Intra- and Interpolyelectrolyte Complexes of Polyampholytes
Abstract
1. Introduction
2. Theory of Polyampholytes (PA) and Polyelectrolyte (PE)–Polyampholyte Complexation
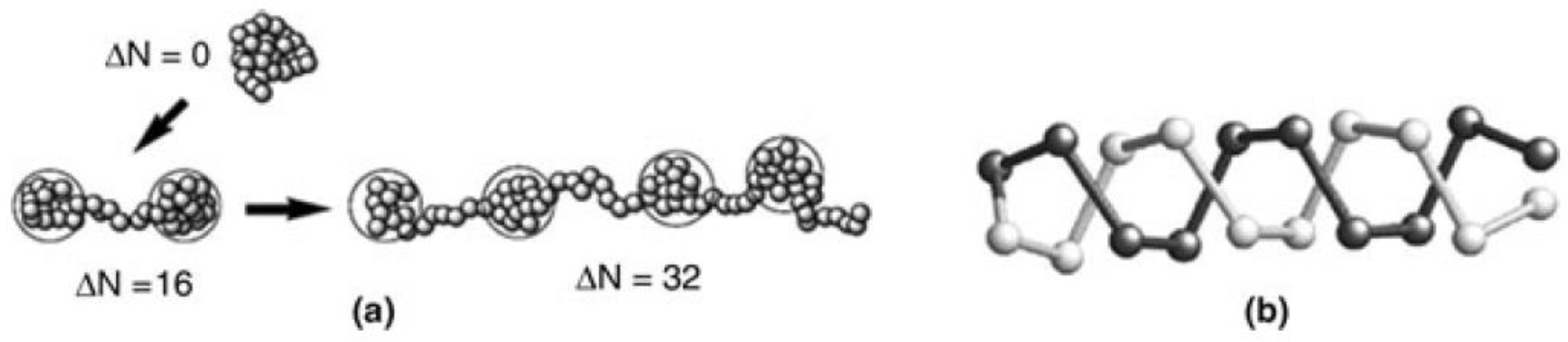
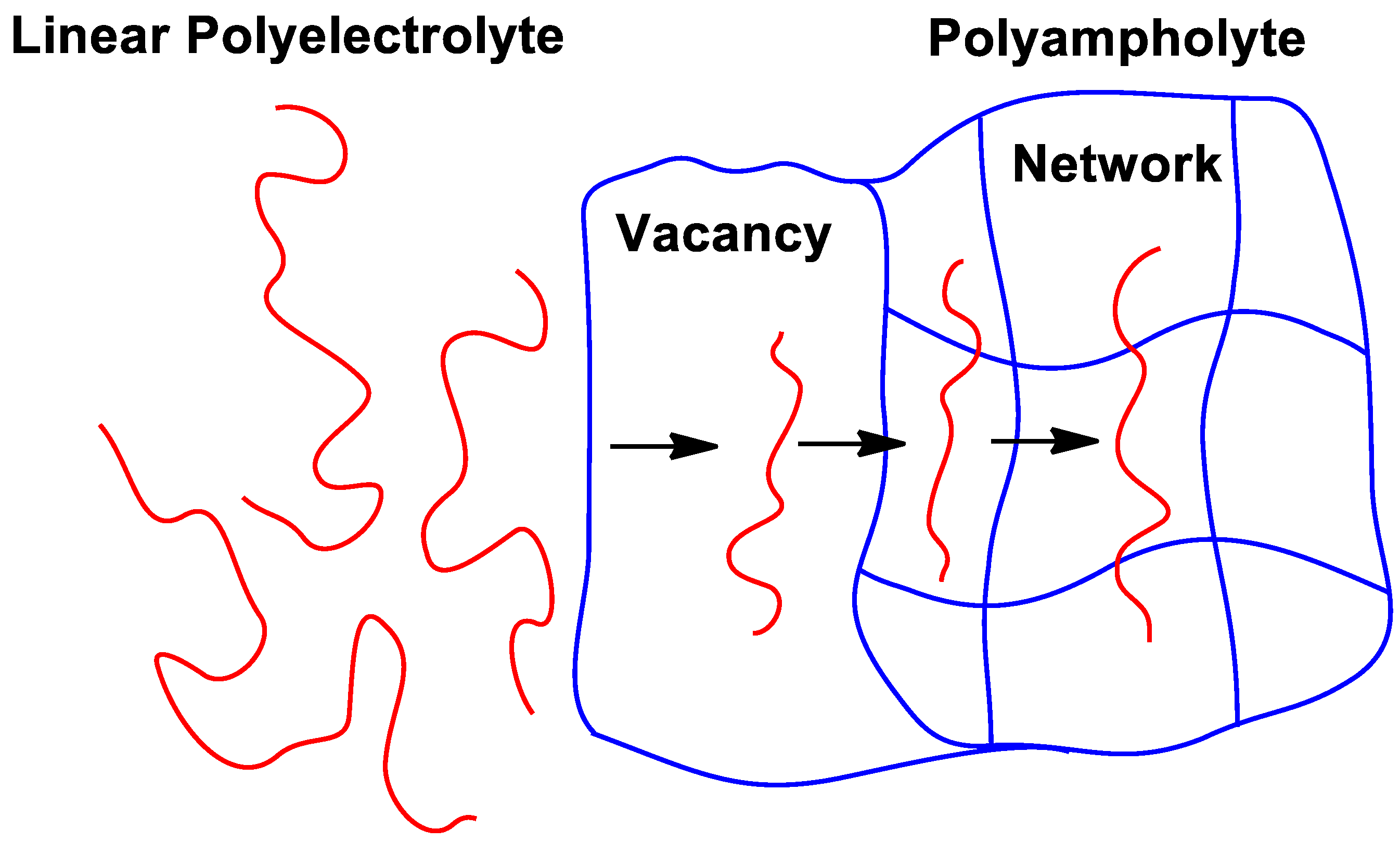
2.1. Theory of Polyampholytes
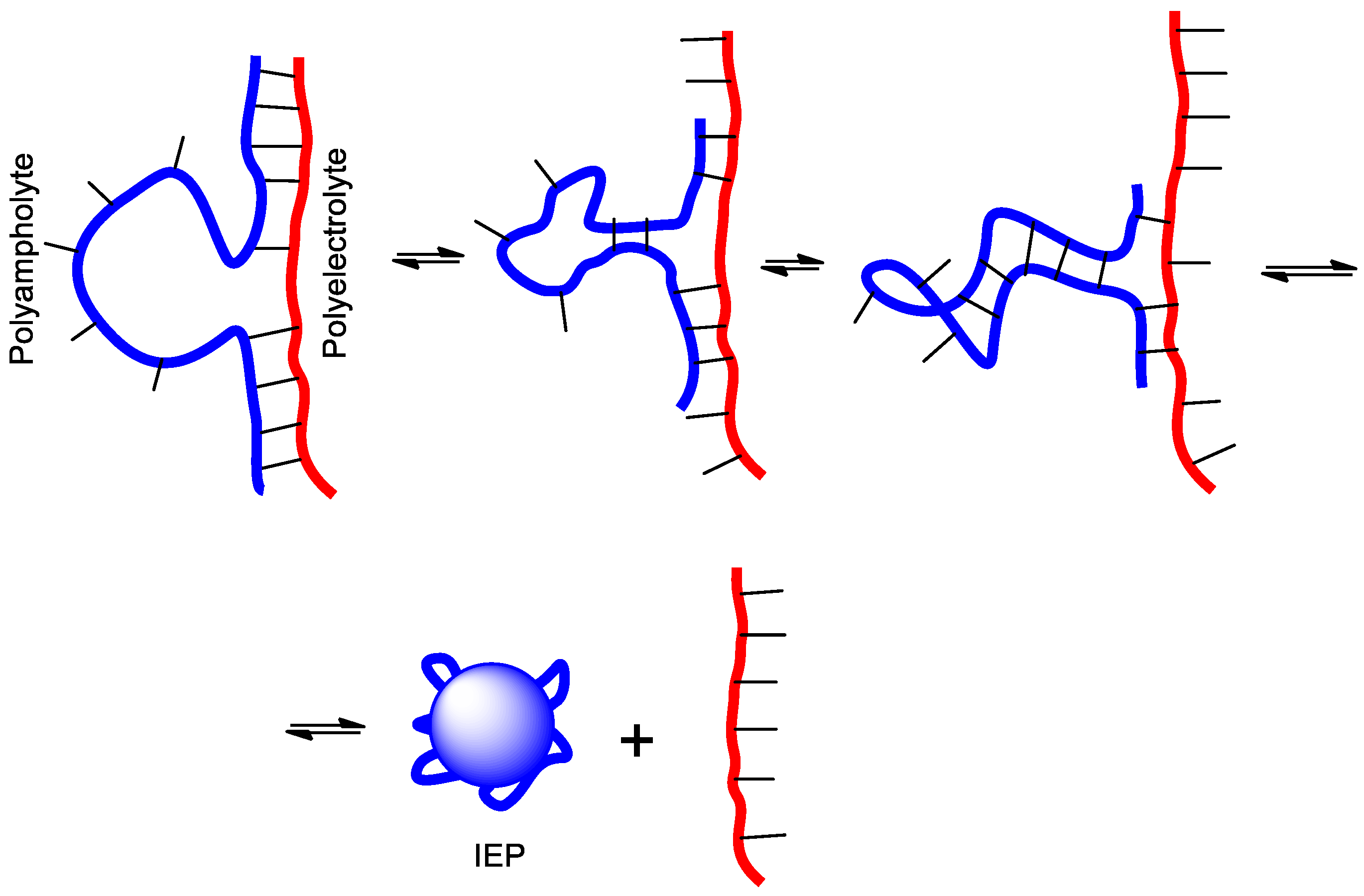
2.2. Theory of Polyelectrolyte–Polyampholyte Complexation
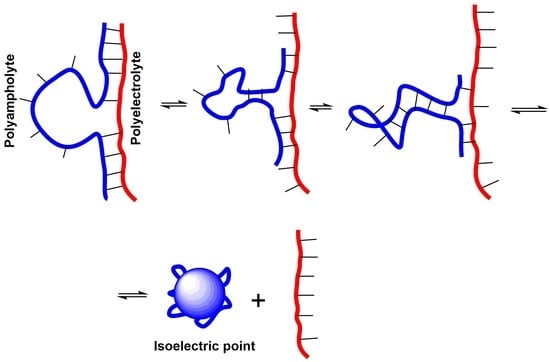
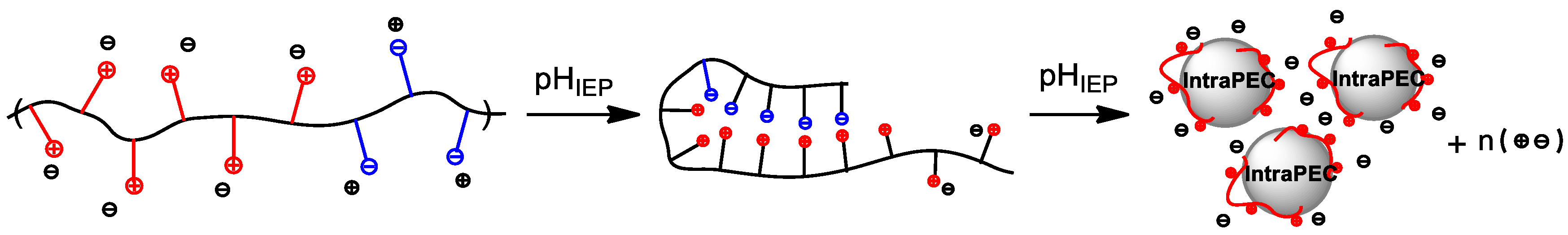
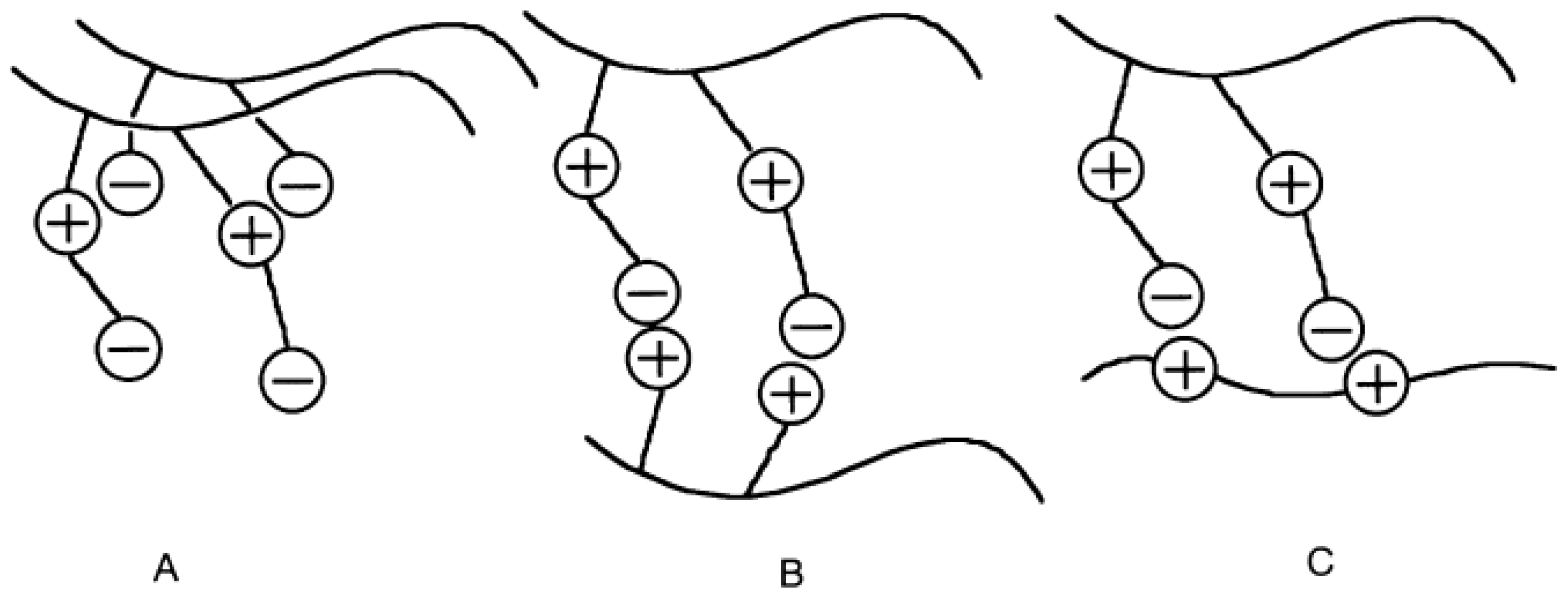
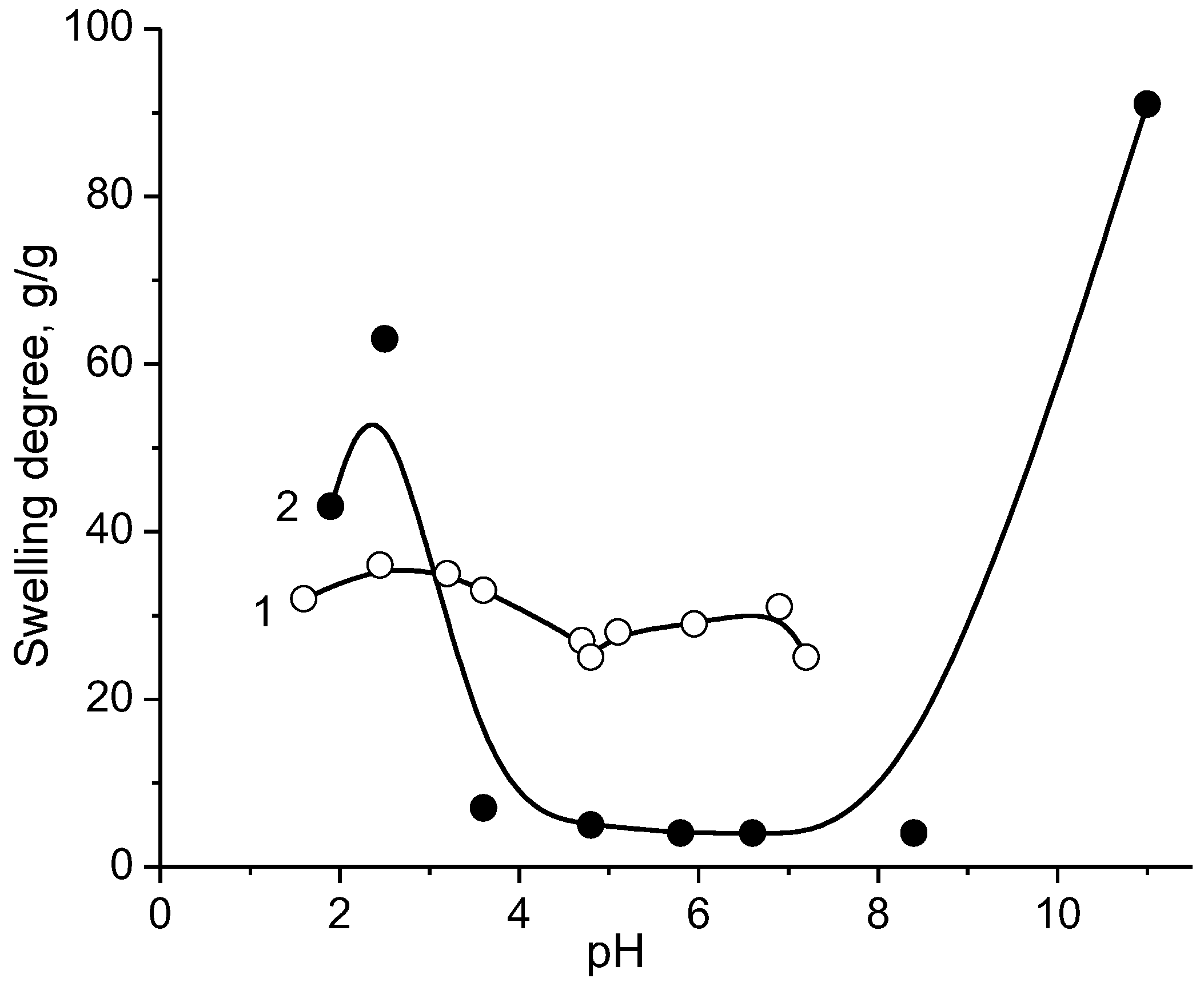
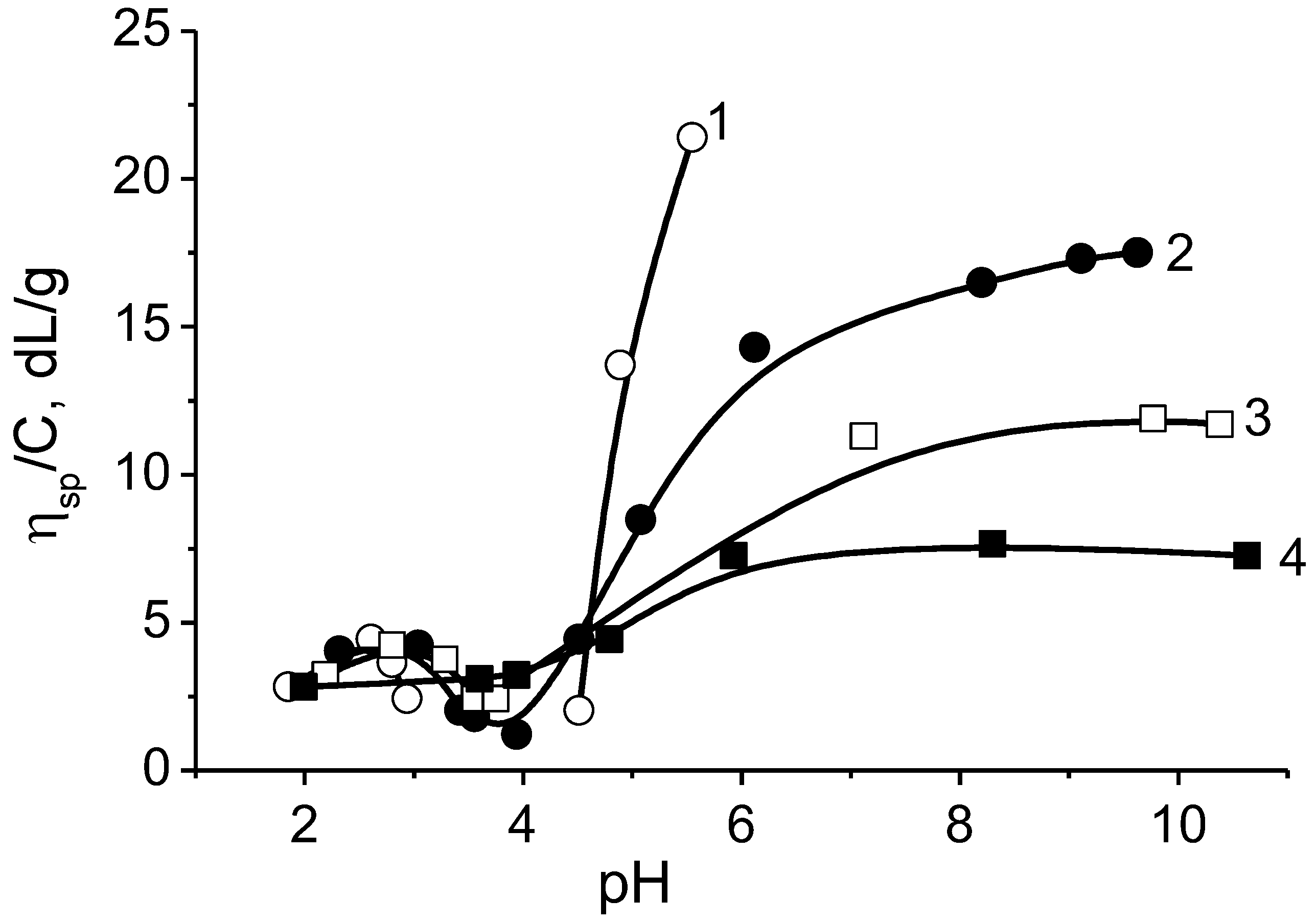
3. Intrapolyelectrolyte Complexes (Intra-PEC) of Polyampholytes
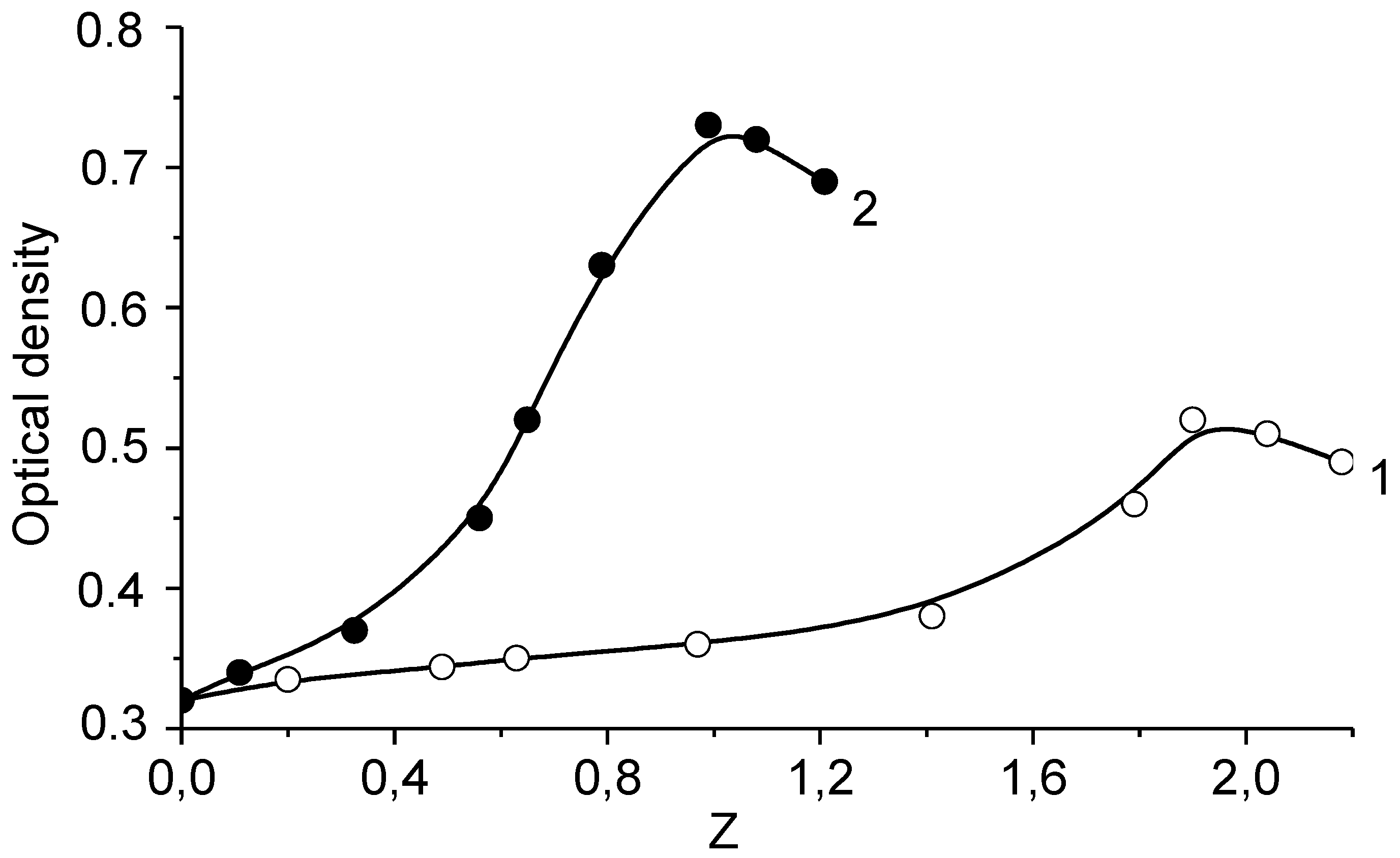
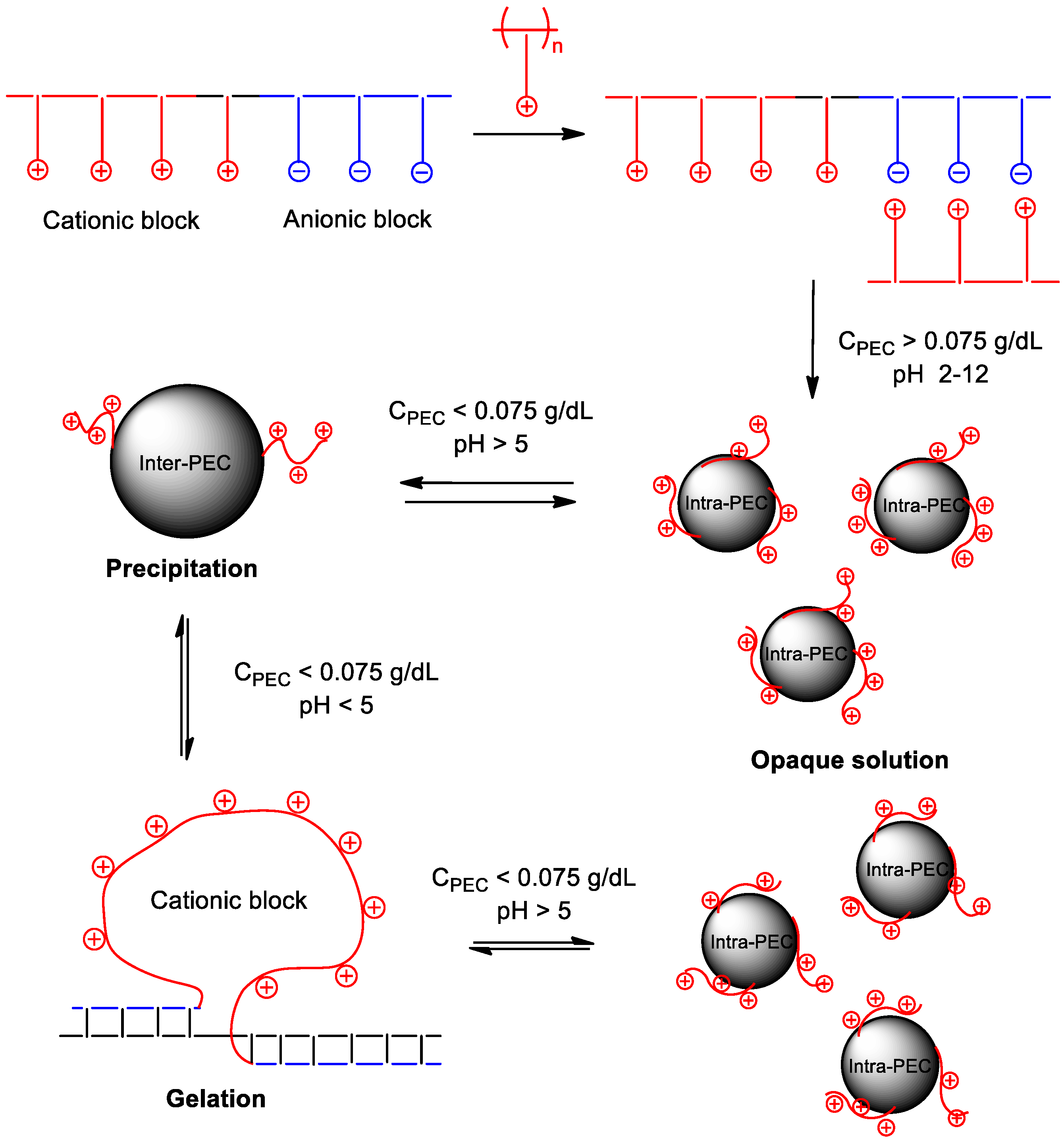
4. Interpolyelectrolyte Complexes of Polyampholytes
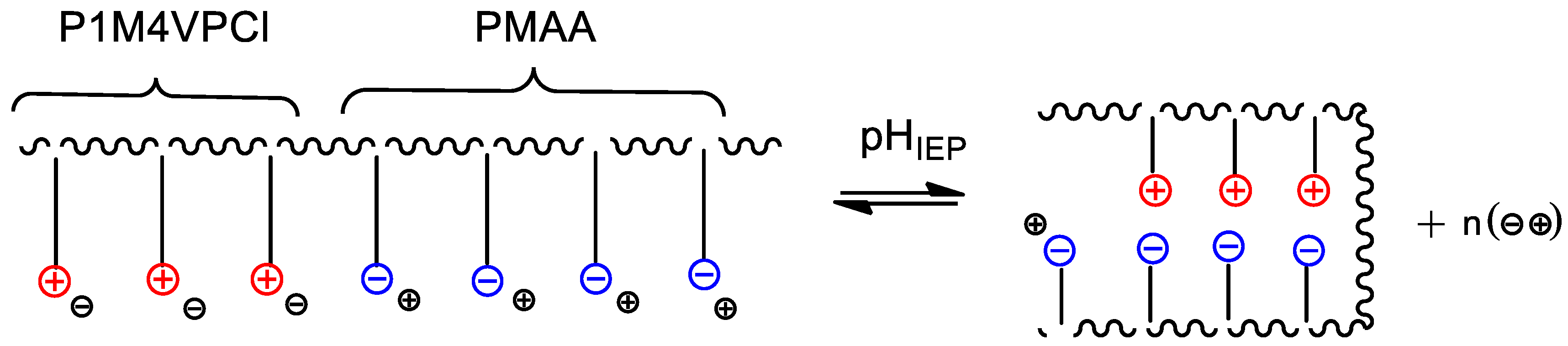
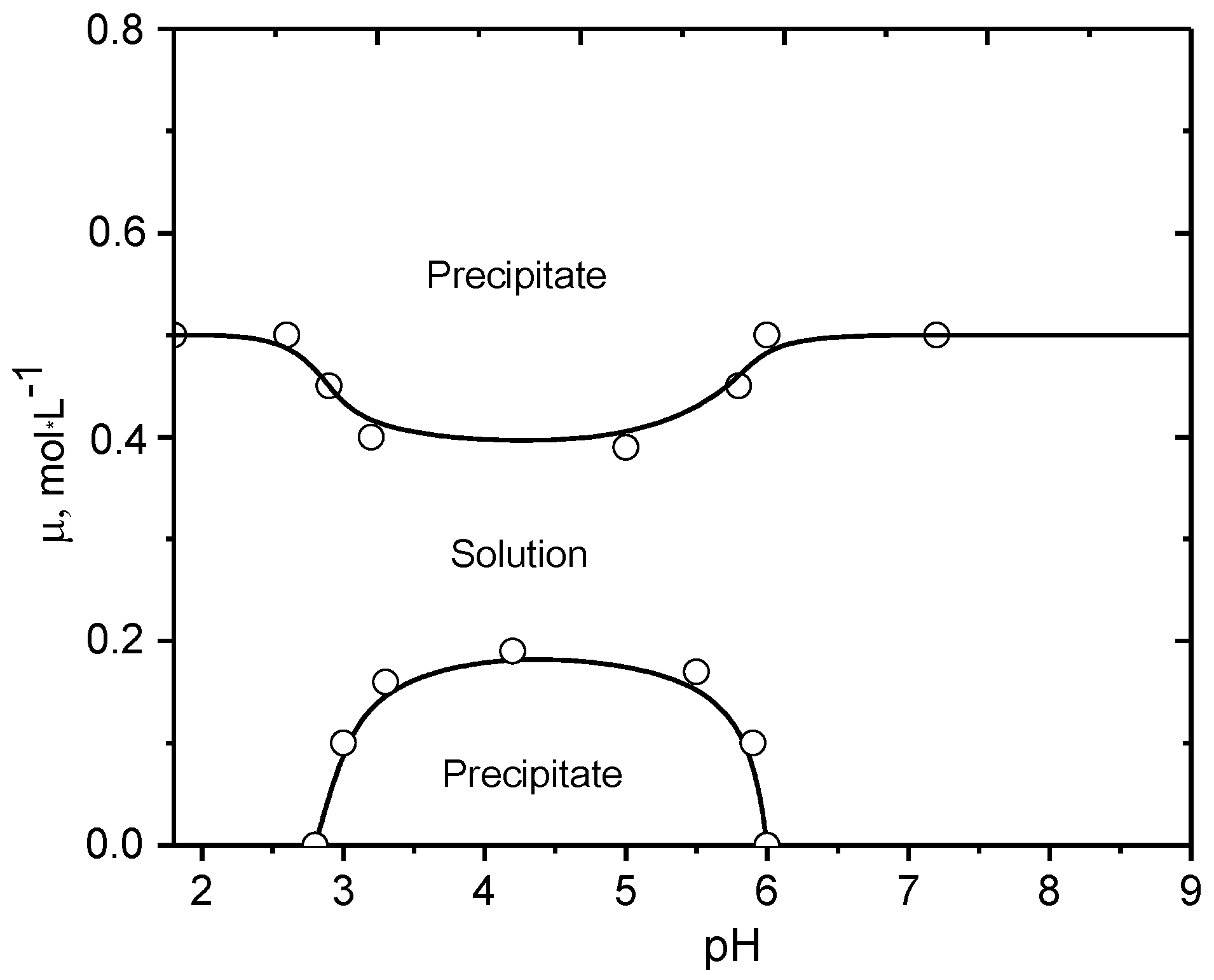
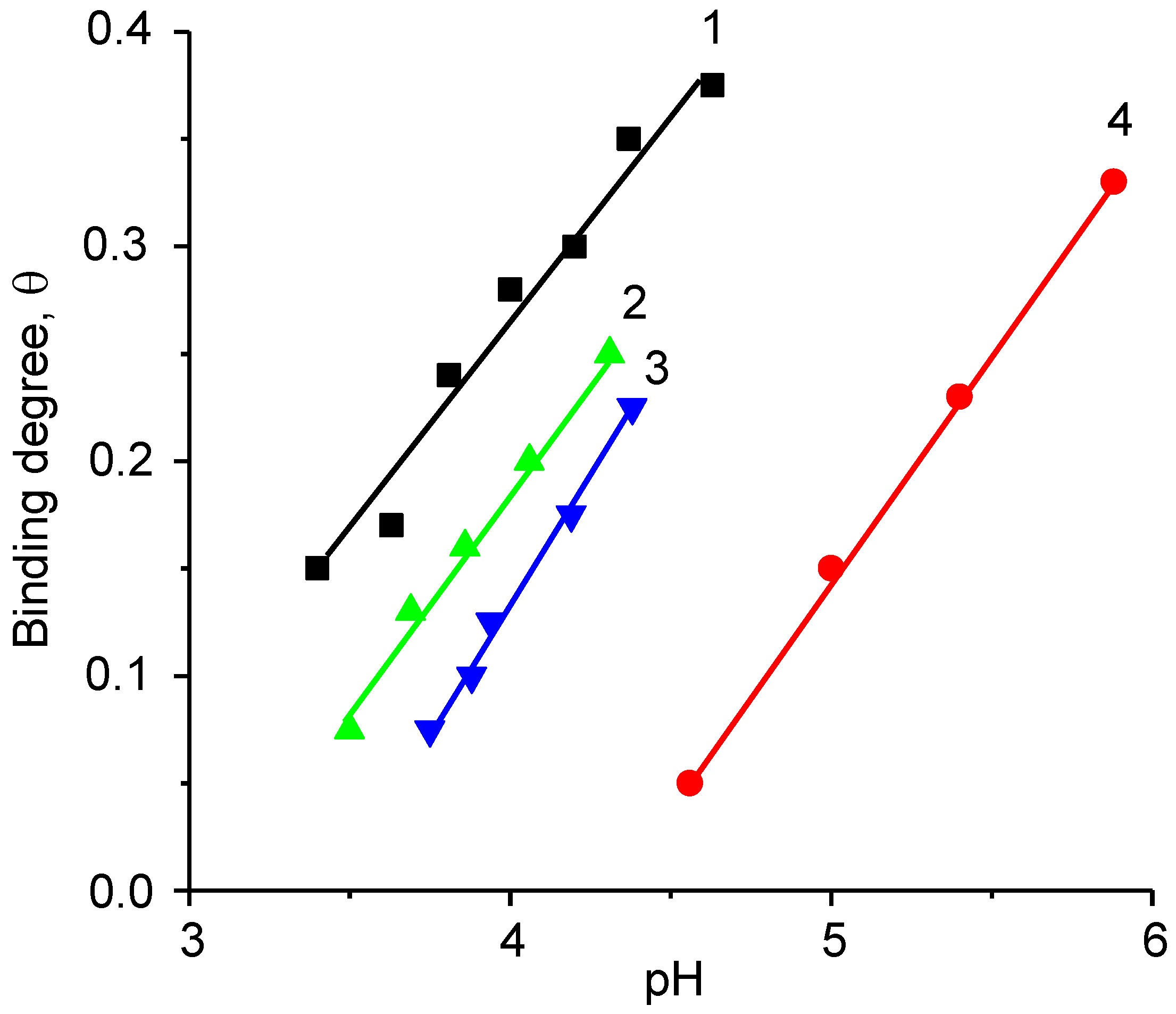
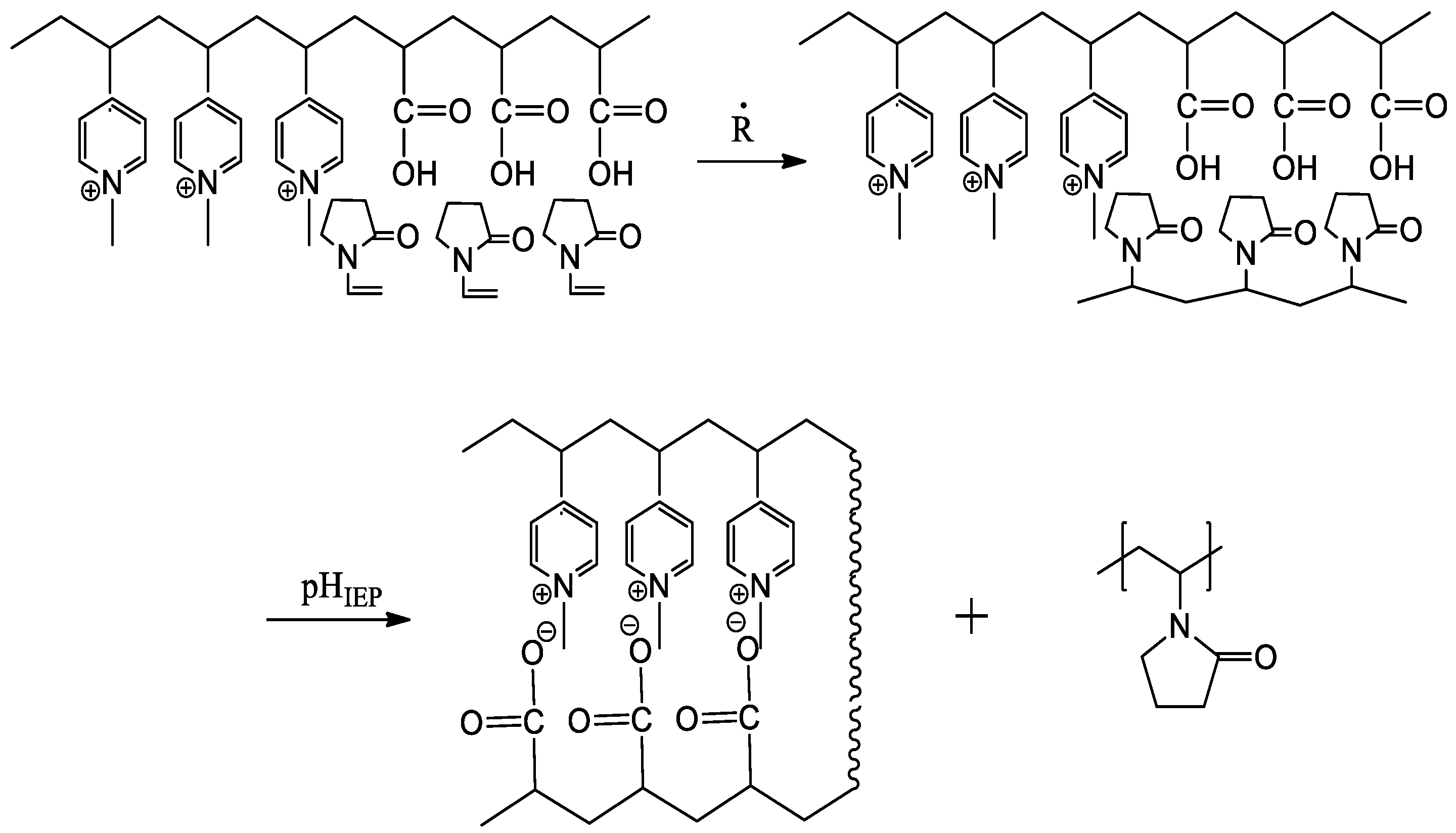
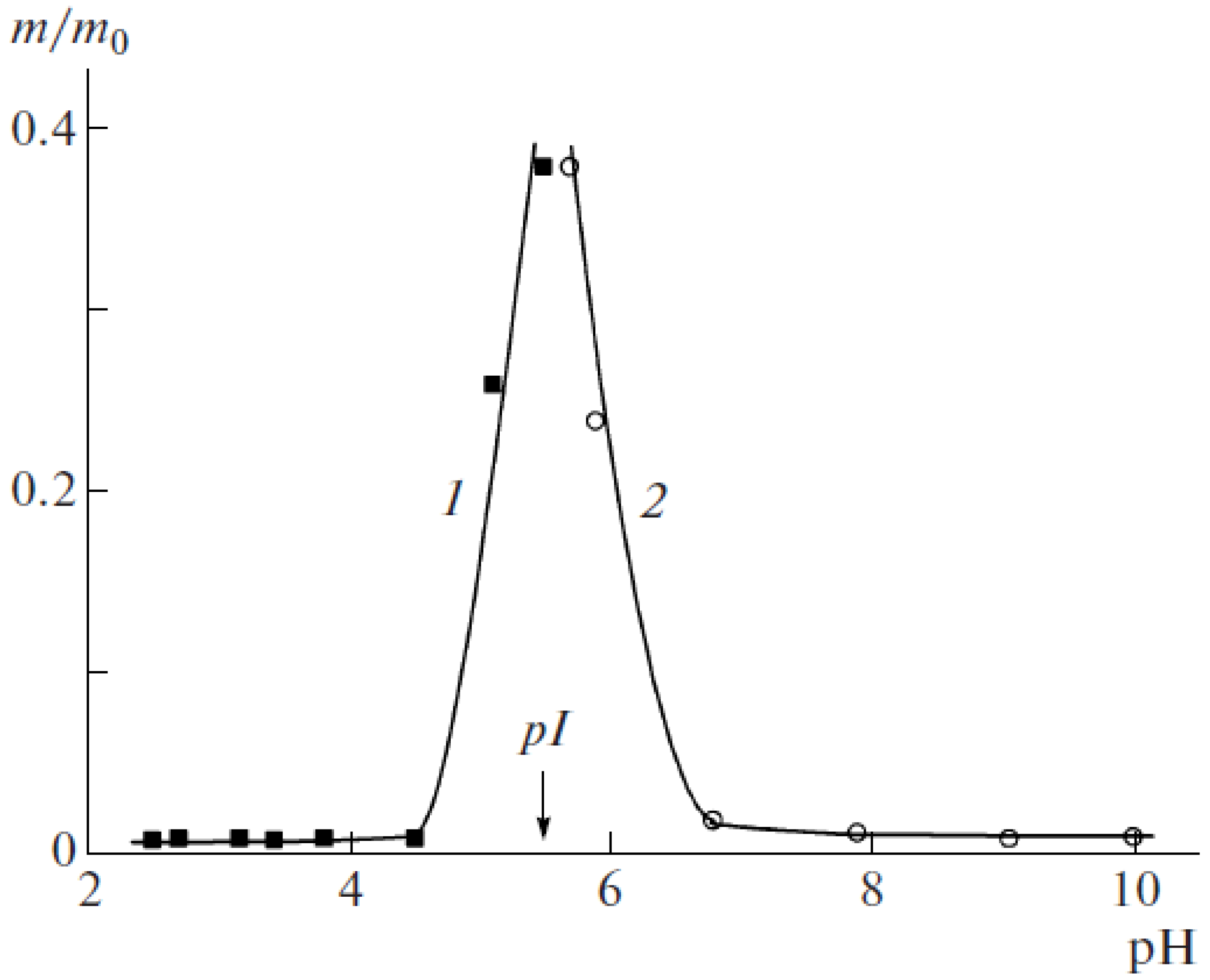
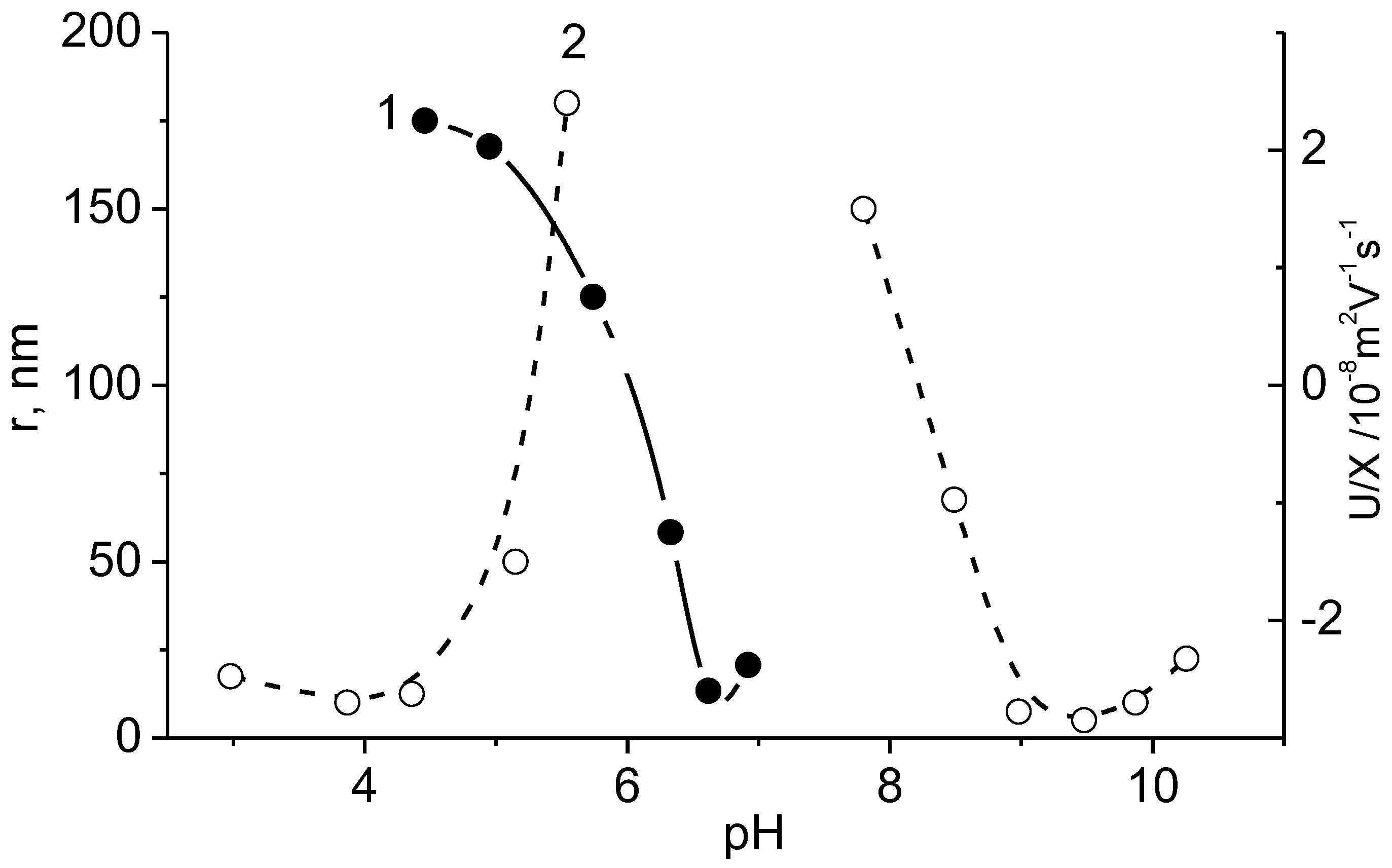
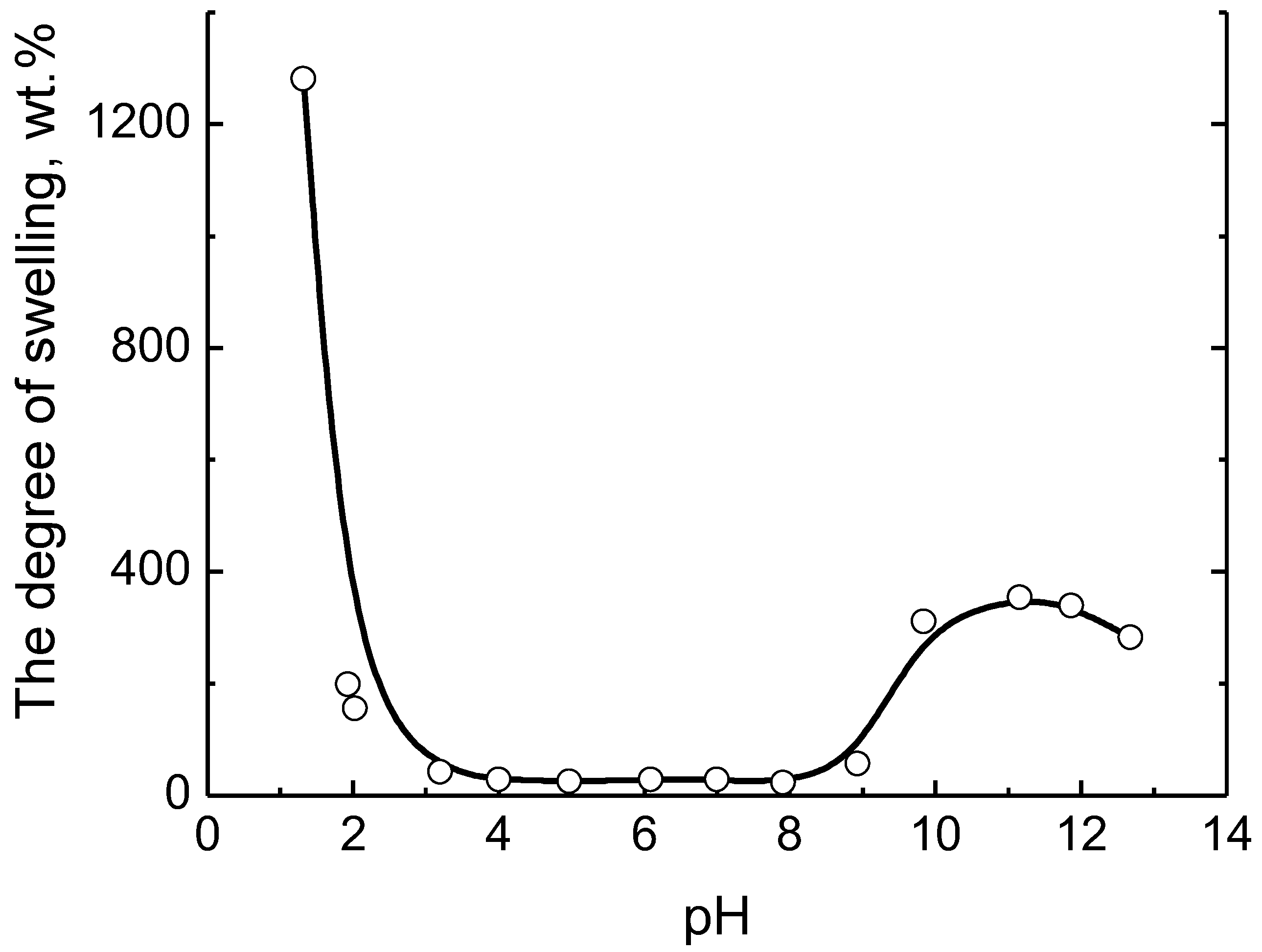
5. Realization of the ‘Isoelectric Effect’ at the Isoelectric Point (IEP) of Polyampholytes
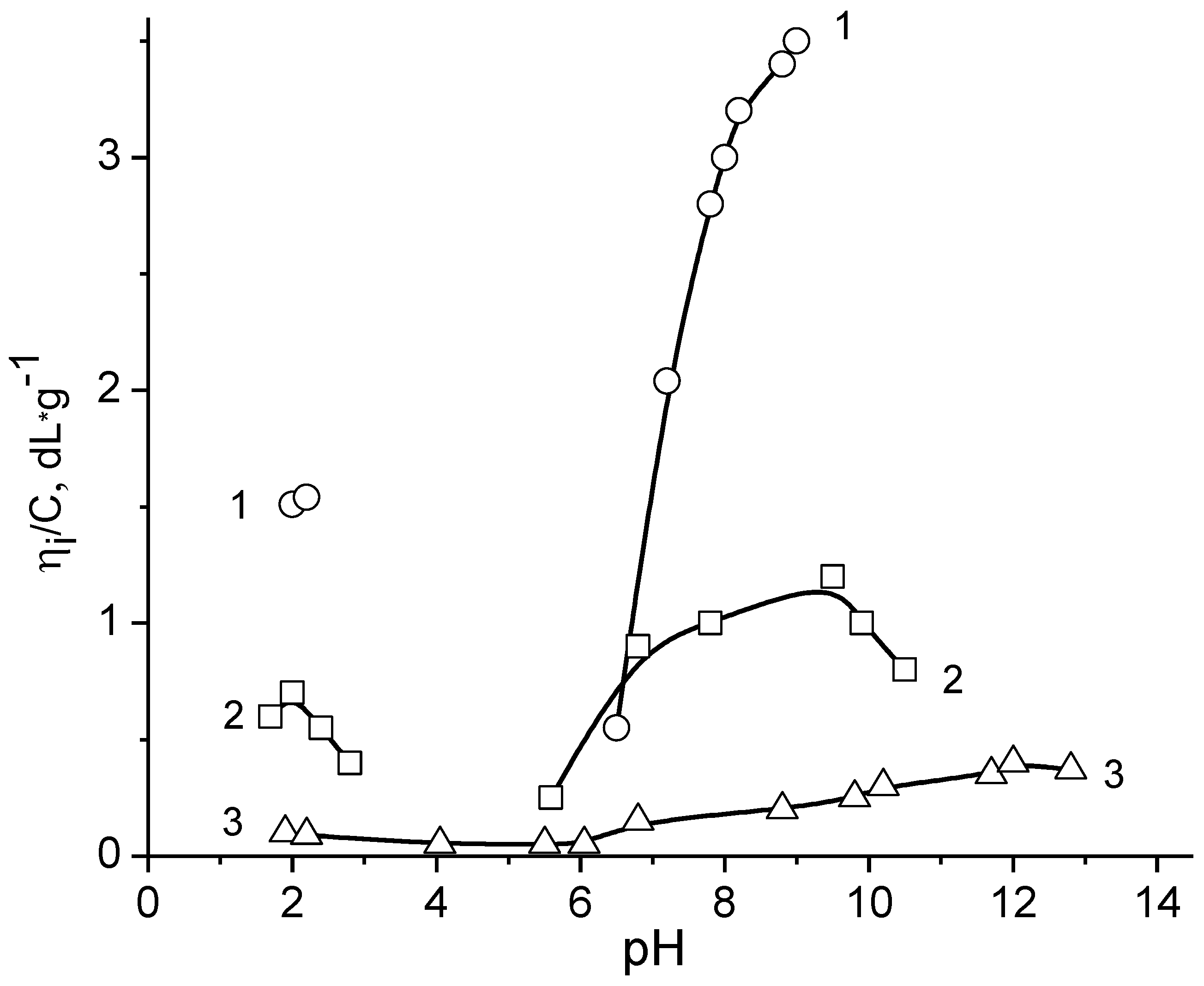
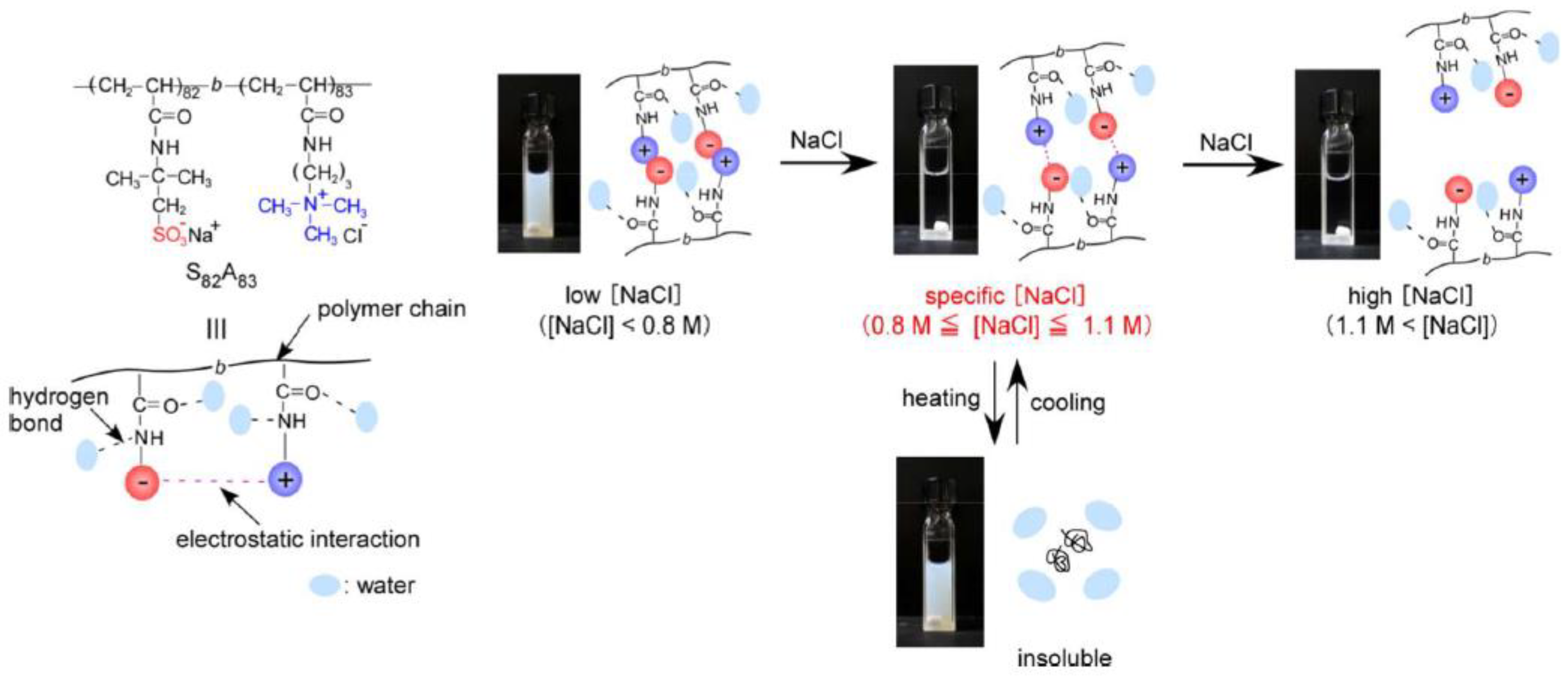
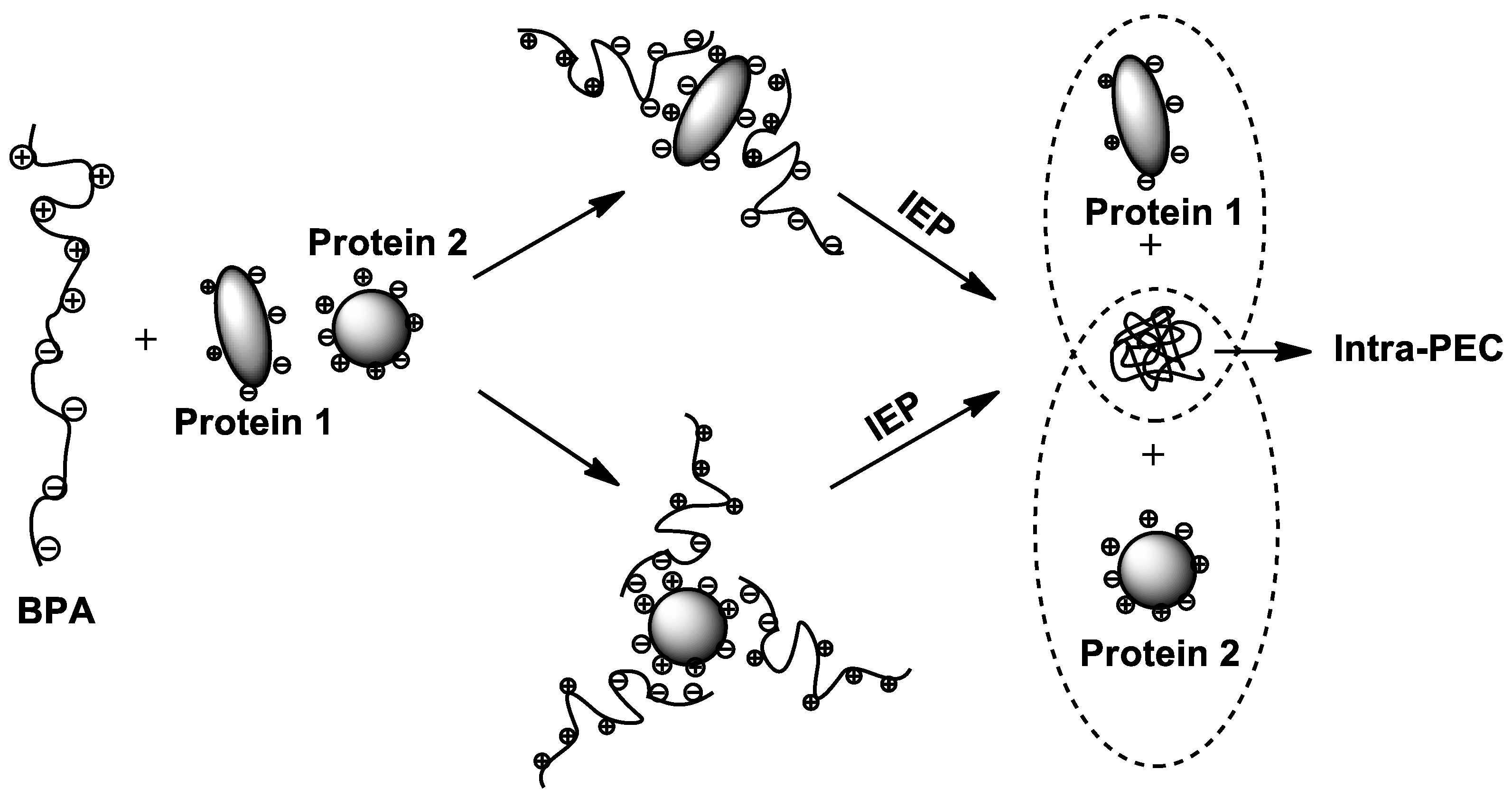
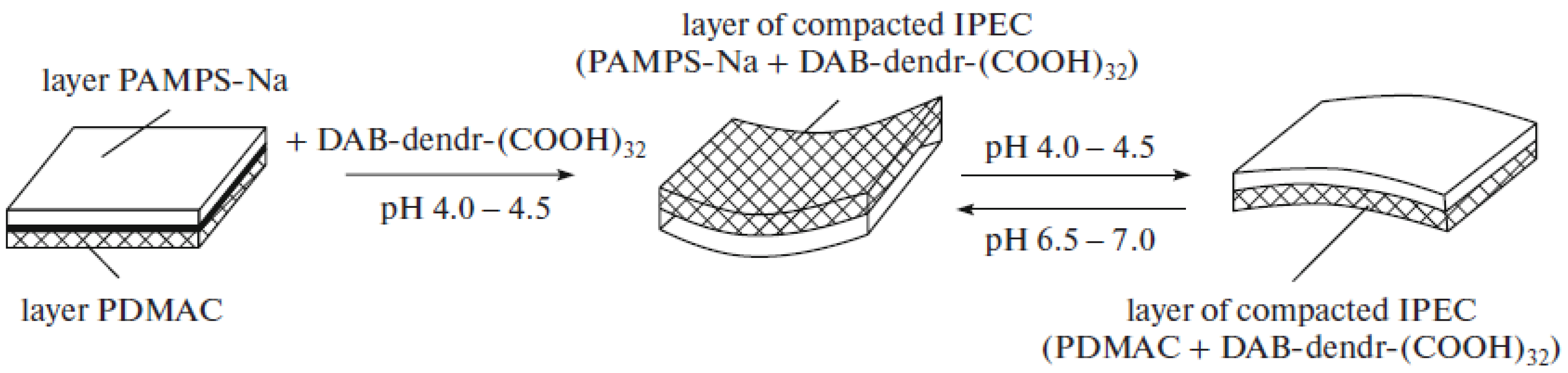
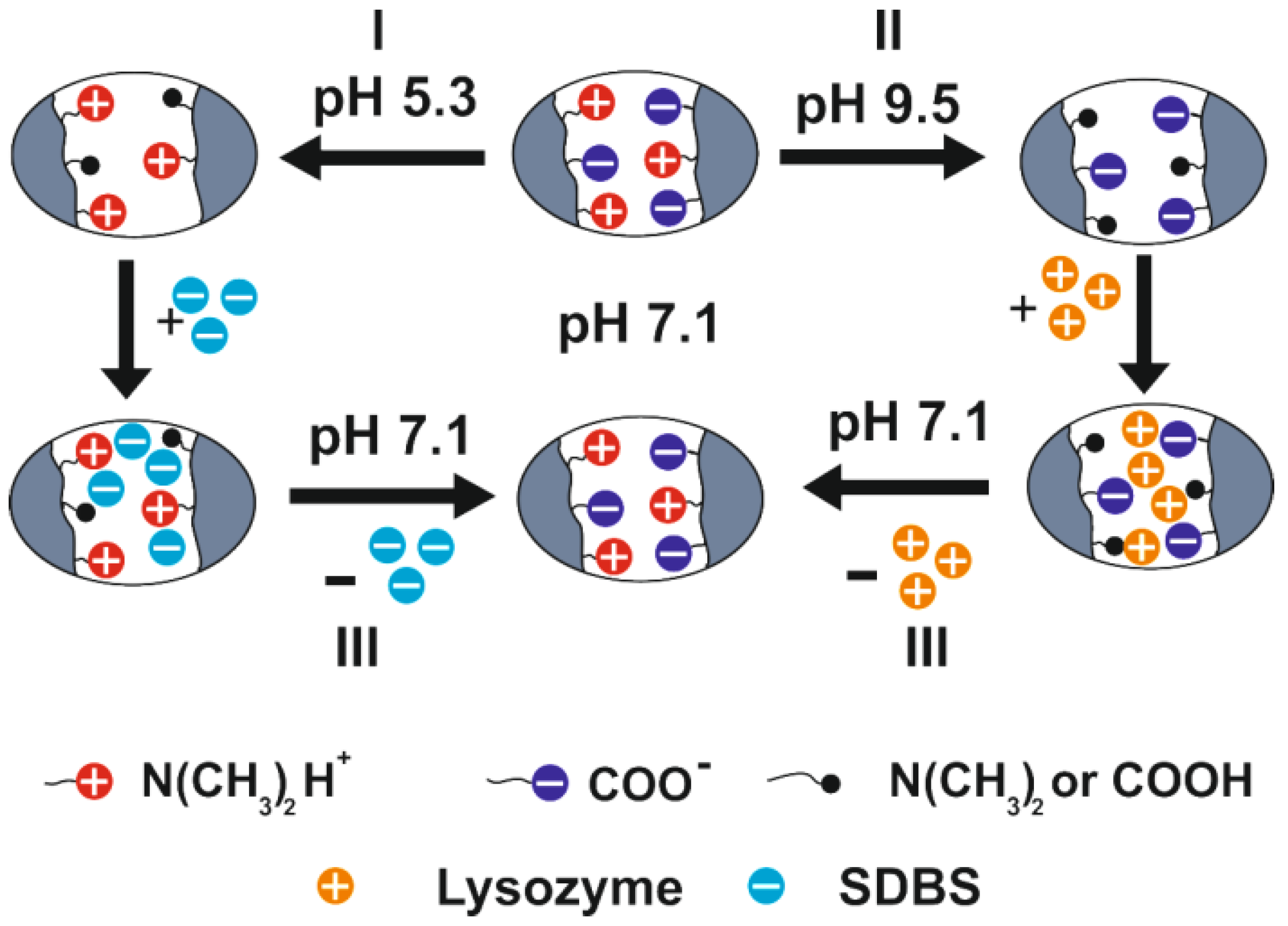
6. Amphoteric Behavior of Interpolyelectrolyte Complexes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Candau, F.; Joanny, J.F. Polyampholytes (Properties in aqueous solution). In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1996; pp. 5476–5488. [Google Scholar]
- Kudaibergenov, S. Polyampholytes: Synthesis, Characterization and Application; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; p. 220. ISBN 978-1-4613-5165-8. [Google Scholar]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Polyampholytes. J. Polym. Sci. Part B 2004, 42, 3513–3538. [Google Scholar] [CrossRef]
- Kudaibergenov, S. Polyampholytes. In Encyclopedia of Polymer Science and Technology; John Wiley Interscience: Hoboken, NJ, USA, 2008; pp. 1–30. [Google Scholar]
- Ciferri, A.; Kudaibergenov, S. Natural and synthetic polyampholytes. 1. Theory and basic structure. Macromol. Rapid Commun. 2007, 28, 1953–1968. [Google Scholar] [CrossRef]
- Hess, M.; Jones, R.G.; Kahovec, J.; Kitayama, T.; Kratochvíl, P.; Kubisa, P.; Mormann, W.; Stepto, R.F.; Tabak, D.; Vohlídal, J.; et al. Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC Recommendations 2006). Pure Appl. Chem. 2006, 78, 2067–2074. [Google Scholar] [CrossRef]
- Kudaibergenov, S.; Jaeger, W.; Laschewsky, A. Polymeric betaines: Synthesis, characterization and application. Adv. Polym. Sci. 2006, 201, 157–224. [Google Scholar]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Luca, C.; Neagu, V.; Vasiliu, S.; Barboiu, V. Synthetic polybetaines. Synthesis and properties. In Focus on Ionic Polymers; Dragan, E.S., Ed.; Research Signpost: Kerala, India, 2005; pp. 1–36. ISBN 81-7736-285-2. [Google Scholar]
- Ascoli, F.; Botre, C. Amphoteric behavior of a copolymer: N,N-diethylallylamine-acrylic acid. J. Polym. Sci. 1962, 62, S56–S59. [Google Scholar] [CrossRef]
- Masuda, S.; Minagawa, K.; Tsuda, M.; Tanaka, M. Spontaneous copolymerization of acrylic acid with 4-vinylpyridine and microscopic acid dissociation of the alternating copolymer. Eur. Polym. J. 2001, 37, 705–710. [Google Scholar] [CrossRef]
- Merle, Y. Synthetic polyampholytes. 5. Influence of nearest-neighbor interaction on potentiometric curves. J. Phys. Chem. 1987, 91, 3092–3098. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Zhaimina, G.M.; Sigitov, V.B.; Bekturov, E.A. Complexation of polyampholyte based on 1-vinylimidazole and acrylic acid with transition metal ions. In Proceedings of the 32nd International Symposium on Macromolecules, Kyoto, Japan, 1–5 August 1988; p. 741. [Google Scholar]
- Su, S.; Wu, R.; Huang, X.; Cao, L.; Wang, J. Effect of the anionic-group/cationic group ratio on the swelling behavior and controlled release of agrochemicals of the amphoteric, superabsorbent polymer poly(acrylic acid-co-diallyldimethylammonium chloride. J. Appl. Polym. Sci. 2006, 102, 986–989. [Google Scholar] [CrossRef]
- Lica, C.G.; Segărceanu, M.; Pleşca, M.; Rikabi, A.A.; Nechifor, G. Synthesis of a new polymer poly(styrene sulfonic acid-co-4-vinyl pyridine) for proton exchange membrane for fuel cell. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2014, 76, 151–158. [Google Scholar]
- Kudaibergenov, S.E.; Frolova, V.A.; Bekturov, E.A.; Rafikov, S.R. Interpolymer reactions with participation of block polyampholyte poly(1-methyl-4-vinylpyridinium chloride)-poly(methacrylic acid). Dokl. Akad. Nauk SSSR 1990, 311, 396–398. [Google Scholar]
- Bekturov, E.A.; Kudaibergenov, S.E.; Khamzamulina, R.E.; Frolova, V.A.; Nurgalieva, D.E.; Schulz, R.C.; Zöller, J. Phase behaviour of block-polyampholytes based on poly(methacrylic acid)-block-poly(1-methyl-4-vinylpyridinium chloride) in aqueous salt solutions. Makromol. Chem. Rapid Commun. 1992, 13, 225–229. [Google Scholar] [CrossRef]
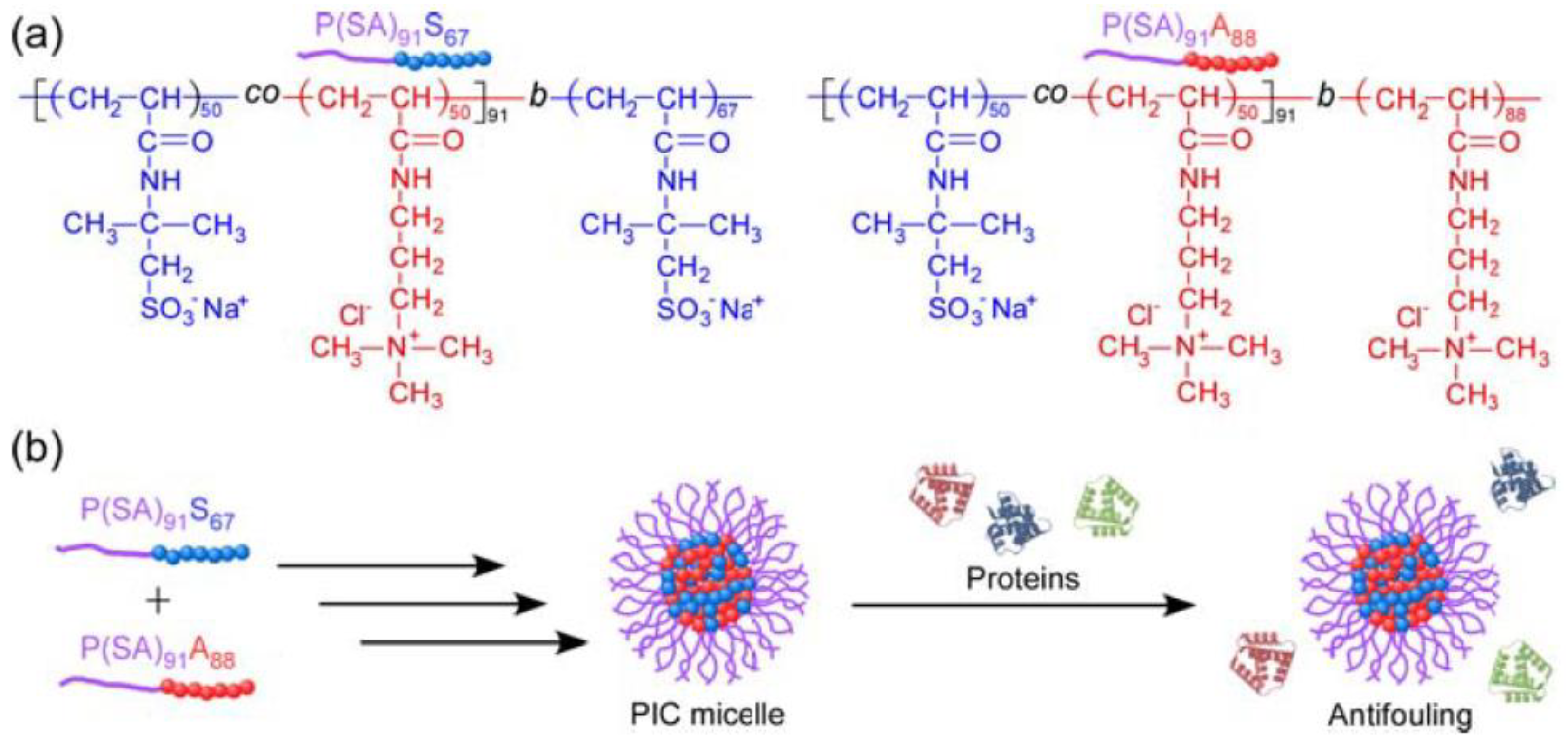
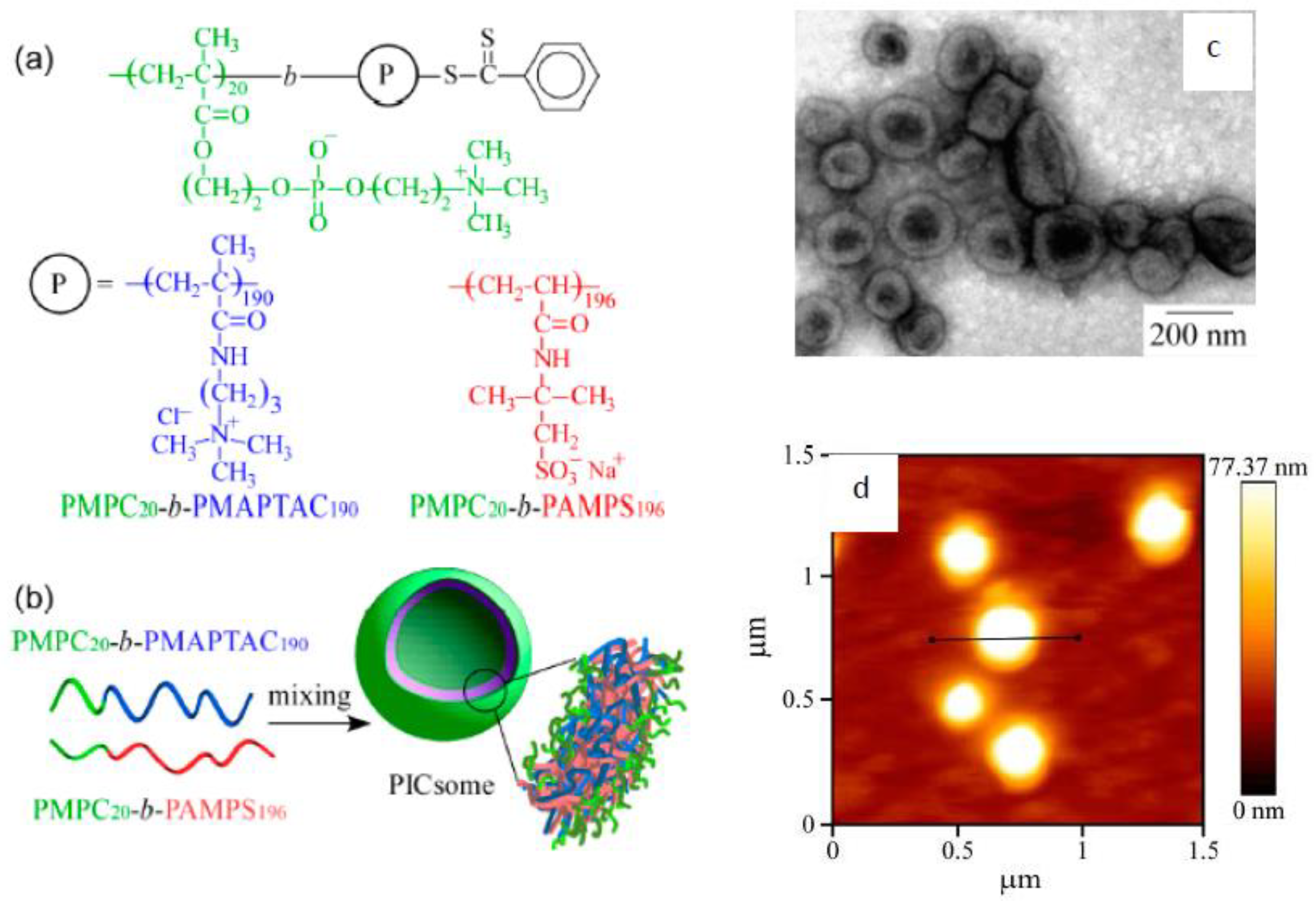
