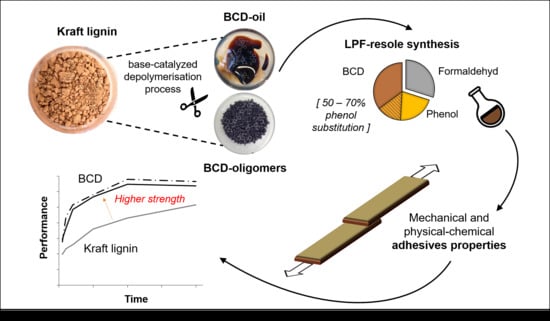
Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
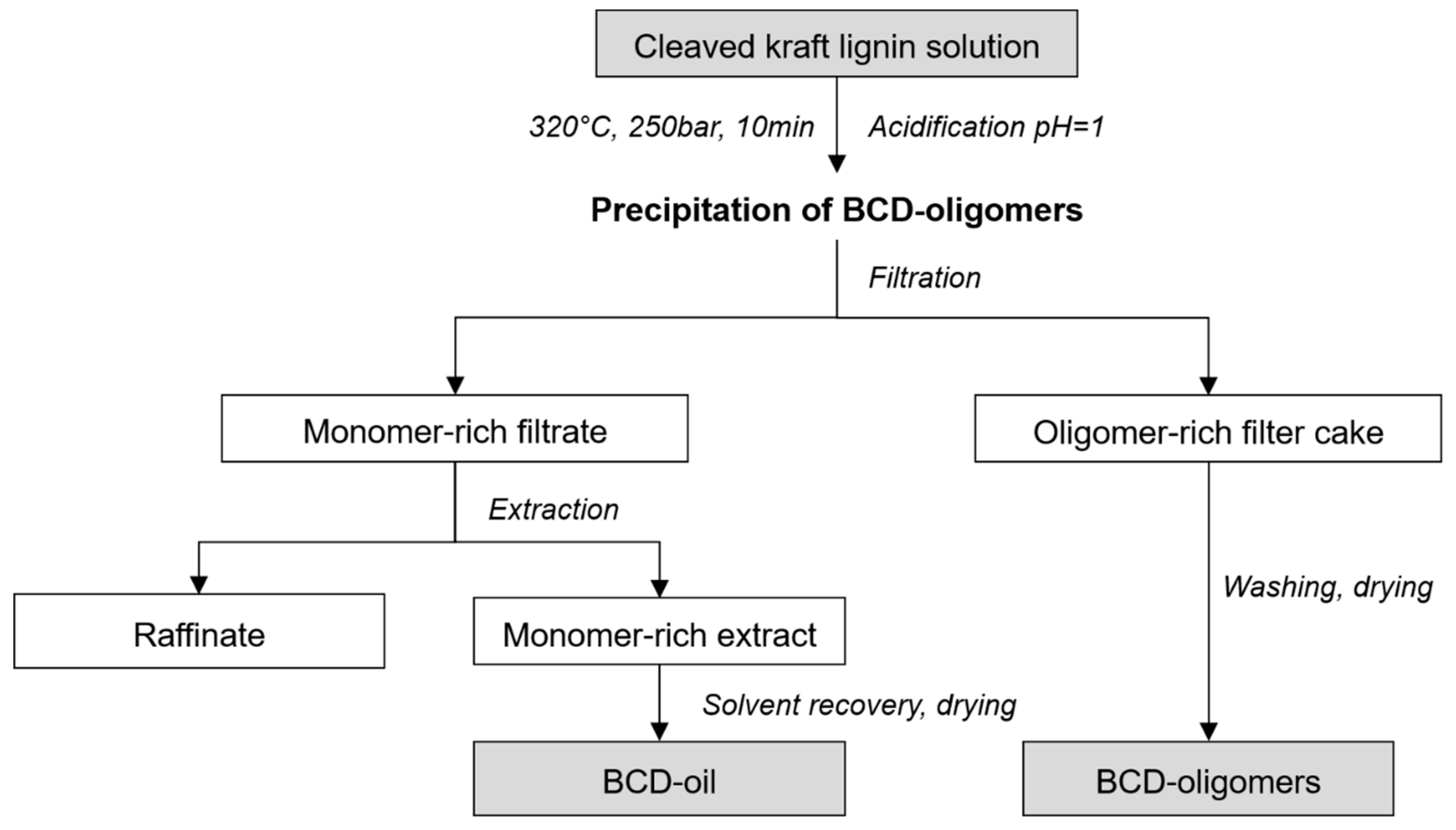
2.2. Base-Catalysed Depolymerisation
2.3. Analytical Characterization of Reaction Products
2.4. Synthesis of Adhesives
2.5. Physical-Chemical Adhesive Characterization
2.6. Development of Bonding Strength
