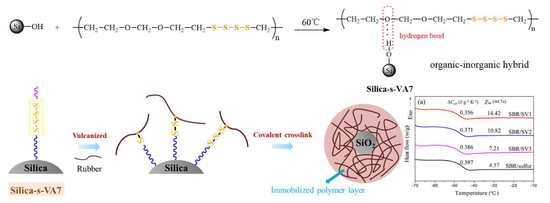
Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
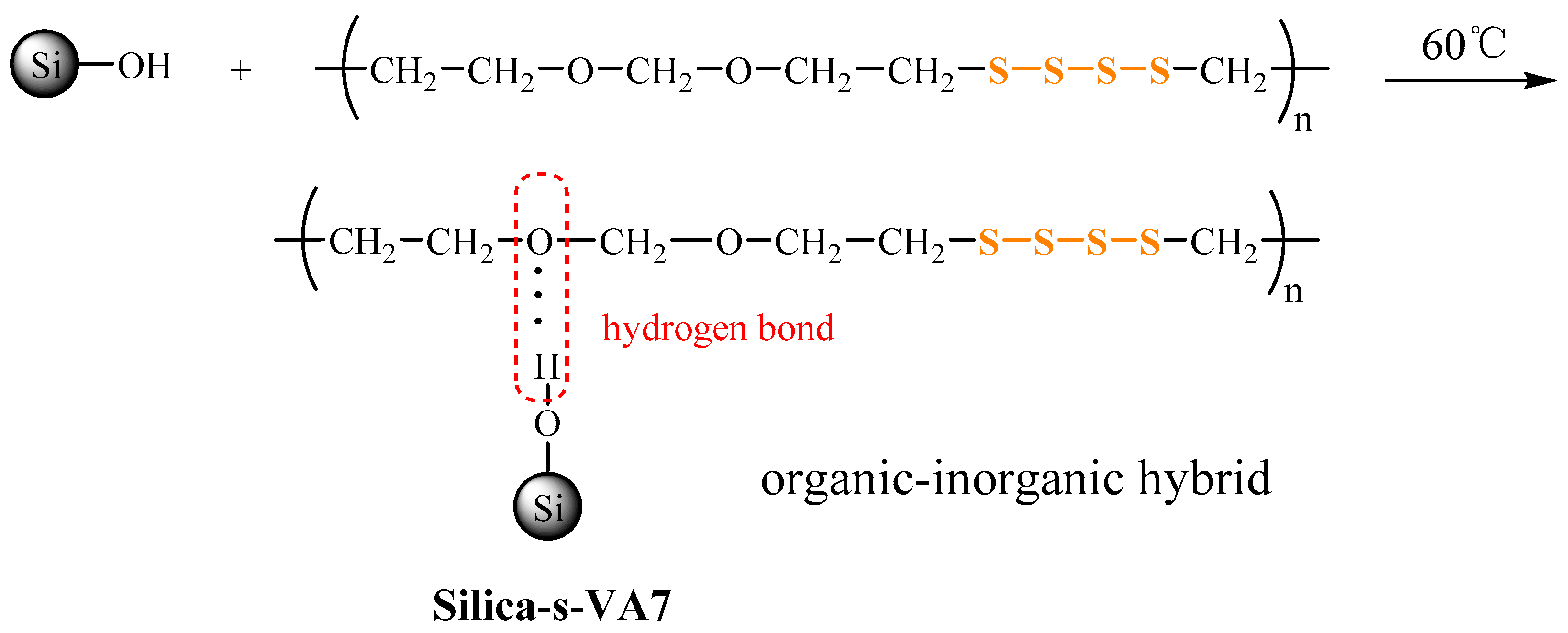
2.2. Preparation of Silica-s-VA7 Vulcanizator
2.3. Preparation of SBR/Silica Nanocomposites
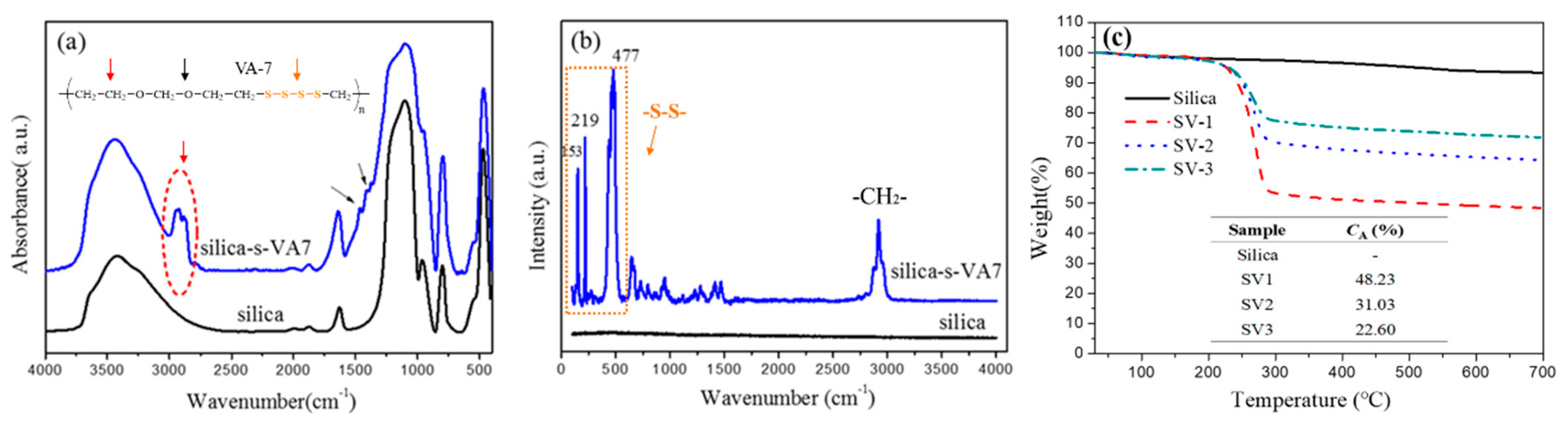
2.4. Characterization
3. Results and Discussion
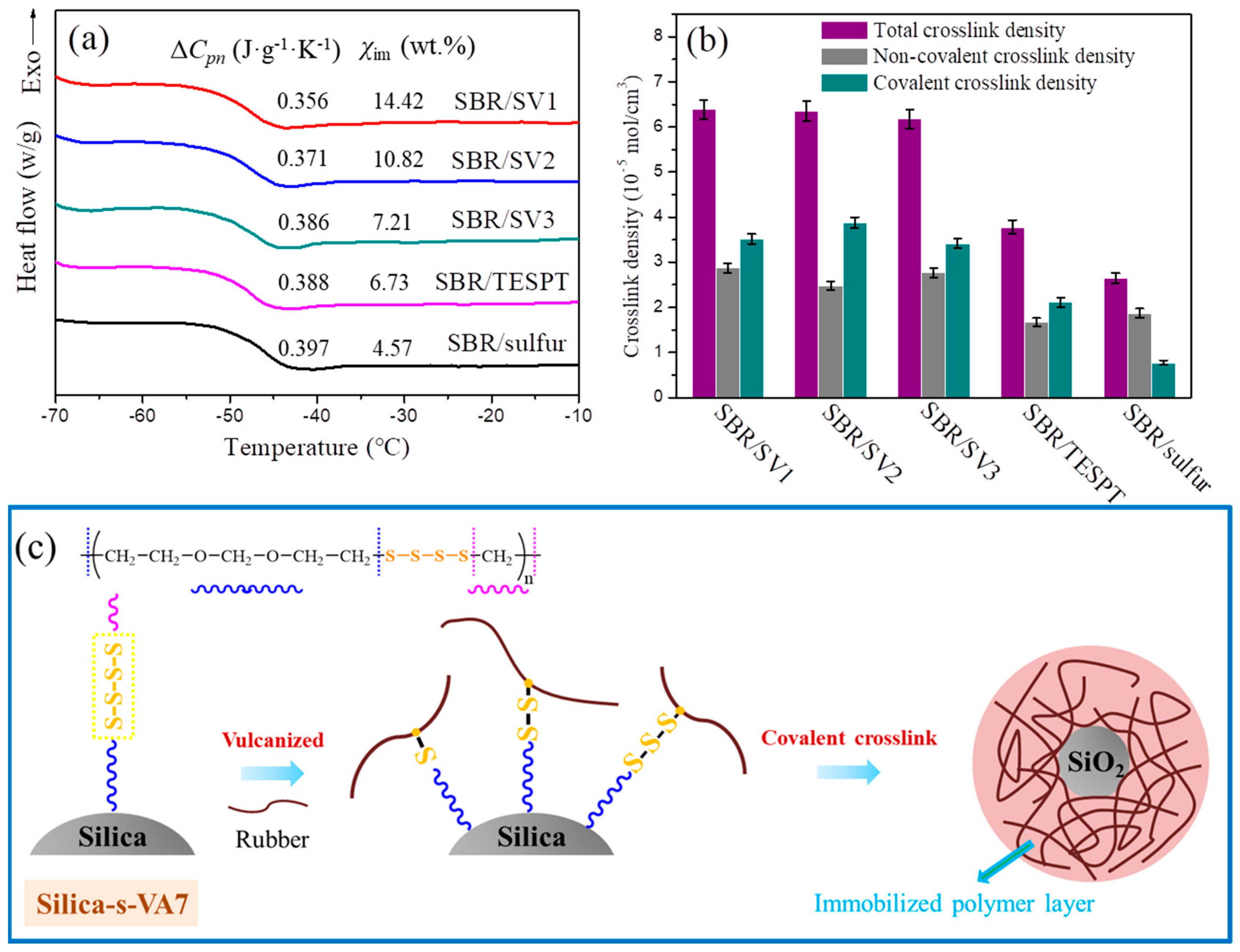
3.1. Aliphatic Ether Polysulfide Supported on a Silica Nanoparticle Surface
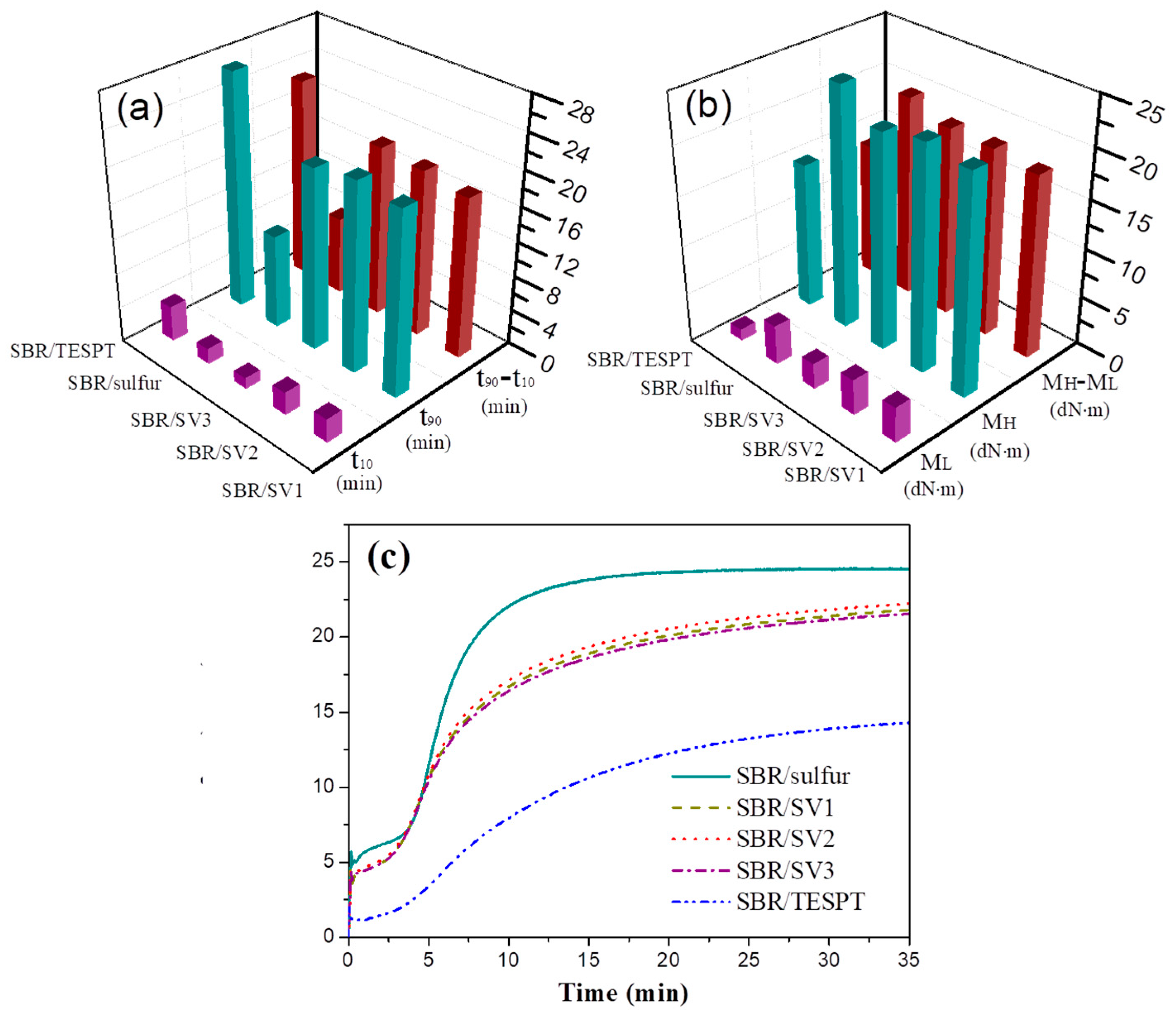
3.2. Vulcanization Characteristics of SBR/Silica Nanocomposites
3.3. Interfacial Interaction Analysis between Filler and Rubber Matrix
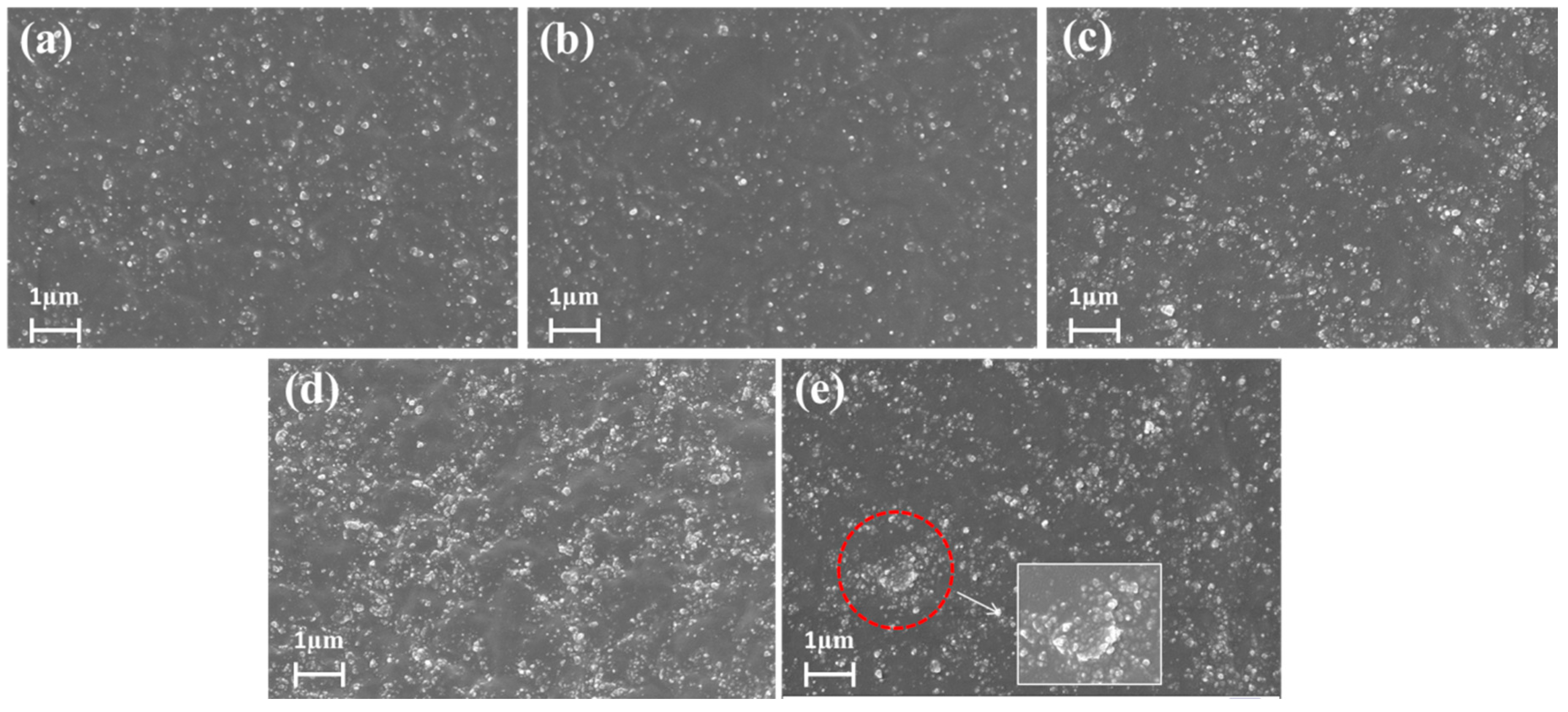
3.4. Immobilized Polymer Layer Anchoring on Filler Surface
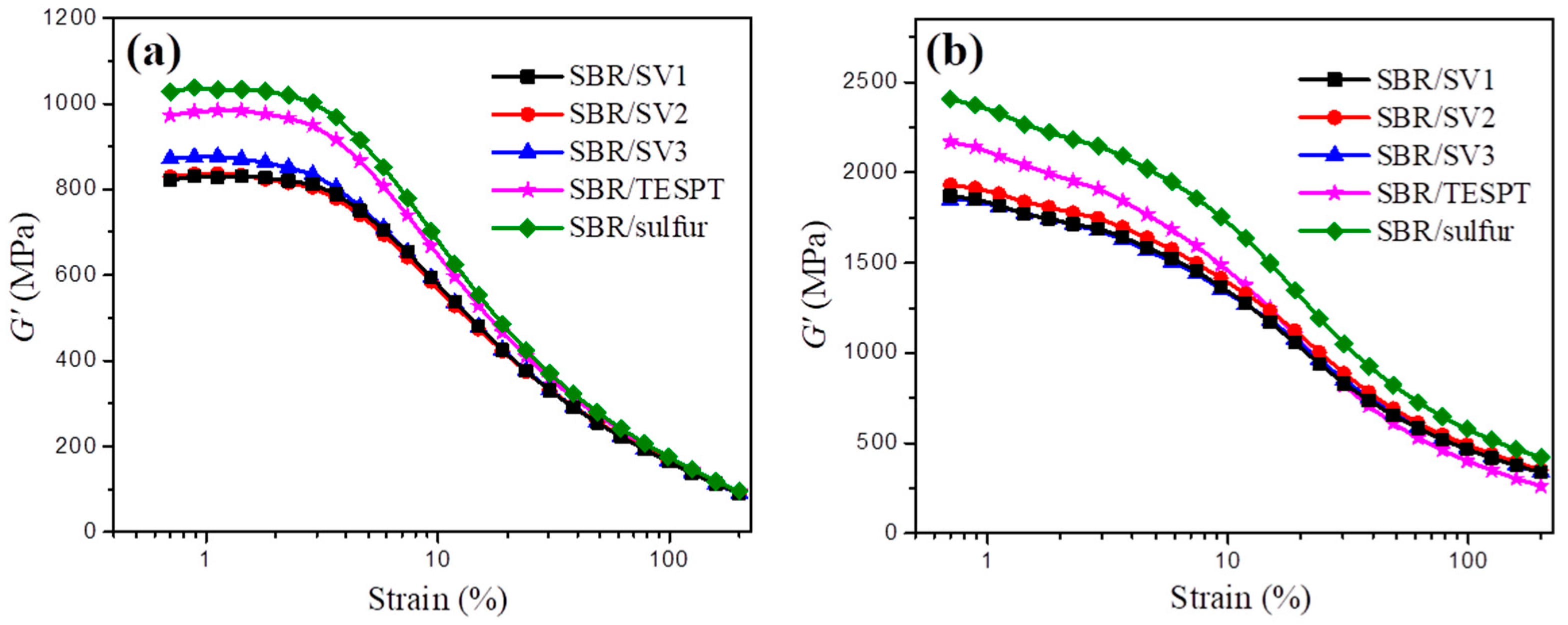
3.5. Mechanical Properties of SBR/Silica Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Tang, Z.; Chen, Y.; Zhang, C.; Guo, B. Engineering of β-hydroxyl esters into elastomer-nanoparticle interface towards malleable, robust and reprocessable vitrimer composites. ACS Appl. Mater. Interfaces 2017, 10, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, X.; Wang, S. Effect of styrene butadiene rubber on the light pyrolysis of the natural rubber. Polym. Degrad. Stab. 2018, 147, 168–176. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeter. Biodegr. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Mansilla, M.A.; Valentín, J.L.; López-Manchado, M.A.; González-Jiménez, A.; Marzocca, A.J. Effect of entanglements in the microstructure of cured NR/SBR blends prepared by solution and mixing in a two-roll mill. Eur. Polym. J. 2016, 81, 365–375. [Google Scholar] [CrossRef]
- Xue, X.; Yin, Q.; Jia, H.; Zhang, X.; Wen, Y.; Ji, Q.; Xu, Z. Enhancing mechanical and thermal properties of styrene-butadiene rubber/carboxylated acrylonitrile butadiene rubber blend by the usage of graphene oxide with diverse oxidation degrees. Appl. Surf. Sci. 2017, 423, 584–591. [Google Scholar] [CrossRef]
- Susanna, A.; Armelao, L.; Callone, E.; Dirè, S.; D’Arienzo, M.; Di Credico, B.; Giannini, L.; Hanel, T.; Morazzoni, F.; Scotti, B. ZnO nanoparticles anchored to silica filler. A curing accelerator for isoprene rubber composites. Chem. Eng. J. 2015, 275, 245–252. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.; Noordermeer, J. Activators in accelerated sulfur vulcanization. Rubber Chem. Technol. 2004, 77, 512–541. [Google Scholar] [CrossRef]