- Nakahata, R.; Yusa, S. Preparation of water-soluble polyion complex (PIC) micelles covered with amphoteric random copolymer shells with pendant sulfonate and quaternary amino groups. Polymers 2018, 10, 205. [Google Scholar] [CrossRef]
- Salamone, J.C.; Rice, W.C. Polyampholytes. In Encyclopedia of Polymer Science and Engineering; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; Wiley: New York, NY, USA, 1987; Volume 11, pp. 514–530. [Google Scholar]
- Tanford, C. Physical Chemistry of Macromolecules; John Wiley and Sons: New York, NY, USA, 1961; p. 710. [Google Scholar]
- Rice, S.A.; Nagasawa, M. Polyelectrolyte Solutions; Academic Press: London, UK; New York, NY, USA, 1961; p. 568. [Google Scholar]
- Armstrong, R.W.; Strauss, U.P. Polyelectrolytes. In Encyclopedia of Polymer Science and Technology, 1st ed.; Mark, H.F., Gaylord, N.G., Bikales, N.M., Eds.; Interscience Publishers: New York, NY, USA, 1969; Volume 10, pp. 781–861. [Google Scholar]
- Selegny, E.; Mandel, M.; Strauss, U.P. (Eds.) Polyelectrolytes; D. Reidel: Dordrecht, The Netherlands; Boston, MA, USA, 1974; p. 533. [Google Scholar]
- Eisenberg, H. Biological Macromolecules and Polyelectrolytes; Clarendon Press: Oxford, UK, 1976; p. 272. [Google Scholar]
- Dautzenberg, H.; Jaeger, W.; Koetz, J.; Philipp, B.; Seidel, C.; Stscherbina, D. Polyelectrolytes: Formation, Characterization, Application; Hanser: Munich, Germany, 1994; p. 343. [Google Scholar]
- Mandel, M. Aqueous solutions of polyelectrolytes. In Chemistry and Technology of Water-Soluble Polymers; Editor Finch, C.A., Ed.; Springer: Boston, MA, USA, 1983; pp. 179–192. [Google Scholar]
- Mandel, M. Polyelectrolytes. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Kroschwitz, J.I., Eds.; Wiley Interscience: New York, NY, USA, 1985; Volume 11, pp. 739–829. [Google Scholar]
- Bekturov, E.A.; Bimendina, L.A.; Kudaibergenov, S.E. Polyelectrolytes (Chain Models of Polyions). In Concise Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 5141–5146. [Google Scholar]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Muthukumar, M. 50th Anniversary Perspective: A perspective on polyelectrolyte solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [PubMed]
- Mathews, C.K.; Van Holde, K.E. Biochemistry; Benjamin/Cummings Publishing: Redwood City, CA, USA, 1990. [Google Scholar]
- Swann, D.A. The Joints and Synovial Fluid; Sokoloff, L., Ed.; Academic Press: New York, NY, USA, 1978; p. 407. [Google Scholar]
- Mattison, K.W.; Brittain, I.J.; Dubin, P.L. Protein-polyelectrolyte phase boundary. Biotechnol. Prog. 1995, 11, 632–637. [Google Scholar] [CrossRef]
- Jiang, J.; Prausnitz, J.M. Molecular thermodynamics for protein precipitation with a polyelectrolyte. J. Phys. Chem. 1999, 103, 5560–5569. [Google Scholar] [CrossRef][Green Version]
- Kokufuta, E. Complexation of proteins with polyelectrolytes in a salt-free system and biochemical characteristics of the resulting complexes. In Macromolecular Complexes in Chemistry and Biology; Dubin, P., Block, J., Davis, R., Schulz, D.N., Thies, C., Eds.; Springer: Berlin, Germany, 1995; pp. 300–325. [Google Scholar]
- Kokufuta, E. Functional immobilized biocatalysts. Prog. Polym. Sci. 1992, 17, 647–697. [Google Scholar] [CrossRef]
- Trubetskoy, V.S.; Hagstrom, J.E.; Budker, V.G.; Wolff, J.A.; Rozema, D.B.; Monahan, S.D. Compositions and methods for drug delivery using pH sensitive molecules. U.S. Patent 6,919,091, 19 July 2005. [Google Scholar]
- Zurick, K.M.; Bernards, M. Recent biomedical advances with polyampholyte polymers. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Khan, M.O.; Akesson, T.; Jonsson, B. Adsorption of polyampholytes to charged surfaces. Macromolecules 2001, 34, 4216–4221. [Google Scholar] [CrossRef]
- Edwards, S.F.; King, P.R.; Pincus, P. Phase change in polyampholytes. Ferroelectrics 1980, 30, 3–6. [Google Scholar] [CrossRef]
- Higgs, P.G.; Joanny, J.F. Theory of polyampholyte solutions. J. Chem. Phys. 1991, 94, 1543–1554. [Google Scholar] [CrossRef]
- Higgs, P.G.; Orland, H. Scaling behavior of polyelectrolytes and polyampholytes: Simulation by an ensemble growth method. J. Chem. Phys. 1991, 96, 4506–4518. [Google Scholar] [CrossRef]
- Wittmer, J.; Johner, A.; Joanny, J.F. Random and alternating polyampholytes. Europhys. Lett. 1993, 24, 263–268. [Google Scholar] [CrossRef]
- Everaers, R.; Johner, A.; Joanny, J.F. Complexation and precipitation in polyampholyte solutions. Europhys. Lett. 1997, 37, 275–280. [Google Scholar] [CrossRef][Green Version]
- Kantor, Y.; Kardar, M. Polymers with random self-interactions. Europhys. Lett. 1991, 14, 421–426. [Google Scholar] [CrossRef][Green Version]
- Kantor, Y.; Li, H.; Kardar, M. Conformations of polyampholytes. Phys. Rev. Lett. 1992, 69, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Kantor, Y.; Kardar, M. Excess charge in polyampholytes. Europhys. Lett. 1994, 27, 643–648. [Google Scholar] [CrossRef]
- Kantor, Y.; Kardar, M. Collapse of randomly self-interacting polymers. Europhys. Lett. 1994, 28, 169–174. [Google Scholar] [CrossRef][Green Version]
- Kantor, Y.; Kardar, M.; Li, H. Statistical mechanics of polyampholytes. Phys. Rev. E 1994, 49, 1383–1392. [Google Scholar] [CrossRef]