2.7. Longitudinal Tensile Shear Strength of Solid Wood Joints
3. Results and Discussion
3.1. Lignin Characterization
3.2. Physical-Chemical Adhesive Characterization
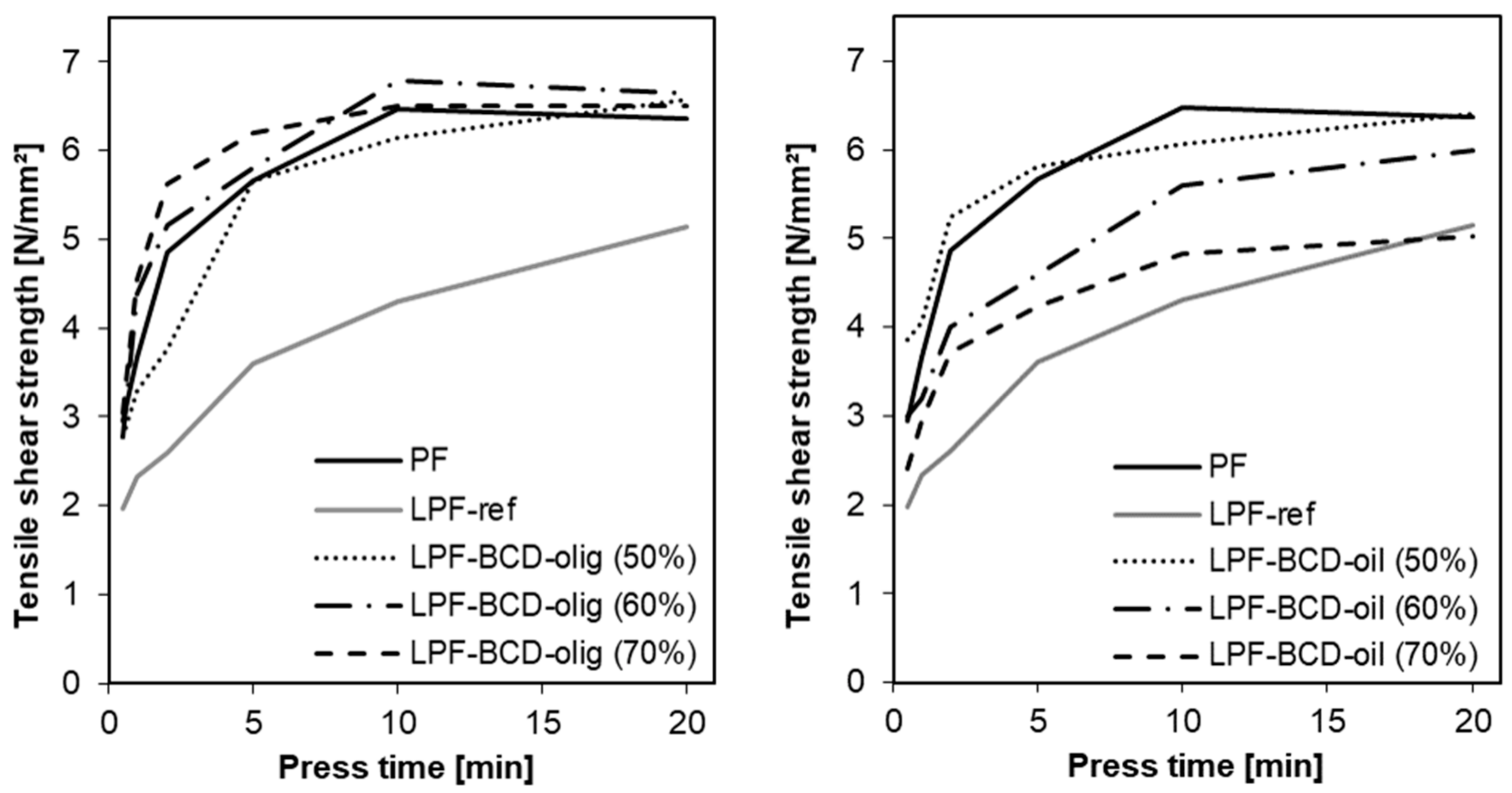
3.3. Development of the Bonding Strength
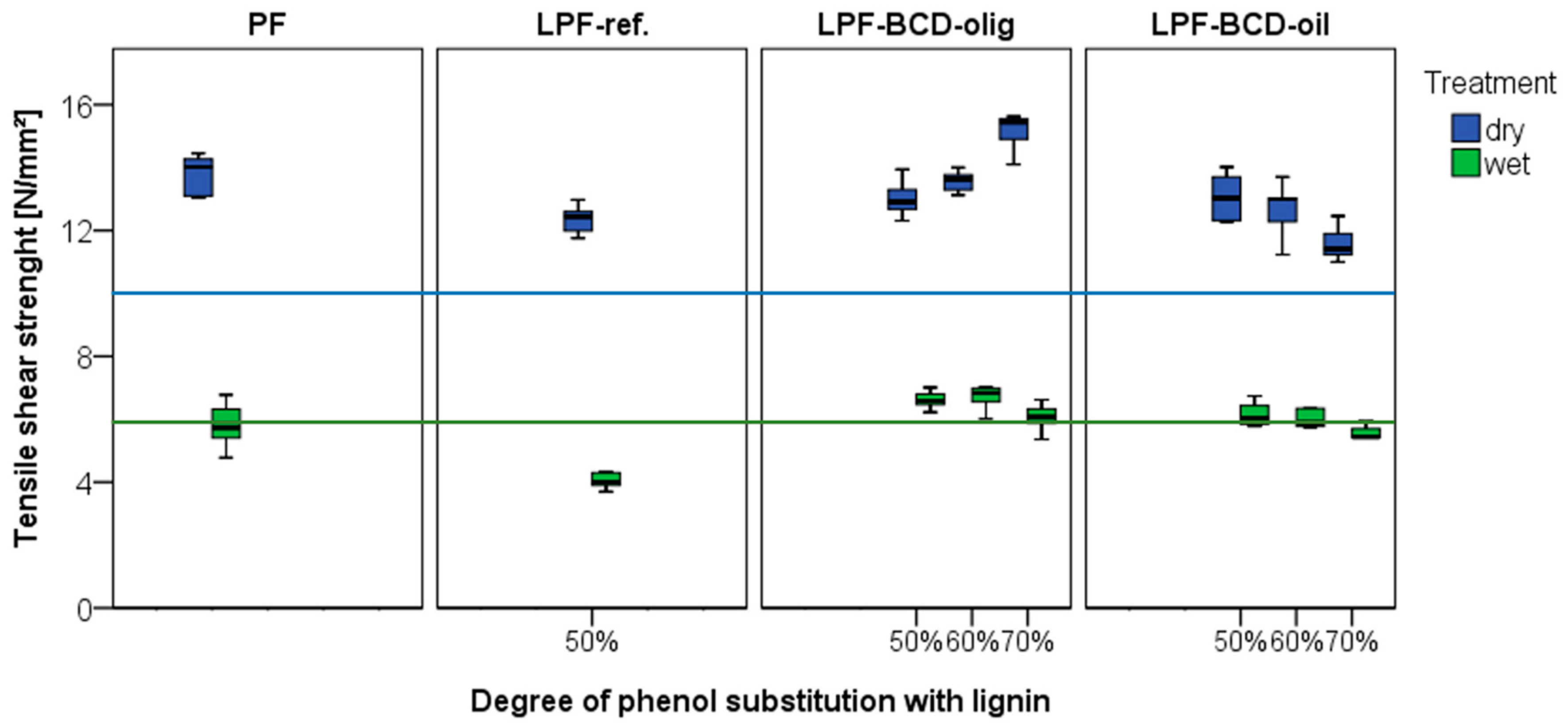
3.4. Longitudinal Tensile Shear Strength
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Umemura, K. Research Trends of Natural Adhesives. Mokuzai Gakkaishi 2014, 60, 123–143. [Google Scholar] [CrossRef] [Green Version]
- In Proceedings of the International Conference on Wood Adhesives, Atlanta, GA, USA, 25–27 October 2017. Available online: http://www.forestprod.org/woodadhesives/ (accessed on 16 October 2018).