- Choi, S.; Kim, I.; Woo, C. Influence of TESPT content on crosslink types and rheological behaviors of natural rubber compounds reinforced with silica. J. Appl. Polym. Sci. 2007, 106, 2753–2758. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Dong, H.; Luo, Y.; Jia, D.; Liu, F. One-step approach to reduce and modify graphene oxide via vulcanization accelerator and its application for elastomer reinforcement. Chem. Eng. J. 2017, 317, 51–59. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Guo, X.; Zhong, B.; Chen, Y.; Luo, Y.; Jia, D. Functionalized HNTs nanocluster vulcanized natural rubber with high filler-rubber interaction. Chem. Eng. J. 2018, 336, 748–756. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Lin, J.; Jia, Z.; Luo, Y.; Jia, D.; Liu, F. Preparation of halloysite nanotubes-silica hybrid supported vulcanization accelerator for enhancing interfacial and mechanical strength of rubber composites. Ind. Eng. Chem. Res. 2017, 56, 9135–9142. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. High reactive sulphide chemically supported on silica surface to prepare functional nanoparticle. Appl. Surf. Sci. 2018, 442, 673–681. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, D.; Yao, P.; Wang, W.; Zhang, L.; Lvov, Y. Highly aging-resistant elastomers doped with antioxidant-loaded clay nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108111. [Google Scholar] [CrossRef]