- Kantor, Y.; Kardar, M. Instabilities of charged polyampholytes. Phys. Rev. E 1995, 51, 1299–1312. [Google Scholar] [CrossRef]
- Kantor, Y.; Kardar, M. Randomly charged polymers: An exact enumeration study. Phys. Rev. E 1995, 52, 835–846. [Google Scholar] [CrossRef]
- Kantor, Y.; Kardar, M.; Ertas, D. Necklace model of randomly charged polymers. Phys. A Stat. Mech. Appl. 1998, 249, 301–306. [Google Scholar] [CrossRef]
- Ertas, D.; Kantor, Y. Randomly charged polymers, random walks, and their extremal properties. Phys. Rev. E 1996, 53, 846–860. [Google Scholar] [CrossRef]
- Levin, Y.; Barbosa, M.C. Conformational phase transition of a polyampholyte in a low dielectric solvent. Europhys. Lett. 1995, 31, 513–518. [Google Scholar] [CrossRef][Green Version]
- Diehl, A.; Barbosa, M.C.; Levin, Y. Neutral polyampholyte in an ionic solution. Phys. Rev. A 1996, 54, 6516–6525. [Google Scholar] [CrossRef]
- Gutin, A.M.; Shakhnovich, E.I. Effect of a net charge on the conformation of polyampholytes. Phys. Rev. E 1994, 50, R3322–R3325. [Google Scholar] [CrossRef]
- Angerman, H.J.; Shakhnovich, E. Freezing in polyampholyte globules: Influence of the long-range nature of the interaction. J. Chem. Phys. 1999, 111, 772–785. [Google Scholar] [CrossRef][Green Version]
- Dobrynin, A.V.; Rubinstein, M. Flory theory of a polyampholyte chain. J. Phys. II 1995, 5, 677–695. [Google Scholar] [CrossRef]
- Bratko, D.; Chakraborty, A.K. A numerical study of polyampholyte configuration. J. Phys. Chem. 1996, 100, 1164–1173. [Google Scholar] [CrossRef]
- Desouza, A.R.; Degreve, L. Monte Carlo simulation of polyampholyte chains. Mol. Simul. 1993, 11, 337–344. [Google Scholar] [CrossRef]
- Srivastava, D.; Muthukumar, M. Sequence dependence of conformations of polyampholytes. Macromolecules 1996, 29, 2324–2326. [Google Scholar] [CrossRef]
- Rabin, Y.; Panyukov, S.; Wilhelm, M. Energy landscapes of interacting randomly and periodically charged rigid polyampholytes. Acta Polym. 1998, 49, 544–548. [Google Scholar] [CrossRef]
- Shimizu, H.; Uehara, K.; Yamamoto, K.; Hiwatari, Y. Structural phase transition of di-block polyampholyte. Mol. Simul. 1999, 22, 285–301. [Google Scholar] [CrossRef]
- Lee, N.; Obukhov, S. Multiple conformations in polyampholytes. Eur. Phys. J. B 1998, 1, 371–376. [Google Scholar] [CrossRef]
- Long, D.; Dobrynin, A.V.; Rubinstein, M.; Ajdari, A. Electrophoresis of polyampholytes. J. Chem. Phys. 1998, 108, 1234–1240. [Google Scholar] [CrossRef]
- Moldakarimov, S.B.; Kramarenko, E.Y.; Khokhlov, A.R.; Kudaibergenov, S.E. Formation of salt bonds in polyampholyte chains. Macromol. Theory Simul. 2001, 10, 780–788. [Google Scholar] [CrossRef]
- Muller-Nedebock, K.K.; Vilgis, T.A. Collective dynamics of random polyampholytes. J. Chem. Phys. 1999, 110, 4651–4657. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Mozuelos, P.; de la Cruz, M.O. Random phase approximation for complex charged systems: Application to copolyelectrolytes (polyampholytes). J. Chem. Phys. 1994, 100, 507–517. [Google Scholar] [CrossRef]
- Baumketner, A.; Shimizu, H.; Isobe, M.; Hiwatari, Y. Helix transition in di-block polyampholyte. J. Phys. Condens. Matter 2001, 13, 10279–10291. [Google Scholar] [CrossRef]
- Yamada, Y.; Ueda, Y.; Kataoka, Y. Replica Exchange Monte Carlo simulations for folding of di-block polyampholyte. J. Comput. Chem. Jpn. 2005, 4, 127–130. [Google Scholar] [CrossRef]
- Shusharina, N.P.; Zhulina, E.B.; Dobrynin, A.V.; Rubinstein, M. Scaling theory of diblock polyampholyte solutions. Macromolecules 2005, 38, 8870–8881. [Google Scholar] [CrossRef]
- Guskova, O.A.; Ryabova, O.A.; Pavlov, A.S. Computer modeling of coil-globule phase transition in polyampholyte systems. Struct. Dyn. Mol. Syst. 2003, 10, 269–272. [Google Scholar]
- Jiang, J.; Feng, J.; Liu, H.; Hu, Y. Phase behavior of polyampholytes from charged hard-sphere chain model. J. Chem. Phys. 2006, 124, 144908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rubinstein, M. Regimes of conformational transitions of a diblock polyampholyte. Macromolecules 2006, 39, 5897–5912. [Google Scholar] [CrossRef]
- Dubin, P.; Block, J.; Davis, R.; Schulz, D.N.; Thies, C. (Eds.) Macromolecular Complexes in Chemistry and Biology; Springer: Berlin, Germany, 1995. [Google Scholar]
- Izumrudov, V.I.; Zhiryakova, M.V.; Kudaibergenov, S.E. Controllable stability of DNA-containing polyelectrolyte complexes in water-salt solutions. Biopolymers 1999, 52, 94–108. [Google Scholar] [CrossRef]
- Joanny, J.F. Adsorption of a polyampholyte chain. J. Phys. II 1994, 4, 1281–1288. [Google Scholar] [CrossRef]
- Van der Eijik, J.M.; Nolte, R.J.M.; Drenth, W.; Hezemans, A.M.F. Optically active polyampholytes derived from L- and D-carbylanayl-L.-histidine. Macromolecules 1980, 13, 1391–1397. [Google Scholar] [CrossRef]
- Jeon, J.; Dobrynin, A.V. Molecular dynamics simulations of polyampholyte-polyelectrolyte complexes in solutions. Macromolecules 2005, 38, 5300–5312. [Google Scholar] [CrossRef]
- Jeon, J.; Dobrynin, A.V. Molecular dynamics simulations of polyampholyte-polyelectrolyte complexes. Effect of solvent quality and salt concentration. J. Phys. Chem. 2006, 110, 24652–24665. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, S.J.; Metzler, R.; Cherstvy, A.G. Critical adsorption of polyelectrolytes onto charged Janus nanospheres. Phys. Chem. Chem. Phys. 2014, 16, 15539–15550. [Google Scholar] [CrossRef] [PubMed]
