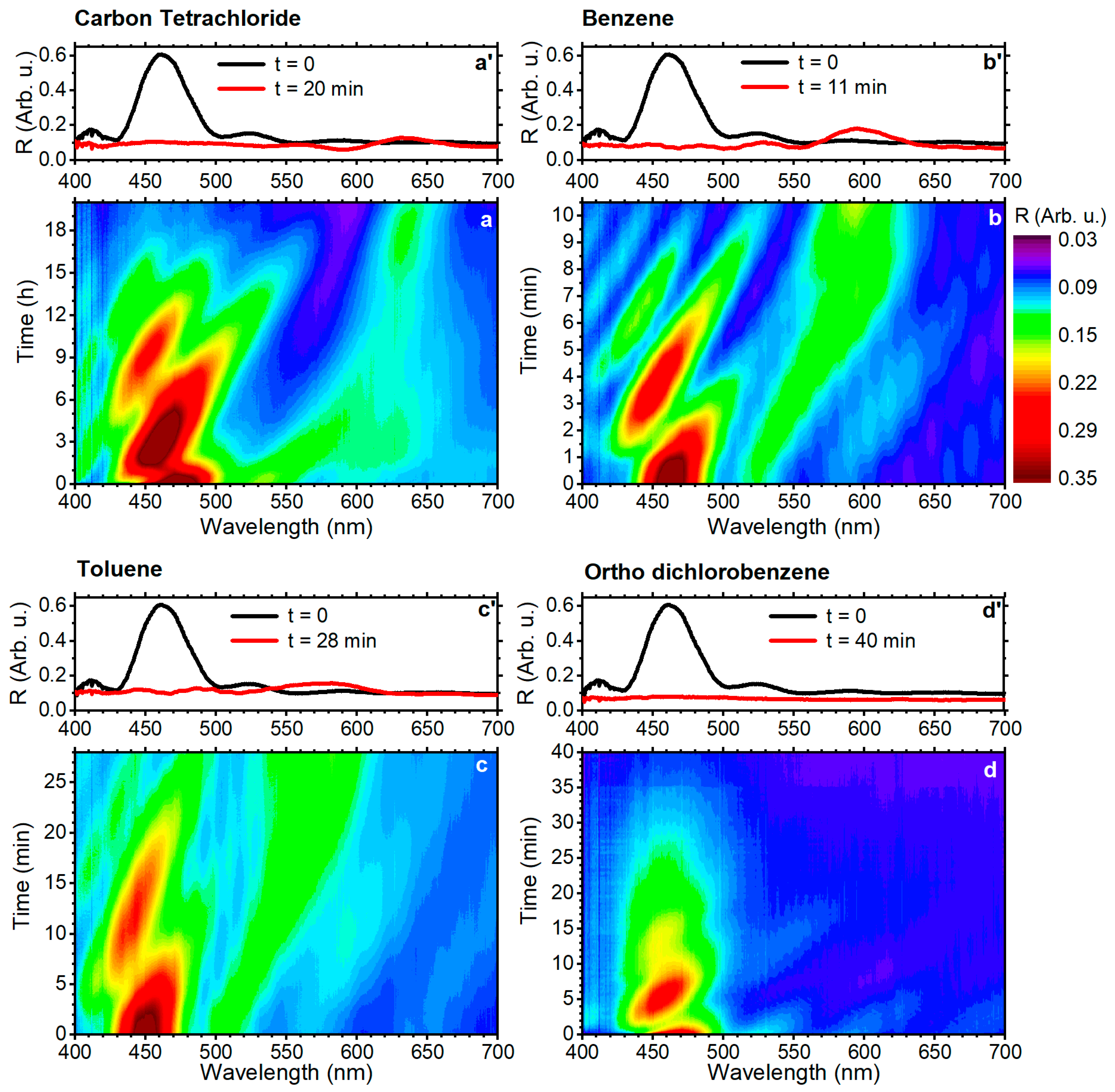
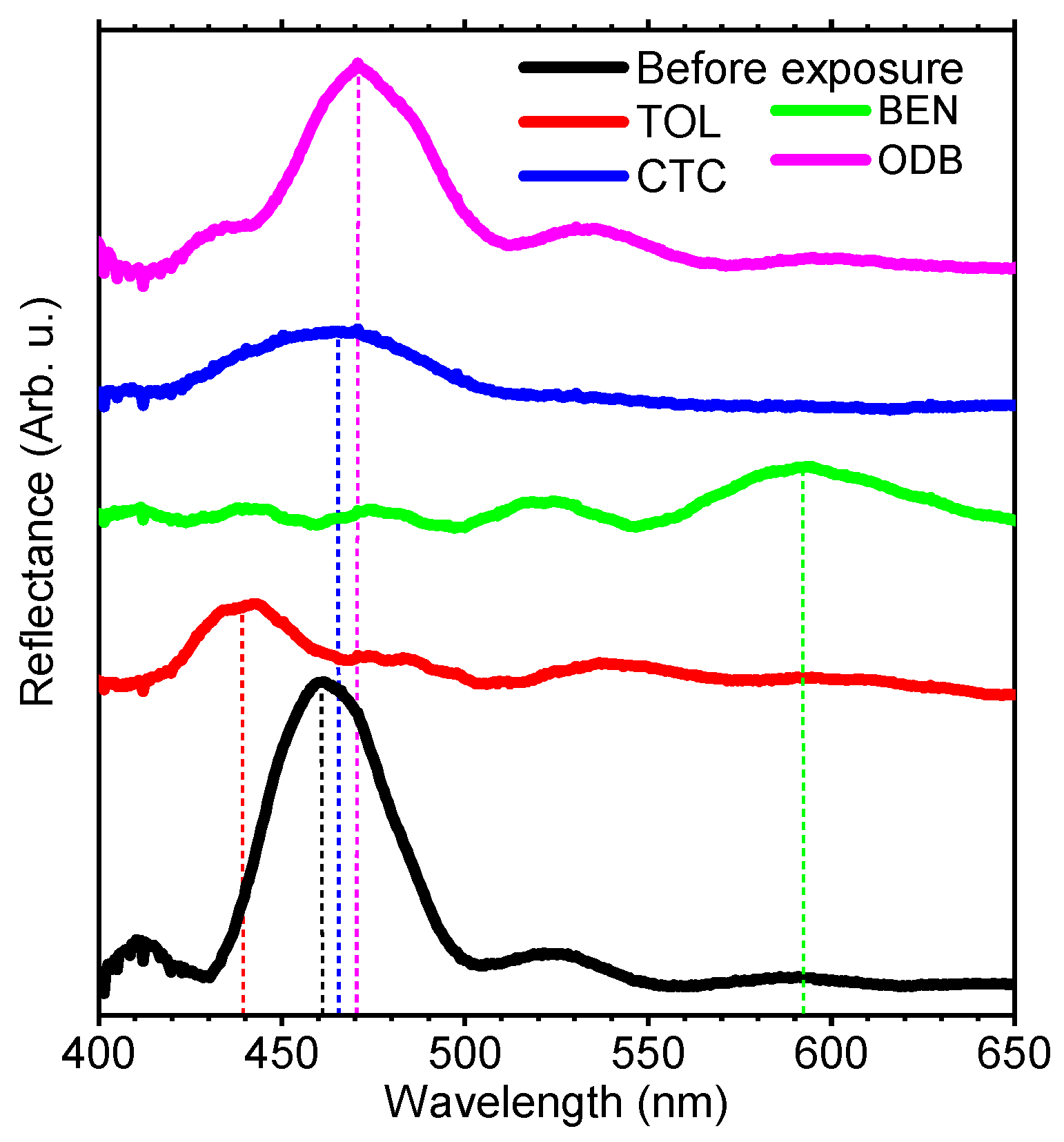
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/index.html (accessed on 28 July 2016).