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Miller, J.; Faleiros, M.; Pilla, L. Lignin: Technology, applications and markets. In Special Market Analysis Study; RISI, Inc.: Cincinnati, OH, USA, 2016. [Google Scholar]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Ghorbani, M.; Liebner, F.; van Herwijnen, H.W.G.; Pfungen, L.; Krahofer, M.; Budjav, E.; Konnerth, J. Lignin Phenol Formaldehyde Resoles: The Impact of Lignin Type on Adhesive Properties. Bioresources 2016, 11, 6727–6741. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, S.; Hübsch, C. Lignin based binder: An industrial reality, latest develoments. In Proceedings of the International Conference on Wood Adhesives, Atlanta, GA, USA, 25–27 October 2017. [Google Scholar]
- El Mansouri, N.-E.; Pizzi, A.; Salvado, J. Lignin-based polycondensation resins for wood adhesives. J. Appl. Polym. Sci. 2007, 103, 1690–1699. [Google Scholar] [CrossRef]
- Prefere_Resins. Biopolymers Put to Industrial Use at Phenolic-Resin Producer Prefere Resins. Available online: http://www.prefereresins.com/ (accessed on 5 July 2018).
- Zhao, M.; Jing, J.; Zhu, Y.; Yang, X.; Wang, X.; Wang, Z. Preparation and performance of lignin–phenol–formaldehyde adhesives. Int. J. Adhes. Adhes. 2016, 64, 163–167. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Adhikari, B. Lignin-modified phenolic resin: Synthesis optimization, adhesive strength, and thermal stability. J. Adhes. Sci. Technol. 2000, 14, 1179–1193. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Zhang, M. Methods to improve lignin’s reactivity as a phenol substitute and as a replacement of other phenolic compounds; A brief review. Bioresources 2011, 6, 3515–3525. [Google Scholar]
- Solt, P.; Jääskeläinen, A.-S.; Lingenfelter, P.; Konnerth, J.; van Herwijnen, H.W.G. Impact of molecular weight of kraft-lignin on adhesive performance of lignin based phenol formaldehyde resins. For. Prod. J. 2018. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 838645. [Google Scholar] [CrossRef]
- Rößiger, B.; Unkelbach, G.; Pufky-Heinrich, D. Base-Catalyzed Depolymerization of Lignin: History, Challenges and Perspectives. In Lignin-Trends and Applications; Poletto, M., Ed.; InTechOpen: London, UK, 2018. [Google Scholar] [Green Version]
- Cheng, S.; Yuan, Z.; Leitch, M.; Anderson, M.; Xu, C. Highly efficient de-polymerization of organosolv lignin using a catalytic hydrothermal process and production of phenolic resins/adhesives with the depolymerized lignin as a substitute for phenol at a high substitution ratio. Ind. Crops Prod. 2013, 44, 315–322. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Soledad Peresin, M.; Rodríguez, A.; Jääskeläinen, A.-S. Aqueous acetone fractionation of kraft, organosolv and soda lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Rößiger, B.; Röver, R.; Unkelbach, G.; Pufky-Heinrich, D. Production of bio-phenols for industrial application: Scale-up of the base-catalyzed depolymerization of lignin. Green Sustain. Chem. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- ISO-1762. Paper, Board and Pulps—Determination of Residue (ash) on Ignition at 525 °C; ISO-International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- EN-827. Adhesives—Determination of Conventional Solids Content and Constant Mass Solids Content; European Committee of Standardization: Brussels, Belgium, 2006. [Google Scholar]
- DIN-16916-2. Reaktionsharze, Phenolharze Prüfverfahren (Reaction Resins, Phenolic Resins Test Methods); German Institute for Standardization: Berlin, Germany, 1987. [Google Scholar]