- Caetano, D.L.Z.; de Carvalho, S.J.; Metzler, R.; Cherstvy, A.G. Critical adsorption of periodic and random polyampholytes onto charged surfaces. Phys. Chem. Chem. Phys. 2017, 19, 23397–23413. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.E.; Nuraje, N.; Khutoryanskiy, V.V. Amphoteric nano-, micro-, and macrogels, membranes, and thin films. Soft Matter 2012, 8, 9302–9321. [Google Scholar] [CrossRef]
- Nair, A.K.N.; Jimenez, A.M.; Sun, S. Complexation behavior of polyelectrolytes and polyampholytes. J. Phys. Chem. B 2017, 121, 7987–7998. [Google Scholar] [CrossRef] [PubMed]
- Goloub, T.; Keizer, A.; Stuart, M.A.C. Association behavior of ampholytic diblock copolymers. Macromolecules 1999, 32, 8441–8446. [Google Scholar] [CrossRef]
- Varoqui, R.; Tran, Q.; Pefferkorn, E. Polycation-polyanion complexes in the linear diblock copolymer of poly(styrene sulfonate)/poly(2-vinylpyridinium) salt. Macromolecules 1979, 12, 831–835. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Kudaibergenov, S.E.; Frolova, V.A.; Schultz, R.S.; Zoller, J. Conformational and complex formation ability of poly(methacrylic acid)-block-poly(1-methyl-4-vinylpyridinium chloride). Makromol. Chem. 1990, 191, 457–463. [Google Scholar] [CrossRef]
- Lifshits, I.M.; Grosberg, A.Y.; Khokhlov, A.R. Volume interactions in the statistical physics of a polymer macromolecule. Sov. Phys. Usp. 1979, 22, 123–142. [Google Scholar] [CrossRef]
- Bazt, M.R.; Skorikova, E.E.; Vikhoreva, L.S.; Galbraikh, L.S. Dilute solution properties of carboxymethyl ester of chitosan. Vysokomol. Soed. Ser. A 1990, 32, 805–809. [Google Scholar]
- Waldo, A.M.; Carlos, P.C. Preparation of a novel polyampholyte from chitosan and citric acid. Makromol. Chem. Rapid Commun. 1993, 14, 73–78. [Google Scholar]
- Patrickios, C.S.; Hertler, W.R.; Abbot, N.L.; Hatton, T.A.; Diblock, A.B.C. Triblock, and random methacrylic polyampholytes: Synthesis by group transfer polymerization and solution behavior. Macromolecules 1994, 27, 930–937. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Sharma, L.R.; Armes, S.P.; Billingham, N.C. Precipitation of a water-soluble ABC triblock methacrylic polyampholyte: Effects of time, pH, polymer concentration, salt type and concentration, and presence of a protein. Langmuir 1999, 15, 1613–1620. [Google Scholar] [CrossRef]
- Santos-Rosas, R.; Licea-Claverie, A.; Arndt, K.F. Statistical copolymers of methacrylic acid derivatives with hydrophobic spacers and N,N’- dimethylaminoethylmethacrylate: New associating polyampholytes. J. Mex. Chem. Soc. 2006, 50, 164–174. [Google Scholar]
- Giebeler, E.; Stadler, R. ABC triblock polyampholytes containing a neutral hydrophobic block, a polyacid and a polybase. Macromol. Chem. Phys. 1997, 198, 3816–3825. [Google Scholar] [CrossRef]
- Kawata, Y.; Kozuka, S.; Yusa, S. Thermo-responsive behavior of amphoteric diblock copolymers bearing sulfonate and quaternary amino pendant groups. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Dyakonova, M.A.; Berezkin, A.V.; Kyriakos, K.; Gkermpoura, S.; Popescu, M.T.; Filippov, S.K.; Stepanek, P.; Di, Z.; Tsitsilianis, C.; Papadakis, C.M. Salt-induced changes in triblock polyampholyte hydrogels: Computer simulations and rheological, structural and dynamic characterization. Macromolecules 2015, 48, 8177–8189. [Google Scholar] [CrossRef]
- Dyakonova, M.A.; Gotzamanis, G.; Niebuur, B.-J.; Vishnevetskaya, N.S.; Raftopoulos, K.N.; Di, Z.; Filippov, S.K.; Tsitsilianis, C.; Papadakis, C.M. pH responsiveness of hydrogels formed by telechelic polyampholytes. Soft Matter 2017, 13, 3568–3579. [Google Scholar] [CrossRef] [PubMed]
- Gotzamanis, G.; Papadimitriou, K.; Tsitsilianis, C. Design of a C-b-(A-co-B)-b-C telechelic polyampholyte pH-responsive gelator. Polym. Chem. 2016, 7, 2121–2129. [Google Scholar] [CrossRef]
- Papadakis, C.M.; Tsitsilianis, C. Responsive hydrogels from associative block copolymers: Physical gelling through polyion complexation. Gels 2017, 3, 3. [Google Scholar] [CrossRef]
- Kudaibergenov, S. Recent advances in the study of synthetic polyampholytes in solutons. Adv. Polym. Sci. 1999, 144, 116–197. [Google Scholar]
- Bixler, H.A.; Michaelis, A.S. Polyelectrolyte complexes. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Gaylord, N.G., Bikales, N.M., Eds.; Interscience Publishers: New York, NY, USA, 1969; Volume 10, p. 765. [Google Scholar]
- Kabanov, V.A.; Zezin, A.B. Soluble interpolymeric complexes as a new class of synthetic polyelectrolytes. Pure Appl. Chem. 1984, 56, 343–354. [Google Scholar] [CrossRef]
- Philipp, B.; Dautzenberg, H.; Linow, K.J.; Koetz, J.; Dawydoff, W. Polyelectrolyte complexes. Recent development, open problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Tsuchida, E.; Abe, K. Interactions between macromolecules and intermacromolecular complexes. Adv. Polym. Sci. 1982, 45, 1–119. [Google Scholar]
- Bekturov, E.A.; Bimendina, L.A. Interpolymer complexes. Adv. Polym. Sci. 1981, 43, 100–147. [Google Scholar]
- Kabanov, V.A. Polyelectrolyte complexes in solution and in the condensed phase. Uspekhi Khimii 2005, 74, 5–23. [Google Scholar] [CrossRef]
