Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Syntheses of Fe3O4 Nanospheres
2.3. Preparation of γ-MPS-Modified Fe3O4 Nanospheres
2.4. Preparation of Fe3O4 Nanospheres Coated with 2-VP
2.5. Material Characterization
2.6. Batch Adsorption
2.7. HPLC Analysis
3. Results and Discussion
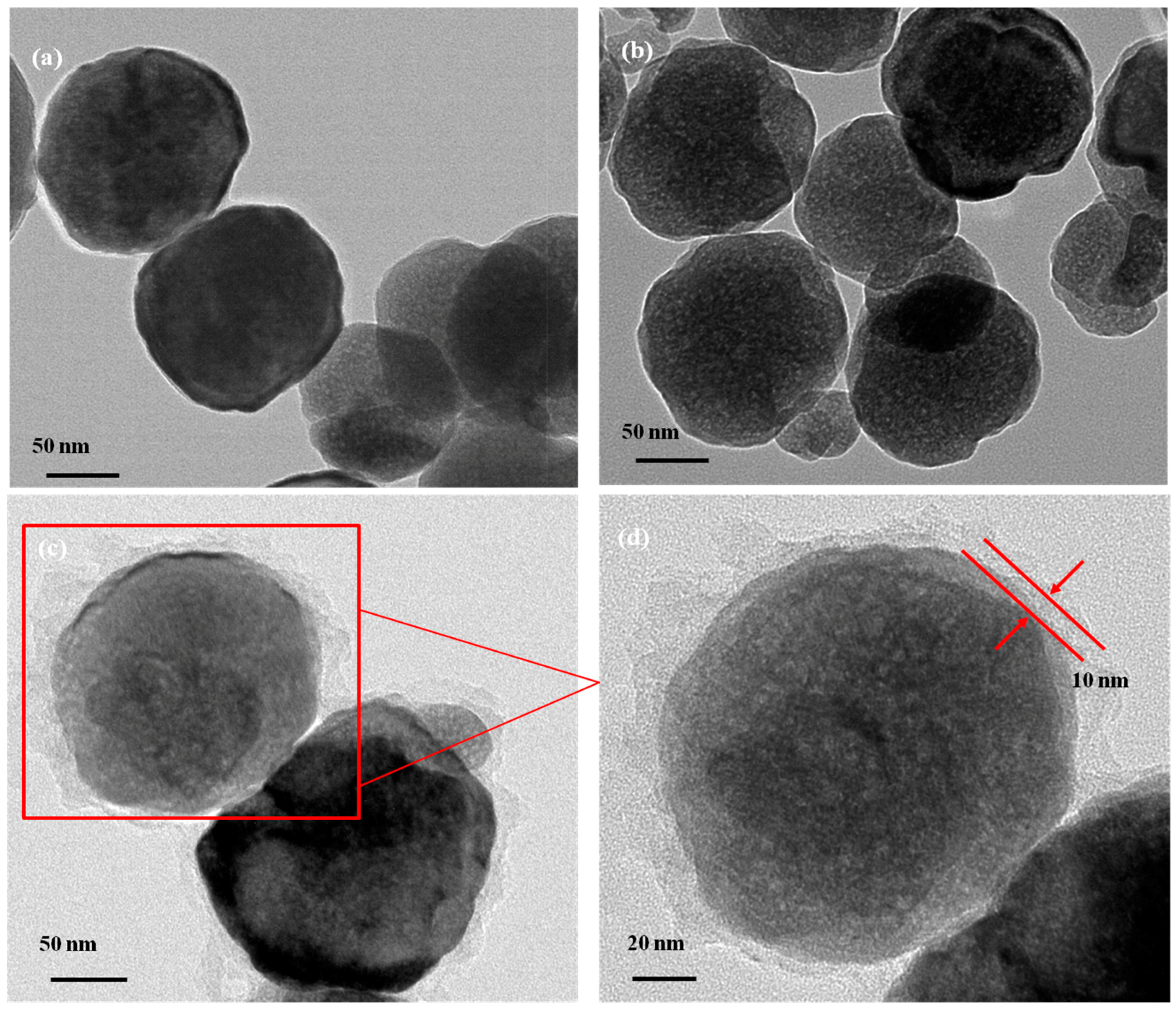
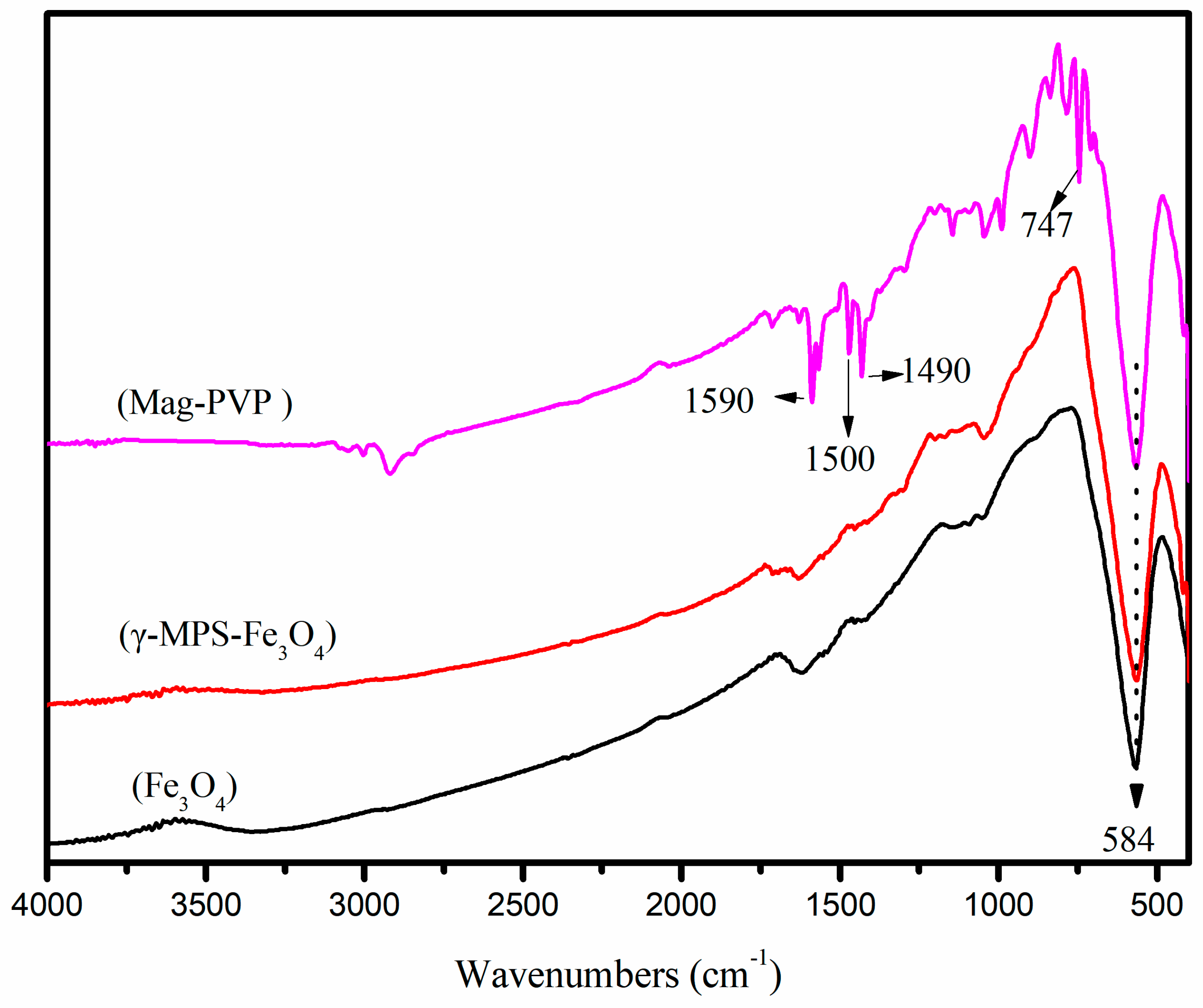
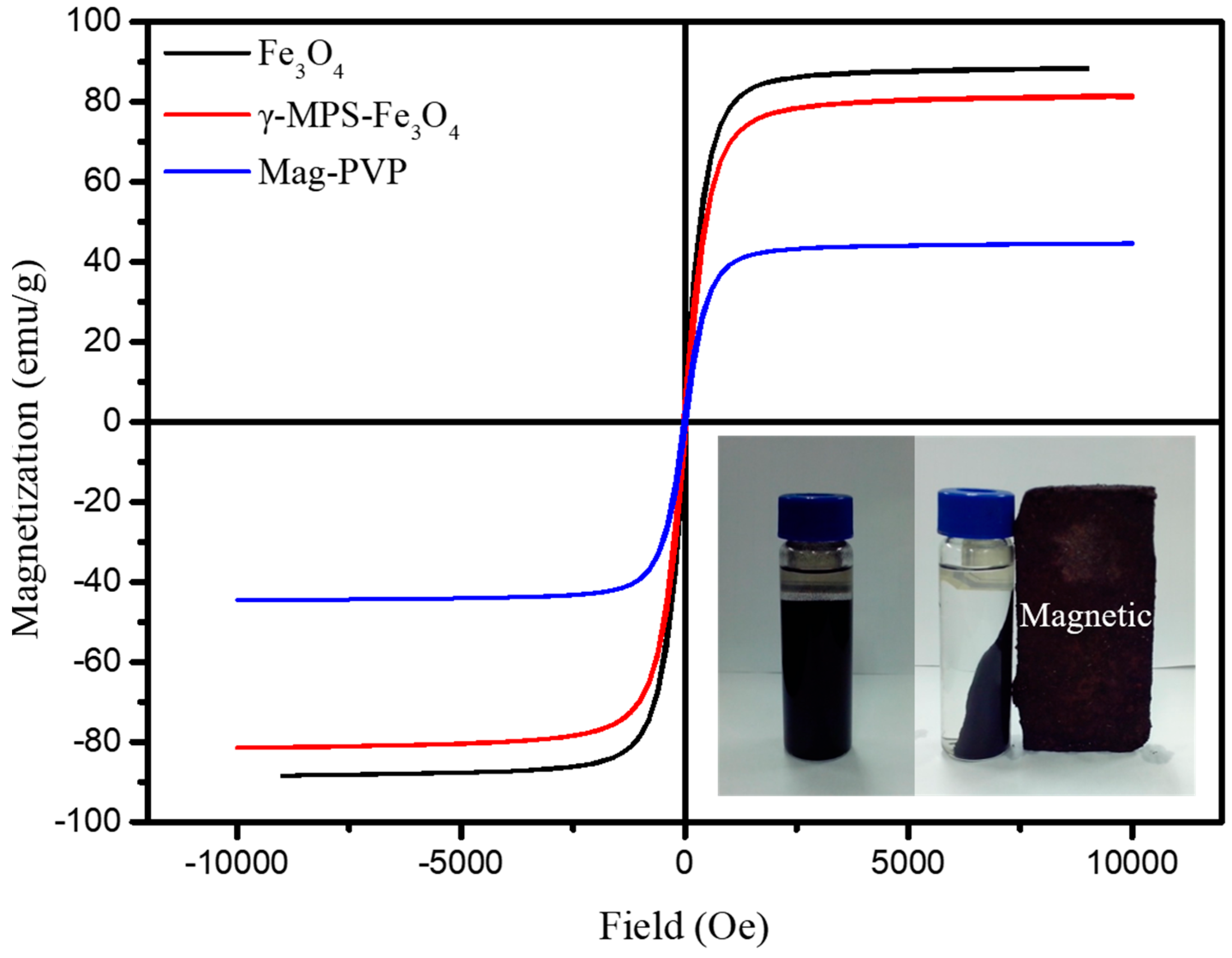
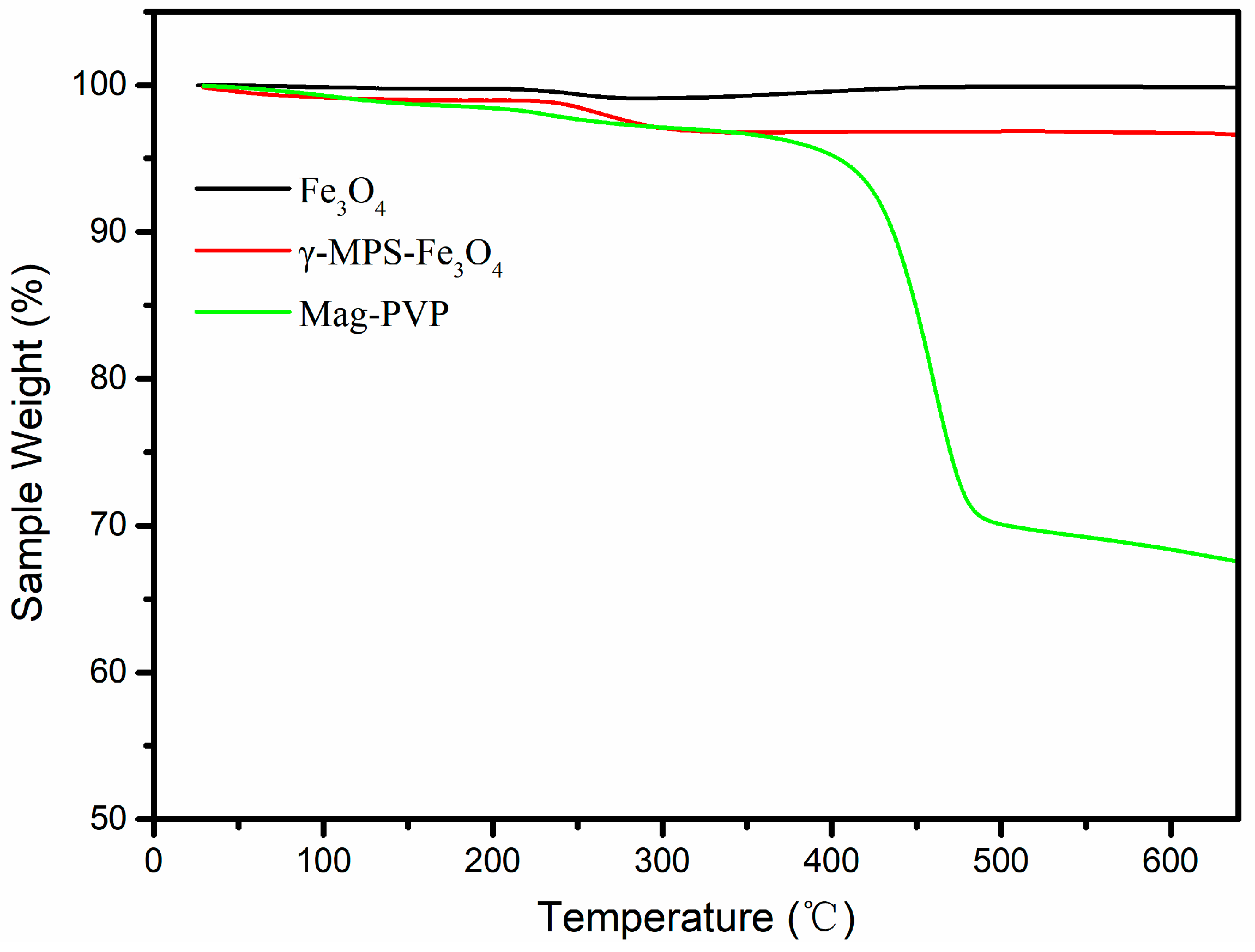
3.1. Characterization
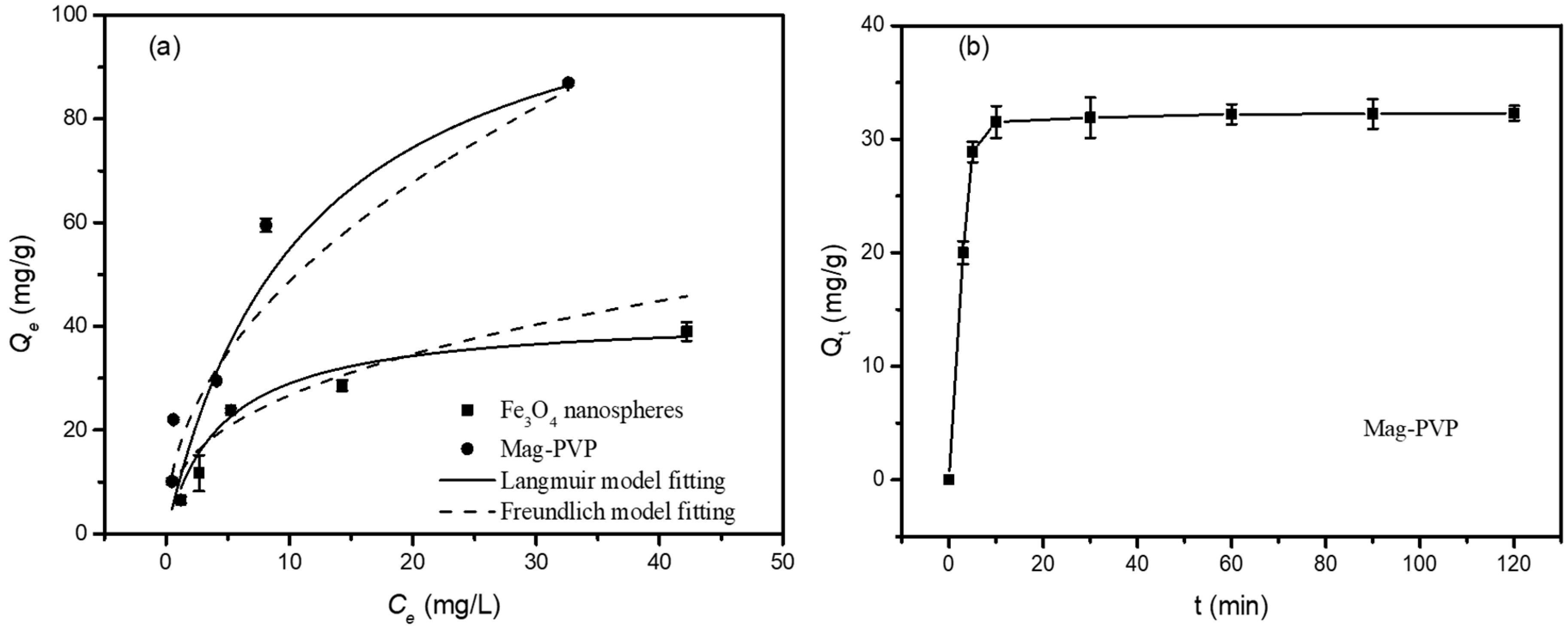