- Marzocca, A.J. Evaluation of the polymer-solvent interaction parameter χ for the system cured styrene butadiene rubber and toluene. Eur. Polym. J. 2007, 43, 2682–2689. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, K.H.; Seo, K.H. Effects of ground rubber having different curing systems on the crosslink structures and physical properties of NR vulcanizates. Mat. Res. Innovat. 2003, 7, 149–154. [Google Scholar] [CrossRef]
- Wei, Z.; Lu, Y.; Yan, S.; Meng, Y.; Zhang, L. Dramatic influence of curing temperature on micro-nano structure transform of HNBR filled with zinc dimethacrylate. J. Appl. Polym. Sci. 2012, 124, 288–295. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Wu, X.; Guo, B.; Zhang, L.; Liu, F. Interface engineering toward promoting silanization by ionic liquid for high-performance rubber/silica composites. Ind. Eng. Chem. Res. 2015, 54, 10747–10756. [Google Scholar] [CrossRef]
- Lucia, C.; Giovanna, C.; Maila, C.; Antonio, T.; Fabio, M.N.; Jean, F.G. Morphology and viscoelastic behaviour of a silica filled styrene/butadiene random copolymer. Macromol. Mater. Eng. 2008, 293, 178–187. [Google Scholar]
- Tang, Z.; Huang, J.; Guo, B.; Zhang, L.; Liu, F. Bioinspired engineering of sacrificial metal-ligand bonds into elastomers with supramechanical performance and adaptive recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Neal, J.; Mozhdehi, D.; Guan, Z. Enhancing mechanical performance of a covalent self-healing material by sacrificial noncovalent bonds. J. Am. Chem. Soc. 2015, 137, 4846–4850. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, K.; Tian, X.; Hu, K.; Wang, R.; Liu, C.; Li, Y.; Cui, P. Double glass transitions and interfacial immobilized layer in in-situ-synthesized poly(vinyl alcohol)/silica nanocomposites. Macromolecules 2010, 43, 1076–1082. [Google Scholar] [CrossRef]
- Rao, Y.; Pochan, J.M. Mechanics of polymer-clay nanocomposites. Macromolecules 2007, 40, 290–296. [Google Scholar] [CrossRef]
- Harton, S.E.; Kumar, S.K.; Yang, H.; Koga, T.; Hicks, K.; Lee, H.; Mijovic, J.; Liu, M.; Vallery, R.S.; Gidley, D.W. Immobilized polymer layers on spherical nanoparticles. Macromolecules 2010, 43, 3415–3421. [Google Scholar] [CrossRef]
- Lorenz, O.; Parks, C.R. The crosslinking efficiency of some vulcanizing agents in natural rubber. J. Polym. Sci. Part A Polym. Chem. 1961, 50, 299–312. [Google Scholar] [CrossRef]