- van der Gucht, J.; Spruijt, E.; Lemmers, M.; Stuart, A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- De Morais, W.A.; Silva, G.T.M.; Nunes, A.O.; Neto, W.; Pereira, M.R.; Fonseca, J.L.S. Interpolyelectrolyte complex formation: From lyophilic to lyophobic colloids. Colloids Surf. A 2016, 498, 112–120. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Muller, A.H.E.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Harikumar, S.L.; Kaur, A. Interpolyelectrolyte complexes as prospective carriers for controlled drug deliver. Int. Res. J. Pharm. 2012, 3, 58–62. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Kudaibergenov, S.E.; Rafikov, S.R. The properties of solutions and complex formation reactions of amphoteric polyelectrolytes. Russ. Chem. Rev. 1991, 60, 410–419. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Staikos, G. (Eds.) Hydrogen Bonded Interpolymer Complexes: Formation, Structure and Applications; World Scientific Pub Co Inc.: Singapore, 2009; p. 376. [Google Scholar]
- Khutoryanskiy, V.V.; Smyslov, R.Y.; Yakimansky, A.V. Modern methods for studying polymer complexes in aqueous and organic solutions. Polym. Sci. Ser. A 2018, 60, 533–576. [Google Scholar] [CrossRef]
- Savinova, I.V.; Fedoseeva, N.A.; Evdakov, V.P.; Kabanov, V.A. Polymer-polymer complexes with participation of synthetic polyampholytes. Vysokomol. Soedin. Ser. A 1976, 18, 2050–2057. [Google Scholar]
- Skorikova, E.E.; Vikhoreva, G.A.; Kalyuzhnaya, R.I.; Zezin, A.B.; Gal’braikh, L.S.; Kabanov, V.A. Polyelectrolyte complexes based on chitosan. Vysokomol. Soedin. Ser. A 1988, 30, 44–49. [Google Scholar] [CrossRef]
- Legkunetz, R.E.; Dzhagiparova, A.T.; Kudaibergenov, S.E.; Bekturov, E.A.; Shayakhmetov, S.S. Study of complexation reaction of polyampholytes based on 1,2,5-trimethyl-4-vinylethynylpiperidinol-4 and acrylic acid with poly(acrylic acid) in aqueous solution. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1982, 6, 54–59. [Google Scholar]
- Kudaibergenov, S.E.; Sigitov, V.B.; Bekturov, E.A.; Koetz, J.; Philipp, B. Studing of novel polyampholytes based on allylamines and maleic acid by 13C NMR and Raman spectroscopy. Vysokomol. Soedin. Ser. B 1989, 31, 132–136. [Google Scholar]
- Khamzamulina, R.E.; Kudaibergenov, S.E.; Bekturov, E.A.; Koetz, J.; Philipp, B.; Hahn, M. Study of interaction of synthetic polyampholyte on the basis of maleic acid and allylamine. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1990, 1, 45–48. [Google Scholar]
- Hahn, M.; Koetz, J.; Ebert, A.; Schmolke, R.; Philipp, B.; Kudaibergenov, S.; Sigitov, V.; Bekturov, E. Synthese, charakterisierung und symplexbildung von polyampholyten aus ungesattigten dicarbonsaure und allylaminderivaten. 2.Mitt.: Spektroskopische untersuchungen an polymeren aus maleinsaure und allylaminderivaten. Acta Polym. 1989, 40, 331–335. [Google Scholar] [CrossRef]
- Koetz, J.; Philipp, B.; Hahn, M.; Evert, A.; Schmolke, R.; Kudaibergenov, S.E.; Sigitov, V.B.; Bekturov, E.A. Synthese, charakterisierung und symplexbildung von polyampholyten aus ungesattigten dicarbonsaure und allylaminderivaten, 4. Mitt.: Spektroskopische untersuchungen und symplexbildung zwischen poly(dimethyldiallylammoniumchlorid) und einem ampholytischen copolymer aus maleinsaure und methyldiallylamin. Acta Polym. 1989, 40, 405–407. [Google Scholar]
- Elschner, T.; Heinze, T. A promising cellulose-based polyzwitterion with pH-sensitive charges. Beilstein J. Org. Chem. 2014, 10, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Ibraeva, Z.E.; Hahn, M.; Jaeger, W.; Bimendina, L.A.; Kudaibergenov, S.E. Solution Properties and Complexation of Polyampholytes based on N,N-Dimethyldiallylammonium Chloride and Maleic Acid or Alkyl (Aryl) Derivatives of Maleamic Acids. Macromol. Chem. Phys. 2004, 205, 2464–2472. [Google Scholar] [CrossRef]
- Zezin, A.; Rogacheva, V.; Skobeleva, V.; Kabanov, V. Controlled uptake and release of proteins by polyelectrolyte gels. Polym. Adv. Technol. 2001, 13, 919–925. [Google Scholar] [CrossRef]
- Zansokhova, M.F.; Rogacheva, V.B.; Gulyaeva, Zh.G.; Zezin, A.B.; Joosten, J.; Brekman, J. Interaction of ampholyte dendrimers with linear polyelectrolytes. Polym. Sci. Ser. A. 2008, 50, 656–664. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Abbot, N.L.; Foss, R.P.; Hatton, T.A. Synthetic polyampholytes for protein partitioning in two-phase aqueous polymer systems. In New Developments in Bioseparation; Ataai, M.M., Sikdar, S.K., Eds.; AIChE Symposium Series; American Institute of Chemical Engineers: New York, NY, USA, 1992; Volume 88, pp. 80–88. [Google Scholar]
- Patrickios, C.S.; Hertler, W.R.; Hatton, T.A. Phase behavior of random and ABC triblock methacrylic polyampholytes with poly(vinyl alcohol) in water: Effect of pH and salt. Fluid Phase Equilib. 1995, 108, 243–254. [Google Scholar] [CrossRef]
- Chen, L.; Honma, Y.; Mizutani, T.; Liaw, D.-J.; Gong, J.P.; Osada, Y. Effect of polyelectrolyte complexation on the UCST of zwitterionic polymer. Polymer 2000, 41, 141–147. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Zelikin, A.N.; Jaeger, W.; Bohrisch, J. Interpolyelectrolyte reactions in solutions of polycarboxybetaines. J. Phys. Chem. B 2003, 107, 7982–7988. [Google Scholar] [CrossRef]