3.2. Adsorption Equilibrium
3.3. Effect of Contact Time
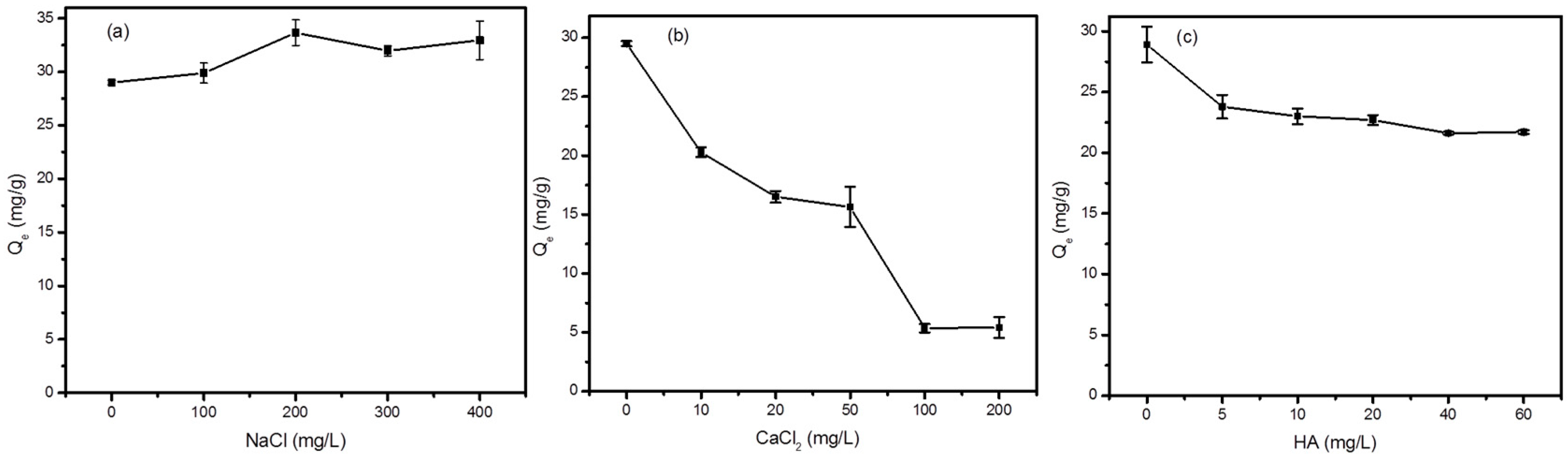
3.4. Effect of Coexisting Ions and HA
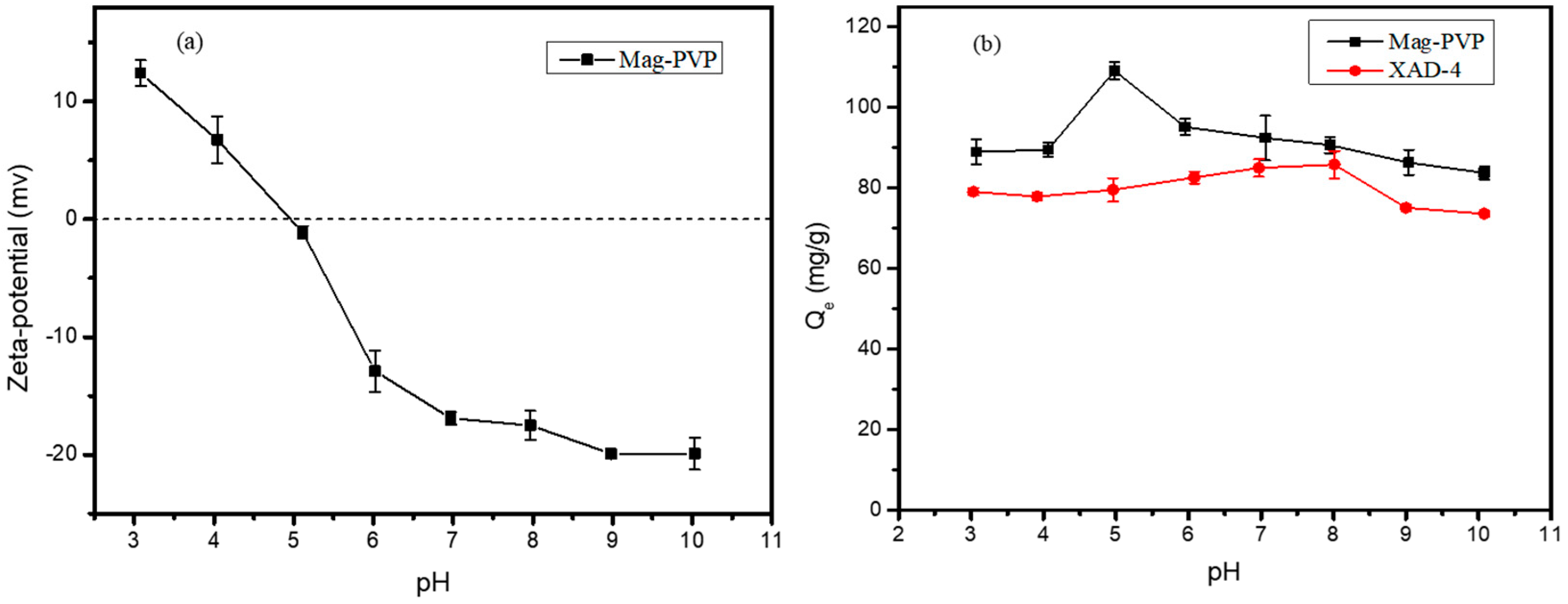
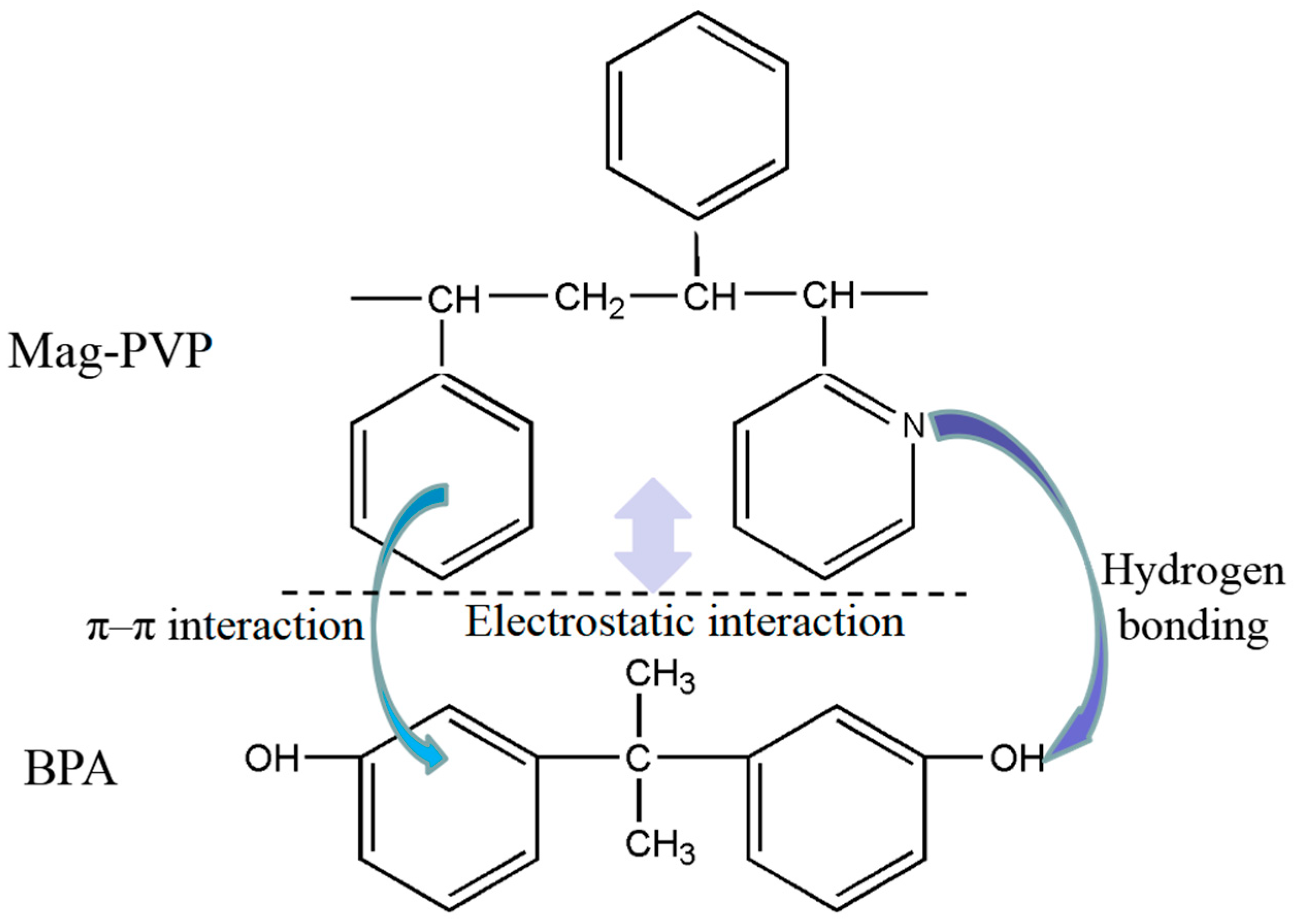
3.5. Effect of pH
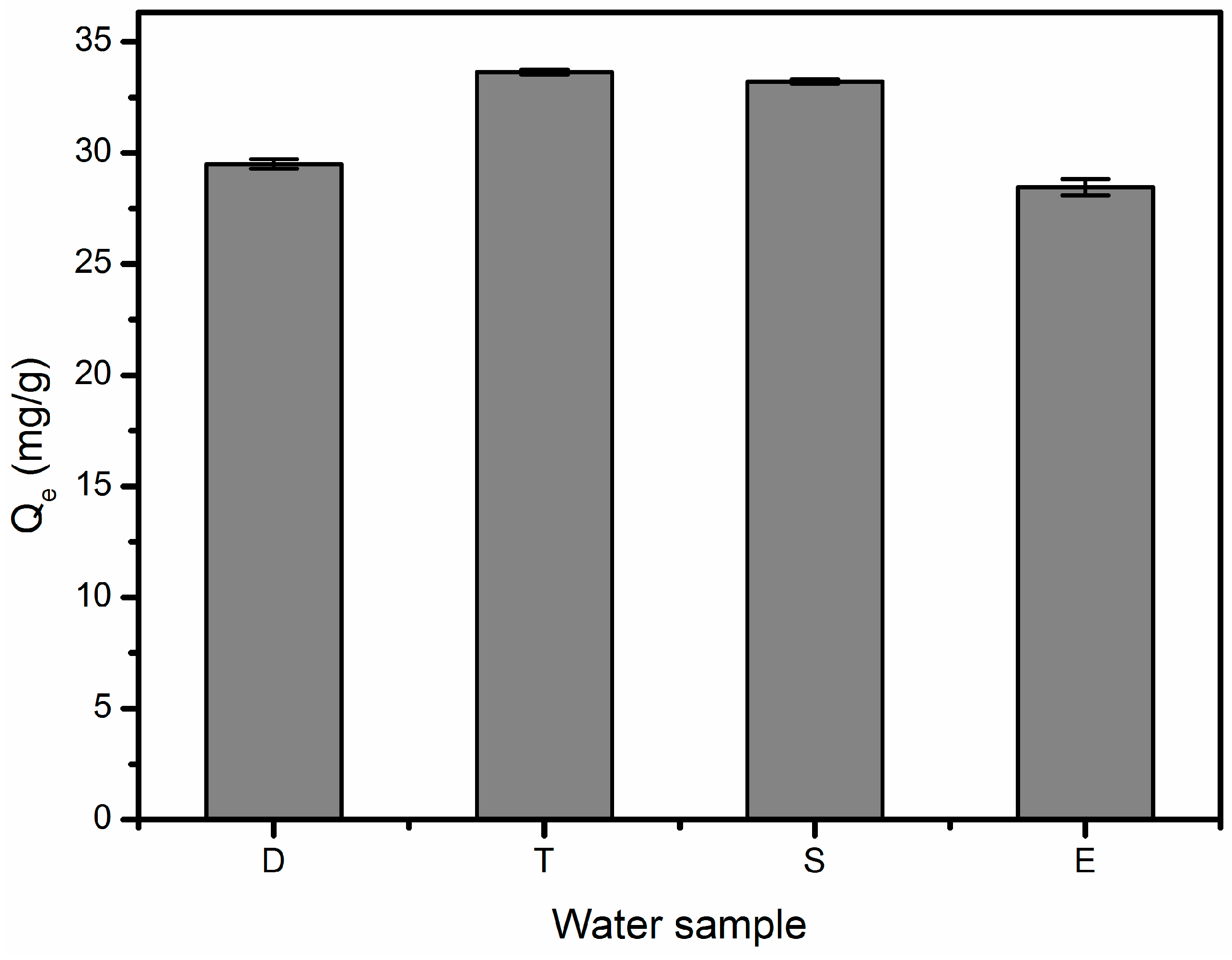
3.6. Effect of Environmental Matrix
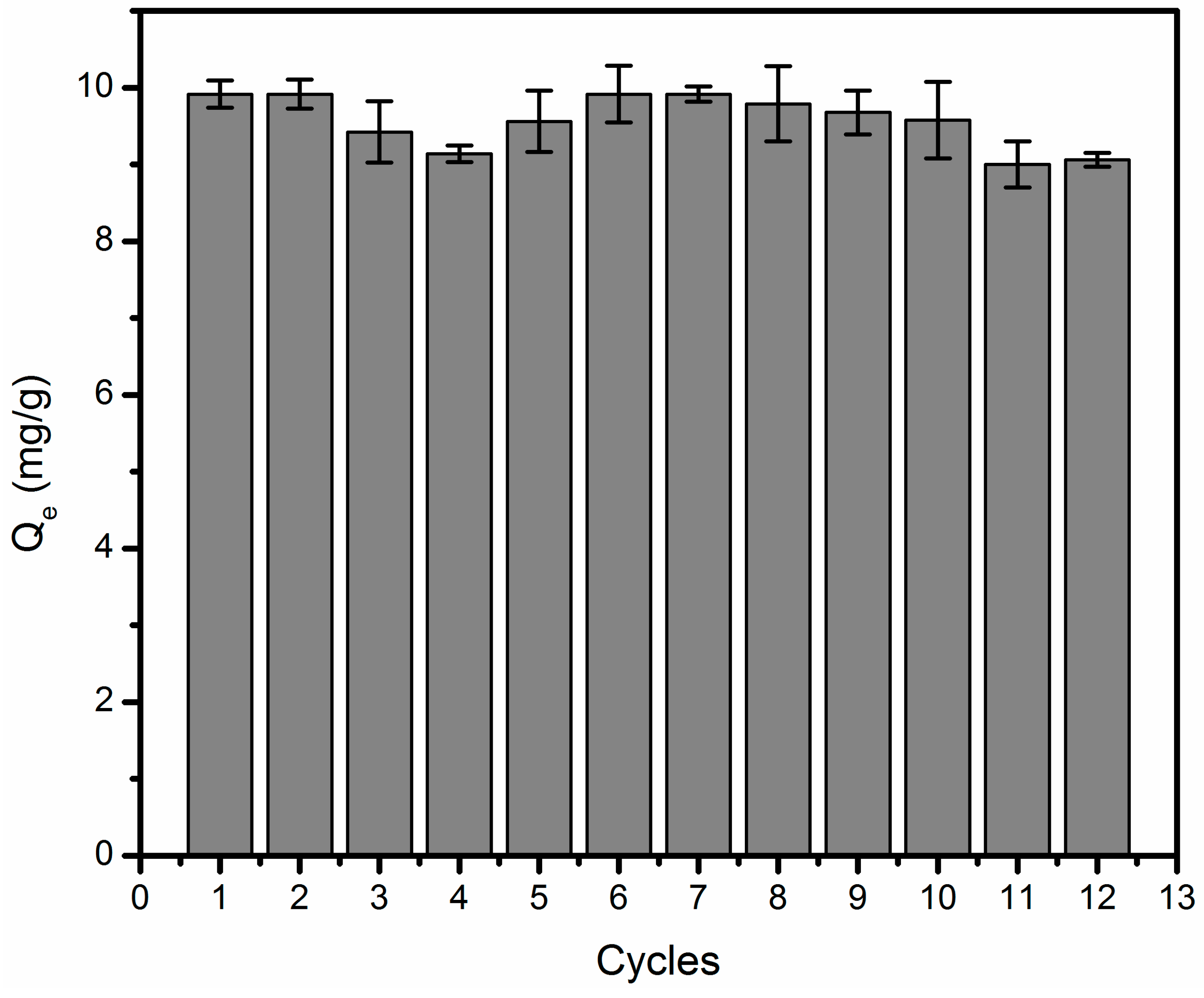
3.7. Regeneration and Reuse
3.8. Comparison of BPA Adsorption on Mag-PVP with Other Adsorbents