- Straif, K.; Cohen, A.; Samet, J. Air Pollution and Cancer; Straif, K., Cohen, A., Samet, J., Eds.; IARC Scientific Publication No. 161; International Agency or Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Hansen, J.; Sato, M.; Ruedy, R.; Lacis, A.; Oinas, V. Global warming in the twenty-first century: An alternative scenario. Proc. Natl. Acad. Sci. USA 2000, 97, 9875–9880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippmann, M. Health effects of ozone a critical review. J. Air Pollut. Control Assoc. 1989, 39, 672–695. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: Hoboken, NJ, USA, 2012; p. 1119. [Google Scholar]
- Vlaanderen, J.; Straif, K.; Ruder, A.; Blair, A.; Hansen, J.; Lynge, E.; Charbotel, B.; Loomis, D.; Kauppinen, T.; Kyyronen, P.; et al. Tetrachloroethylene exposure and bladder cancer risk: A meta-analysis of dry-cleaning-worker studies. Environ. Health Perspect. 2014, 122, 661–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.Y.; Anderson, R.L. Non-Dispersive Infrared Gas Measurement; IFSA Publishing: Barcelona, Spain, 2012; p. 120. [Google Scholar]
- Baur, X. International workshop 2011: “How to handle import containers safely”. Int. Marit. Health 2012, 63, 140–147. [Google Scholar] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L. Introduction to Organic Laboratory Techniques: A Small Scale Approach, 2nd ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005; Volume 1, p. 1021. [Google Scholar]
- Lova, P.; Manfredi, G.; Boarino, L.; Comite, A.; Laus, M.; Patrini, M.; Marabelli, F.; Soci, C.; Comoretto, D. Polymer distributed bragg reflectors for vapor sensing. ACS Photonics 2015, 2, 537–543. [Google Scholar] [CrossRef]
- Lova, P.; Bastianini, C.; Giusto, P.; Patrini, M.; Rizzo, P.; Guerra, G.; Iodice, M.; Soci, C.; Comoretto, D. Label-free vapor selectivity in poly(p-phenylene oxide) photonic crystal sensors. ACS Appl. Mater. Interfaces 2016, 8, 31941–31950. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Manfredi, G.; Comoretto, D. Advances in functional solution processed planar one-dimensional photonic crystals. Adv. Opt. Mater. 2018. [Google Scholar] [CrossRef]
- Mönch, W.; Dehnert, J.; Prucker, O.; Rühe, J.; Zappe, H. Tunable bragg filters based on polymer swelling. Appl. Opt. 2006, 45, 4284–4290. [Google Scholar] [CrossRef] [PubMed]
- Mönch, W.; Dehnert, J.; Jaufmann, E.; Zappe, H. Flory-huggins swelling of polymer bragg mirrors. Appl. Phys. Lett. 2006, 89, 164104. [Google Scholar] [CrossRef]
- Gao, S.; Tang, X.; Langner, S.; Osvet, A.; Harreiβ, C.; Barr, M.; Spiecker, E.; Bachmann, J.; Brabec, C.J.; Forberich, K. Time-resolved analysis of dielectric mirrors for vapor sensing. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- Bellingeri, M.; Chiasera, A.; Kriegel, I.; Scotognella, F. Optical properties of periodic, quasi-periodic, and disordered one-dimensional photonic structures. Opt. Mater. 2017, 72, 403–421. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.H.; Aviles, M.; Crescimanno, M.; Dawson, N.J.; Mazzocco, A.; Petrus, J.B.; Singer, K.D.; Baer, E.; Song, H. Thermo-spectral study of all-polymer multilayer lasers. Opt. Mater. Express 2013, 3, 1152–1160. [Google Scholar] [CrossRef]
- Ponting, M.; Hiltner, A.; Baer, E. Polymer nanostructures by forced assembly: Process, structure, and properties. Macromol. Symp. 2010, 294, 19–32. [Google Scholar] [CrossRef]
- Zhou, J.; Singer, K.D.; Lott, J.; Song, H.; Wu, Y.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. All-polymer distributed feedback and distributed bragg-reflector lasers produced by roll-to-roll layer-multiplying co-extrusion. Nonlinear Opt. Quantum Opt. 2010, 41, 59–71. [Google Scholar]
- Hou, L.; Hou, Q.; Mo, Y.; Peng, J.; Cao, Y. All-organic flexible polymer microcavity light-emitting diodes using 3M reflective multilayer polymer mirrors. Appl. Phys. Lett. 2005, 87, 243504. [Google Scholar] [CrossRef]
- 3M DICHROIC. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/3M-Dichroic-Films-for-Architectural-Laminated-Glass/?N=5002385+3291680356&rt=rud (accessed on 5 October 2018).
- Chamaleonlab. Available online: http://chameleonlab.nl/ (accessed on 5 October 2018).