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir 2013, 29, 9651–9661. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Ishihara, K.; Kappl, M.; Fujii, S.; Nakamura, Y.; Yusa, S. Polyion complex vesicles with solvated phosphobetaine shells formed from oppositely charged diblock copolymers. Polymers 2017, 9, 49. [Google Scholar] [CrossRef]
- Nakahata, R.; Yusa, S. Solution properties of amphoteric random copolymers bearing pendant sulfonate and quaternary ammonium groups with controlled structures. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Ibraeva, Z.E.; Hahn, M.; Jaeger, W.; Laschewsky, A.; Bimendina, L.A.; Kudaibergenov, S.E. Swelling behavior and complex formation ability of ternary amphoteric gels based on allylamine derivatives and maleic acid. Macromol. Mater. Eng. 2005, 290, 769–777. [Google Scholar] [CrossRef]
- Guang, C.; Guojun, W.; Liming, W.; Zhang, S.; Su, Z. Layer-by-layer assembly of single-charged ions with a rigid polyampholyte. Chem. Commun. 2008, 15, 1741–1743. [Google Scholar] [CrossRef]
- Tokuda, Y.; Miyaqishima, T.; Tomida, K.; Wanq, B.; Takahashi, S.; Sato, K.; Anzai, J. Dual pH-sensitive layer-by-layer films containing amphoteric poly(diallylamine-co-maleic acid). J. Colloid Interface Sci. 2013, 399, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tokuda, Y.; Tomida, K.; Takahashi, S.; Sato, K. Use of amphoteric copolymer films as sacrificial layers for constructing free-standing layer-by-layer films. Materials 2013, 6, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.E.; Bekturov, E.A. Peculiarities of synthetic polyampholytes at the isoelectric point. Vestnik Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1985, 4, 36–38. [Google Scholar]
- Kudaibergenov, S.E.; Bekturov, E.A. New properties of synthetic polyampholytes in solution. Vestnik Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1988, 12, 41–44. [Google Scholar]
- Bekturov, E.A.; Kudaibergenov, S.E.; Dzhumadilov, T.K. Complexes of water-soluble polymers. In Ionic Polymers; Ordered Polymers for High Performance Materials, Biomaterials; Materials Research Society: Pittsburg, CA, USA, 1989; pp. 19–33. [Google Scholar]
- Koetz, J.; Philipp, B.; Kudaibergenov, S.E.; Bekturov, E.A. Untersuchungen zur gleichzeitigen homo-und heterosymplexbildung mit synthetischen polyampholyten vom pendant typ. Acta Polym. 1991, 42, 181–185. [Google Scholar] [CrossRef]
- Koetz, J.; Hahn, M.; Philipp, B.; Bekturov, E.A.; Kudaibergenov, S.E. Inter- and intramolecular interactions in polyelectrolyte complex formation with polyampholytes. Makromol. Chem. 1993, 194, 397–409. [Google Scholar] [CrossRef]
- Kulagina, E.M. Intermolecular Association of Water-Soluble Amphoteric Polyelectrolytes Based on (Meth)acrylic Acid with Cationic Surfactants. Ph.D. Thesis, Kazan State Technological University, Kazan, Russia, 1995. [Google Scholar]
- Kudaibergenov, S.E.; Zhaimina, G.M.; Bekturov, E.A. Recovery of Transition Metal Ions by Polyampholytes; Author Certificate of the USSR No.1231810; Moscow, Russia, 1984; pp. 1–5. [Google Scholar]
- Bekturov, E.A.; Kudaibergenov, S.E.; Saltybaeva, S.S.; Kyaturka, V.G.; Radzhunas, L.V. Study of complex formation properties of synthetic polyampholyte based on N,N-dimethylaminoethylmethacrylate and methacrylic acid. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1990, 1, 33–38. [Google Scholar]
- Kudaibergenov, S.E.; Bekturov, E.A. Influence of the coil-globule confromational transition in polyampholytes on sorption and desorption of polyelectrolytes and human serum albumin. Vysokomol. Soedin. Ser. A 1989, 31, 2614–2617. [Google Scholar]
- Kudaibergenov, S.E. Complexation reactions with participation of synthetic polyampholytes. Ph.D. Thesis, Moscow State University, Moscow, Russia, 1991. [Google Scholar]
- Patrickios, C.S.; Jang, C.J.; Hertler, W.R.; Hatton, T.A. Protein interactions with acrylic polyampholytes. Polym. Prepr. 1993, 34, 954–955. [Google Scholar]
- Patrickios, C.S.; Jang, C.J.; Hertler, W.R.; Hatton, T.A. Protein interactions with acrylic polyampholytes. In Macro-Ion Characterization from Dilute Solutions to Complex Fluids; Schmitz, K.S., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 548, Chapter 19; pp. 257–267. [Google Scholar]
- Nath, S.; Patrickios, C.S.; Hatton, T.A. A turbidimetric titration study of the interaction of proteins with block and random acrylic polyampholytes. Biotechnol. Prog. 1995, 11, 99–103. [Google Scholar] [CrossRef]
- Nath, S. Complexation behavior of proteins with polyelectrolytes and random acrylic polyampholytes using turbidimetric titration. J. Chem. Technol. Biotechnol. 1995, 62, 295–300. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Hertler, W.R.; Hatton, T.A. Protein complexation with acrylic polyampholytes. Biotechnol. Bioeng. 1994, 44, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.E.; Bekturov, E.A. Natural and synthetic polyampholytes: From fundamentals to application. In Polymeric Electrolytes, Hydrogels, Complexes and Catalysts; Gylym: Almaty, Kazakhstan, 2007; pp. 7–44. [Google Scholar]
- Kudaibergenov, S.; Ciferri, A. Natural and synthetic polyampholytes. 2. Functions and applications. Macromol. Rapid Commun. 2007, 28, 1969–1986. [Google Scholar] [CrossRef]