- ASTM-D7998-15. Standard Test Method for Measuring the Effect of Temperature on the Cohesive Strength Development of Adhesives Using Lap Shear Bonds under Tensile Loading; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Humphrey, P.E. Device for Testing Adhesive Bonds. U.S. Patent 5,176,028, 5 January 1993. [Google Scholar]
- EN-302-1. Adhesives for Load-Bearing Timber Structures—Test Methods—Part 1: Determination of Longitudinal Tensile Shear Strength; European Committee of Standardization: Brussels, Belgium, 2004. [Google Scholar]
- EN-301. Adhesives, Phenolic and Aminoplastic, for Load-Bearing Timber Structures—Classification and Performance Requirements; European Committee of Standardization: Brussels, Belgium, 2017. [Google Scholar]
- Dunky, M. Bindemittel und Verleimung. In Holzwerkstoffe und Leime—Technologie und Einflussfaktoren; Dunky, M., Niemz, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Humphrey, P.E. Temperature and reactant injection effects on the bonding dynamics of thermosetting adhesives. In Wood Adhesives; Adhesive Evaluation Systems. Inc.: San Diego, CA, USA, 1994; pp. 311–316. [Google Scholar]
- Konnerth, J.; Gindl, W.; Harm, M.; Müller, U. Comparing dry bond strength of spruce and beech wood glued with different adhesives by means of scarf-and lap joint testing method. Holz als Roh-und Werkst. 2006, 64, 269–271. [Google Scholar] [CrossRef]
- Ma, C.; Mei, X.; Fan, Y.; Zhang, Z. Oxidative depolymerizaton of kraft lignin and its application in the synthesis of lignin-phenol- formaldehyde resin. BioResources 2018, 13, 1223–1234. [Google Scholar] [CrossRef]
- Danielson, B.; Simonson, R. Kraft lignin in phenol formaldehyde resin. Part 1. Partial replacement of phenol by kraft lignin in phenol formaldehyde adhesives for plywood. J. Adhes. Sci. Technol. 1998, 12, 923–939. [Google Scholar] [CrossRef]
| Lignin Type | Yield | Ash Content a | Mn | Mw | PD | Texture |
|---|---|---|---|---|---|---|
| (%) | (%) | (g/mol) | (g/mol) | [Viscosity (mPa·s)] | ||
| Kraft lignin | 1.3 | 1350 | 9850 | 7.3 | Powder | |
| BCD-oligomers | 40 | 0.18 | 750 | 2250 | 3.0 | Powder |
| BCD-oil | 15 | 0.02 | 150 | 300 | 2.0 | Liquid [750 mPa·s] |
| Resins | Phenol | Solid | pH b | Viscosity a | Free HCHO b | Free Phenol b | B-Time |
|---|---|---|---|---|---|---|---|
| Substitution | Content a | 140 °C a | |||||
| (%) | (%) | (mPa·s) | (%) | (%) | (s) | ||
| PF | 43.6 | 12.1 | 580 | 0.2 | <0.01 | 92 | |
| LPF-ref. | 50 | 46.7 | 12.4 | 520 | 0.4 | 0.1 | 120 |
| LPF-BCD-olig. | 50 | 45.7 | 12.7 | 540 | 0.3 | <0.1 | 89 |
| 60 | 45.0 | 12.6 | 1720 | 0.3 | <0.1 | 96 | |
| 70 | 45.3 | 12.4 | 5580 | n.a. | <0.1 | 125 | |
| LPF-BCD-oil | 50 | 44.2 | 12.5 | 830 | 0.5 | 0.3 | 81 |
| 60 | 43.7 | 12.4 | 450 | 0.7 | 0.3 | 75 | |
| 70 | 43.0 | 12.4 | 220 | 0.9 | 0.3 | 71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solt, P.; Rößiger, B.; Konnerth, J.; Van Herwijnen, H.W.G. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers 2018, 10, 1162. https://doi.org/10.3390/polym10101162
Solt P, Rößiger B, Konnerth J, Van Herwijnen HWG. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers. 2018; 10(10):1162. https://doi.org/10.3390/polym10101162
Chicago/Turabian StyleSolt, Pia, Björn Rößiger, Johannes Konnerth, and Hendrikus W. G. Van Herwijnen. 2018. "Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin" Polymers 10, no. 10: 1162. https://doi.org/10.3390/polym10101162
APA StyleSolt, P., Rößiger, B., Konnerth, J., & Van Herwijnen, H. W. G. (2018). Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers, 10(10), 1162. https://doi.org/10.3390/polym10101162