- Pangamol, P.; Sirisinha, C.; Hu, Y.; Urquhart, S.G. Effectiveness of by-product sulfur from petroleum refining as a rubber vulcanizing agent: A XANES investigation. Ind. Eng. Chem. Res. 2013, 52, 17179–17183. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Coleman, M.; Shelton, J.; Koenig, J. Sulfur vulcanization of hydrocarbon diene elastomers. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 154–166. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D. A method to improve the mechanical performance of styrene-butadiene rubber via vulcanization accelerator modified silica. Compos. Sci. Technol. 2015, 117, 46–53. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Luo, Y.; Zhang, D.; Jia, Z.; Jia, D.; Liu, F. Simultaneous reduction and functionalization of graphene oxide via antioxidant for highly aging resistant and thermal conductive elastomer composites. Compos. Sci. Technol. 2017, 151, 156–163. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, B.; Chen, Z.; Zhao, J. Reinforcement and antioxidation effects of antioxidant functionalized silica in styrene-butadiene rubber. Mater. Design 2013, 50, 558–565. [Google Scholar] [CrossRef]

| Sample | Component (phr) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBR | SV1 | SV2 | SV3 | Silica | S | CBS | ZnO | SA | TESPT | DMPPD | |
| SBR/SV1 | 100 | 8 | 0 | 0 | 32 | 0 | 2 | 4 | 2 | 0 | 2 |