- Rogacheva, V.B.; Panova, T.V.; Bykova, E.V.; Zezin, A.B.; Joosten, J.; Brekman, J. Interaction of ampholyte dendrimers with network polycations and polyanions. Polym. Sci. Ser. A 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Tatykhanova, G.S.; Klivenko, A.N. Complexation of macroporous amphoteric cryogels based on N,N-dimethylaminoethylmethacrylate and methacrylic acid with dyes, surfactant, and protein. J. Appl. Polym. Sci. 2016, 133, 43784. [Google Scholar] [CrossRef]
- Janiak, D.S.; Ayyub, O.B.; Kofinas, P. Effects of charge density on the recognition properties of molecularly imprinted polyampholyte hydrogels. Polymer 2010, 51, 665–670. [Google Scholar] [CrossRef]
- Lago, M.A.; Grinberg, V.Y.; Burova, T.V.; Concheiro, A.; Alvarez-Lorenzo, C. Ionic and polyampholyte N-isopropylacrylamide-based hydrogels prepared in the presence of imprinting ligands: Stimuli-responsiveness and adsorption/release properties. J. Funct. Biomater. 2011, 2, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, X.-L.; Liu, Y.-R.; Wang, J.; Tian, L.-L.; Zhang, Y.; Hu, X.-Y. Charged groups synergically enhance protein imprinting in amphoteric polyacrylamide cryogels. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Huang, J.T.; Zhang, J.; Zhang, J.Q.; Zheng, S.H. Template imprinting amphoteric polymer for the recognition of proteins. J. Appl. Polym. Sci. 2005, 95, 358–361. [Google Scholar] [CrossRef]
- Haag, S.L.; Barnards, M.T. Polyampholyte hydrogels in biomedical applications. Gels 2017, 3, 41. [Google Scholar] [CrossRef]
- Su, E.; Okay, O. Polyampholyte hydrogels formed via electrostatic and hydrophobic interactions. Eur. Polym. J. 2017, 88, 191–204. [Google Scholar] [CrossRef]
- Zezin, A.B.; Kabanov, V.A. A new class of water-soluble polyelectrolytes-polyelectrolyte complexes. Uspekhi Khimii 1982, 51, 1447–1483. [Google Scholar]
- Kudaibergenov, S.E.; Khamzamulina, R.E.; Bekturov, E.A.; Bimendina, L.A.; Frolova, V.A.; Askarova, M.Z. Polyelectrolyte complex formation on a dimeric interface. Macromol. Chem. Rapid Commun. 1994, 15, 943–947. [Google Scholar] [CrossRef]
- Bimendina, L.A.; Iskaraeva, S.B.; Kudaibergenov, S.E.; Bekturov, E.A. Polyelectrolyte complexes on a dimeric interface. Int. J. Polym. Anal. Charact. 1996, 2, 135–140. [Google Scholar] [CrossRef]
- Bimendina, L.A.; Kudaibergenov, S.E.; Bekturov, E.A. Polymer complexes at interfaces. J. Macromol. Sci. Rev. Macromol. Chem. Phys. C 2003, 43, 27–44. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Bimendina, L.A.; Zhumadilova, G.T. Design, structure and behavior of interpolymer complex membranes. In Advanced Macromolecular and Supramolecular Materials and Processes; Kluwer Academic/Plenum Publishers: Dordrecht, The Netherlands, 2003; Chapter 10; pp. 149–162. [Google Scholar]
- Zhumadilova, G.T.; Gazizov, A.D.; Bimendina, L.A.; Kudaibergenov, S.E. Properties of polyelectrolyte complex membranes based on some weak polyelectrolytes. Polymer 2001, 42, 2985–2989. [Google Scholar] [CrossRef]
- Gazizov, A.D.; Zhumadilova, G.T.; Bimendina, L.A.; Kudaibergenov, S.E. Interpolymer complexes of some vinyl copolymers in a solution and on the boundary of two liquid phases. Polymer 2000, 41, 5793–5797. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Mun, G.A.; Khutoryanskiy, V.V.; Gazizov, A.D. Design of Composite Films and Ultrathin Membranes of Interpolymer Complexes. Polym. Adv. Technol. 2000, 11, 15–19. [Google Scholar] [CrossRef]
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000, 21, 319–348. [Google Scholar] [CrossRef]
- Arys, X.; Jonas, A.M.; Laschewsky, A.; Legras, R. Supramolecular polyelectrolyte assemblies. In Supramolecular Polymers; Ciferri, A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2000. [Google Scholar]
- Richardson, J.J.; Cui, J.; Bjornmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in layer-by-layer assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [PubMed]
- Izumrudov, V.A.; Mussabayeva, B.K.; Murzagulova, K.B. Polyelectrolyte multilayers: Preparation and application. Russ. Chem. Rev. 2018, 87, 192–200. [Google Scholar] [CrossRef]
- Koetz, J.; Philipp, B.; Schultz, W.; Kudaibergenov, S.E.; Bekturov, E.A. Amphoteric character of polyelectrolyte complex particles as revealed by isotachophoresis and viscometry. Colloid Polym. Sci. 1988, 266, 906–912. [Google Scholar] [CrossRef]
- Koetz, J.; Philipp, B.; Kudaibergenov, S.E.; Sigitov, V.B.; Bekturov, E.A. Influence of the charge density of polyelectrolytes on the properties of polyelectrolyte complexes. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1989, 3, 54–58. [Google Scholar]
- Fedorova, I.P.; Korshunov, M.A.; Varkholeva, N.F.; Gorpenyuk, L.V.; Senitch, V.M. Conformational behavior of equimolar polyampholytes on the basis of vinyl- and styrenesulfonic acids and aminoalkylmethacrylates. Vestnik Kievskogo Universiteta. Khimiya 1986, 27, 18–25. [Google Scholar]
- Kudaibergenov, S.E.; Nurgalieva, D.E.; Bekturov, E.A. Study of polyampholyte hydrogels and interpenetrating polyelectrolyte networks based on 4(but-3-en-1-ynyl)-1-methylpiperidin-4-ol. Macromol. Chem. Phys. 1994, 195, 3033–3038. [Google Scholar] [CrossRef]