- Lova, P.; Cortecchia, D.; Krishnamoorthy, H.N.S.; Giusto, P.; Bastianini, C.; Bruno, A.; Comoretto, D.; Soci, C. Engineering the emission of broadband 2D perovskites by polymer distributed bragg reflectors. ACS Photonics 2018, 5, 867–874. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Rastogi, P.; Krahne, R.; Comoretto, D. Lasing from dot-in-rod nanocrystals in planar polymer microcavities. RSC Adv. 2018, 8, 13026–13033. [Google Scholar] [CrossRef] [Green Version]
- Lova, P.; Grande, V.; Manfredi, G.; Patrin, M.; Herbst, S.; Würthner, F.; Comoretto, D. All-polymer photonic microcavities doped with perylene bisimide j-aggregates. Adv. Opt. Mater. 2017, 5, 1700523. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Krahne, R.; Comoretto, D. Directional fluorescence spectral narrowing in all-polymer microcavities doped with CdSe/CdS dot-in-rod nanocrystals. ACS Photonics 2017, 4, 1761–1769. [Google Scholar] [CrossRef]
- Manfredi, G.; Mayrhofer, C.; Kothleitner, G.; Schennach, R.; Comoretto, D. Cellulose ternary photonic crystal created by solution processing. Cellulose 2016, 23, 2853–2862. [Google Scholar] [CrossRef]
- Giusto, P.; Lova, P.; Manfredi, G.; Gazzo, S.; Srinivasan, B.; Radice, S.V.; Comoretto, D. Colorimetric detection of perfluorinated compounds by all-polymer photonic transducers. ACS Omega 2018, 3, 7517–7522. [Google Scholar] [CrossRef]
- Song, H.; Singer, K.; Lott, J.; Wu, Y.; Zhou, J.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. Continuous melt processing of all-polymer distributed feedback lasers. J. Mater. Chem. 2009, 19, 7520–7524. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Boarino, L.; Laus, M.; Urbinati, G.; Losco, T.; Marabelli, F.; Caratto, V.; Ferretti, M.; Castellano, M.; et al. Hybrid ZnO: Polystyrene nanocomposite for all-polymer photonic crystals. Phys. Status Solidi C 2015, 12, 158–162. [Google Scholar] [CrossRef]
- Sun, D.; Wong, M.; Sun, L.; Li, Y.; Miyatake, N.; Sue, H.-J. Purification and stabilization of colloidal ZnO nanoparticles in methanol. J. Sol-Gel Sci. Technol. 2007, 43, 237–243. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill Professional: New York, NY, USA, 1999; Volume 1, p. 2704. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
| Effects | Compounds |
|---|---|
| Toxic to organ systems [1] | Toluene, xylenes, chlorobenzene, dichlorobenzenes, styrene, carbon disulfide, acrolein, chloroform, bromoform, 2-butanone, 1,3-butadiene, tetrachloroethane, dichloroethene, chloroethane, dichloropropenes, bromomethan, hydrazines. |
| Mutagen and developmental [1] | Xylenes, n-hexane, ethylene glycol, vinyl chloride, acrylonitrile, acrylamide, ethylbenzene, chloroform, chloroethane, dichlorobenzenes, phalates, ethylene oxide. |
| Neurological [1] | Fuels and mineral oils, toluene, acetone, n-hexane, benzene, xylenes, acrylonitrile, pyridine, trichloroethane, carbon tetrachloride, chloroform, 1,3-butadiene, ethylbenzene, mercaptanes, naphtalenes. |
| Potential carcinogen [2] | Polycyclic aromatic hydrocarbons, acrylonitrile, acrylamide, nitrobenzene, styrene, hydrazines, naphtalenes, halogenated hydrocarbons (e.g., chloroform, carbon tetrachloride, dichlorobenzenes, trichloroethylene, hexachloroethane polyhalogenated biphenyles) |
| Carcinogen [2] | Benzene, formaldehyde, vinyl chloride, ethylene oxide, benzidine, 1,3-butadiene, bis(chloromethyl) ether. |
| Analyte | V (Å2) [16] | χPS [38] | χCA [38] | χeff [38] | Δλeq (nm) | teq (min) | Vapor Pressure (kPa) |
|---|---|---|---|---|---|---|---|
| benzene | 89.4 | 0.001 | 1.635 | 1.183 | 130 | 11 | 10.5 |
| toluene | 106.8 | 0.006 | 2.090 | 1.512 | 120 | 28 | 2.8 |
| o-dichlorobenzene | 112.8 | 0.088 | 1.223 | 0.887 | -- | 40 | 0.13 |
| carbon tetrachloride | 97.1 | 0.008 | 1.942 | 1.406 | 175 | >1200 | 11.95 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lova, P. Selective Polymer Distributed Bragg Reflector Vapor Sensors. Polymers 2018, 10, 1161. https://doi.org/10.3390/polym10101161
Lova P. Selective Polymer Distributed Bragg Reflector Vapor Sensors. Polymers. 2018; 10(10):1161. https://doi.org/10.3390/polym10101161
Chicago/Turabian StyleLova, Paola. 2018. "Selective Polymer Distributed Bragg Reflector Vapor Sensors" Polymers 10, no. 10: 1161. https://doi.org/10.3390/polym10101161
APA StyleLova, P. (2018). Selective Polymer Distributed Bragg Reflector Vapor Sensors. Polymers, 10(10), 1161. https://doi.org/10.3390/polym10101161