| SBR/SV2 | 100 | 0 | 12 | 0 | 28 | 0 | 2 | 4 | 2 | 0 | 2 |
| SBR/SV3 | 100 | 0 | 0 | 16 | 24 | 0 | 2 | 4 | 2 | 0 | 2 |
| SBR/TESPT | 100 | 0 | 0 | 0 | 38 | 0 | 2 | 4 | 2 | 8 | 2 |
| SBR/sulfur | 100 | 0 | 0 | 0 | 38 | 2 | 2 | 4 | 2 | 0 | 2 |
| Content (%) | |||
|---|---|---|---|
| C | H | S | |
| Silica | 0 | 1.56 | 0 |
| SV1 | 13.15 | 2.21 | 26.85 |
| SV2 | 8.37 | 1.60 | 17.52 |
| SV3 | 6.55 | 1.30 | 13.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Chen, Y.; Jia, D. Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction. Polymers 2018, 10, 1138. https://doi.org/10.3390/polym10101138
Chen L, Guo X, Luo Y, Jia Z, Chen Y, Jia D. Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction. Polymers. 2018; 10(10):1138. https://doi.org/10.3390/polym10101138
Chicago/Turabian StyleChen, Lijuan, Xiaohui Guo, Yuanfang Luo, Zhixin Jia, Yongjun Chen, and Demin Jia. 2018. "Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction" Polymers 10, no. 10: 1138. https://doi.org/10.3390/polym10101138
APA StyleChen, L., Guo, X., Luo, Y., Jia, Z., Chen, Y., & Jia, D. (2018). Inorganic and Organic Hybrid Nanoparticles as Multifunctional Crosslinkers for Rubber Vulcanization with High-Filler Rubber Interaction. Polymers, 10(10), 1138. https://doi.org/10.3390/polym10101138