- Nurgalieva, D.E.; Sigitov, V.B.; Kudaibergenov, S.E.; Bekturov, E.A. Synthesis and investigation of polyampholyte hydrogels and interpenetrating networks based on 1-methyl-4vinylethynylpiperidinol-4. Izvestia Akademii nauk Respubliki Kazakhstan. Seria khimicheskaia 1993, 4, 71–74. [Google Scholar]
- Kudaibergenov, S.E. Synthesis and characterization of polyampholyte gels. Ber. Bunsenges. Phys. Chem. 1996, 100, 1079–1082. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Wang, Z. Studies on chitosan and poly(acrylic acid) interpolymer complex. 1. Preparation, structure, pH-sensitivity, and salt sensitivity of complex forming-poly(acrylic acid): Chitosan semi-interpenetrating polymer network. J. Appl. Polym. Sci. 1997, 65, 1445–1450. [Google Scholar] [CrossRef]
- Bimendina, L.A.; Zhumadilova, G.T.; Kudaibergenov, S.E. Phase transitions in interpolymer complexes of some linear and crosslinked polymers. In Polymer Gels. Fundamentals and Applications; Bohidar, H.B., Dubin, P., Osada, Y., Eds.; ACS Symposium Series; ACS: Washington, DC, USA, 2002; Volume 833, Chapter 9; pp. 137–148. [Google Scholar]
- Chen, W.Y.; Alexandridis, P.; Su, C.K.; Patrickios, C.S.; Hertler, W.R.; Hatton, T.A. Effect of block size and sequence on the micellization of ABC triblock methacrylic polyampholytes. Macromolecules 1995, 28, 8604–8611. [Google Scholar] [CrossRef]
- Katchalsky, A.; Spangler, R. Dynamics of membrane processes. Q. Rev. Biophys. 1968, 1, 137–175. [Google Scholar] [CrossRef]
- Wang, Q.; Schlenoff, J.B. The polyelectrolyte complex/Coacervate continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving forces for oppositely charged polyion association in aqueous solutions: Enthalpic, entropic, but not electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, G.; Doty, P. Macro-ions. III. The solution behavior of a polymeric ampholyte. J. Am. Chem. Soc. 1954, 76, 3764–3777. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Shayakhmetov, S.S.; Rafikov, S.R.; Bekturov, E.A. On hydrodynamic properties of amphoteric copolymers. Dokl. Acad. Nauk SSSR 1979, 246, 147–149. [Google Scholar]
- Puglia, G.P. Solution properties of polymers. 1. A measure of the ionic strength dependence of polyampholyte solutions. Annu. Tech. Conf. Soc. Plast. Eng. 1992, 2280–2281. [Google Scholar]
- Zheng, G.-Z.; Meshitsuka, G.; Ishizu, A. Inter- and intramolecular ionic interactions of polyampholyte: Carboxymethyl-2-diethylaminoethylcellulose. Polym. Int. 1994, 34, 241–248. [Google Scholar] [CrossRef]
- Ali, S.A.; Rasheed, A.; Wazeer, M.I. Synthesis and solution properties of a quaternary ammonium polyampholyte. Polymer 1999, 40, 2439–2446. [Google Scholar] [CrossRef]



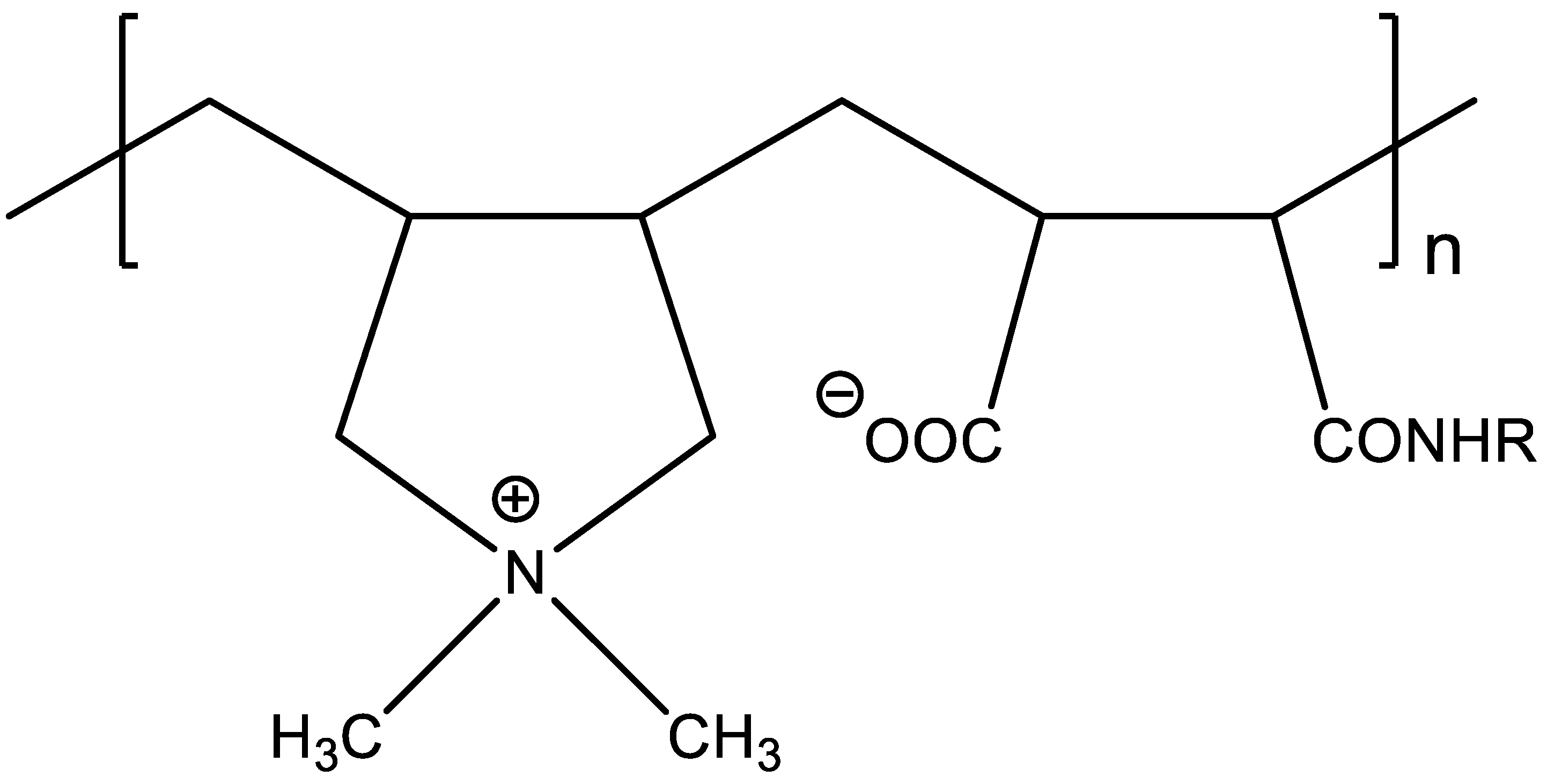

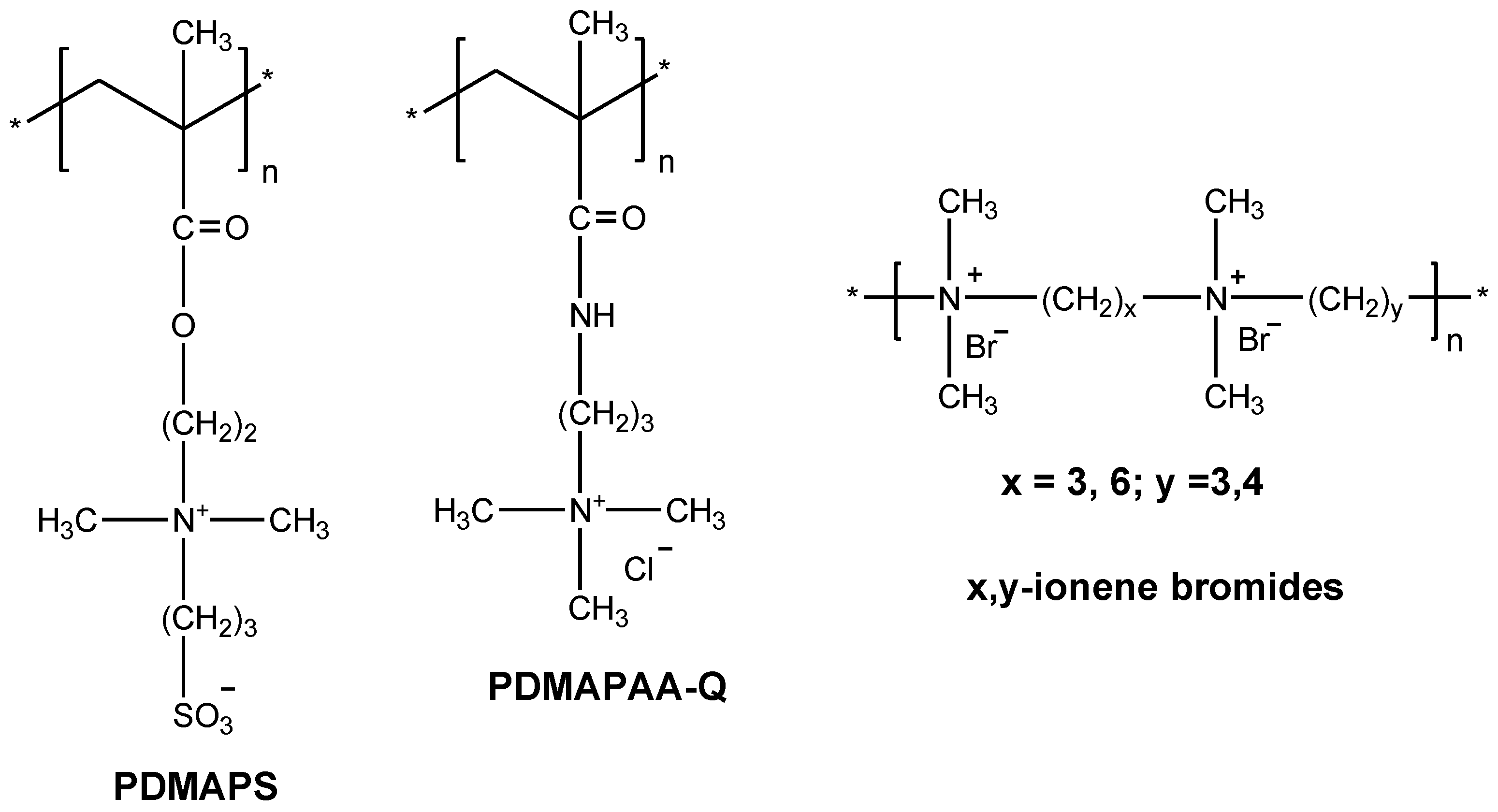


| Chemical Structure of Monomer Units | Name Acronym | Type of Polyampholyte | Refs |
|---|---|---|---|
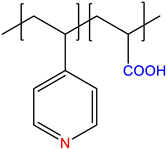 | 4-vinylpyridine-co-acrylic acid, P(4VPy-co-AA) | Annealed | [12] |
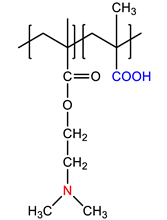 | N,N-dimethylaminoethylmethacrylate- co-methacrylic acid, P(DMAEM-co-MAA) | Annealed | [13] |
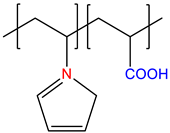 | N-vinylimidazole-co-acryic acid, P(VI-co-AA) | Annealed | [14] |
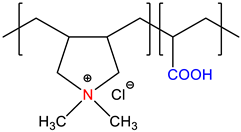 | N,N’-dimethyl-N,N’-diallylammonium chloride-co-acrylic acid, P(DMDAAC-co-AA) | Self-quenched * | [15] |
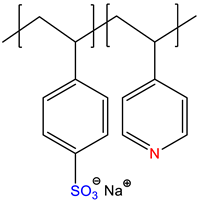 | sodium styrene sulfonate-co-4-vinylpyridine, P(NaSS-co-4VPy) | Self-quenched * | [16] |
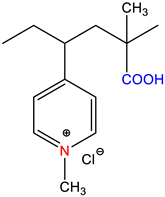 | poly(1-methyl-4-vinylpyridinium chloride)-block-poly(methacrylic acid), P1M4VPCl-b-PMAA | Self-quenched * | [17,18] |
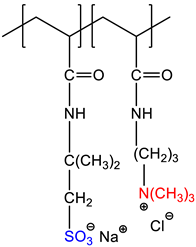 | 2-acrylamido-2-methyl-1-propanesulfonic acid sodium salt-co-(3-acrylamidopropyl)trimethylammonium chloride, P(AMPSNa-co-APTAC) | Quenched | [19] |
 | 2-methacryloyloxyethyltrimethylammonium-co-2-methacryloyloxyethanesulfonate, P(METMA-co-MES) | Quenched or zwitterionic | [20] |
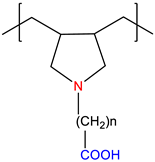 | Poly(carbobetaine), PCB | Annealed | [7] |
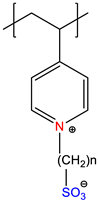 | Poly(sulfobetaine), PSB | Quenched or zwitterionic | [7] |
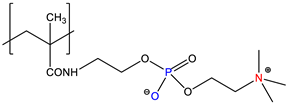 | Poly(phosphobetaine), PPB | Quenched or zwitterionic | [7] |
| Polyampholytes | Polyelectrolytes (PE) | PPC, n = [APA]/[PE] |
|---|---|---|
| APA-1 | PAA | 1:1 |
| NaPSS | 3:1 | |
| APA-2 | PAA | 1:4 |
| NaPSS | 5:2 | |
| APA-3 | PAA | 1:2 |
| NaPSS | 5:1 | |
| APA-4 | PAA | 1:2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudaibergenov, S.E.; Nuraje, N. Intra- and Interpolyelectrolyte Complexes of Polyampholytes. Polymers 2018, 10, 1146. https://doi.org/10.3390/polym10101146
Kudaibergenov SE, Nuraje N. Intra- and Interpolyelectrolyte Complexes of Polyampholytes. Polymers. 2018; 10(10):1146. https://doi.org/10.3390/polym10101146
Chicago/Turabian StyleKudaibergenov, Sarkyt E., and Nurxat Nuraje. 2018. "Intra- and Interpolyelectrolyte Complexes of Polyampholytes" Polymers 10, no. 10: 1146. https://doi.org/10.3390/polym10101146
APA StyleKudaibergenov, S. E., & Nuraje, N. (2018). Intra- and Interpolyelectrolyte Complexes of Polyampholytes. Polymers, 10(10), 1146. https://doi.org/10.3390/polym10101146