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tang, J.Y.; McCarty, S.; Glenn, E.; Neale, P.A.; Warne, M.S.J.; Escher, B.I. Mixture effects of organic micropollutants present in water: Towards the development of effect-based water quality trigger values for baseline toxicity. Water Res. 2013, 47, 3300–3314. [Google Scholar] [CrossRef] [PubMed]
- Huntscha, S.; Singer, H.P.; McArdell, C.S.; Frank, C.E.; Hollender, J. Multiresidue analysis of 88 polar organic micropollutants in ground, surface and wastewater using online mixed-bed multilayer solid-phase extraction coupled to high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1268, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Molkenthin, M.; Olmez-Hanci, T.; Jekel, M.R.; Arslan-Alaton, I. Photo-Fenton-like treatment of BPA: Effect of UV light source and water matrix on toxicity and transformation products. Water Res. 2013, 47, 5052–5064. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wong, C.; Zheng, J.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, H.; Zhao, L.; Lin, J.M. Selective determination of bisphenol A (BPA) in water by a reversible fluorescence sensor using pyrene/dimethyl β-cyclodextrin complex. Anal. Chim. Acta 2006, 556, 313–318. [Google Scholar] [CrossRef]
- Välitalo, P.; Perkola, N.; Seiler, T.-B.; Sillanpää, M.; Kuckelkorn, J.; Mikola, A.; Hollert, H.; Schultz, E. Estrogenic activity in Finnish municipal wastewater effluents. Water Res. 2016, 88, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Toledo, I.; Ferro-Garcia, M.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Vegas Fernández, F. Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, W.; Li, A. Adsorption of phenol, bisphenol A and nonylphenol ethoxylates onto hypercrosslinked and aminated adsorbents. React. Funct. Polym. 2011, 71, 994–1000. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kim, M.; Teslic, S.; Alaee, M.; Smyth, S. Bisphenol-A removal in various wastewater treatment processes: Operational conditions, mass balance, and optimization. J. Environ. Manag. 2015, 152, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Ghadermazi, M.; Bhatnagar, A.; Sadighara, P.; Jahed-Khaniki, G.; Heibati, B.; McKay, G. Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. J. Environ. Chem. Eng. 2016, 4, 2647–2655. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Zaib, Q.; Khan, I.A.; Berge, N.D.; Park, Y.-G.; Saleh, N.B.; Yoon, Y. Removal of bisphenol A and 17α-ethinyl estradiol from landfill leachate using single-walled carbon nanotubes. Water Res. 2011, 45, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Fu, L.; Li, A. Enhanced adsorption of bisphenol A from water by acetylaniline modified hyper-cross-linked polymeric adsorbent: Effect of the cross-linked bridge. Chem. Eng. J. 2012, 191, 171–176. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, D.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gong, Y.; Yang, Y.; He, C.; Hu, L.; Zhu, L.; Sun, L.; Shu, D. Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem. Eng. J. 2015, 260, 231–239. [Google Scholar] [CrossRef]
- Pan, J.; Yao, H.; Li, X.; Wang, B.; Huo, P.; Xu, W.; Ou, H.; Yan, Y. Synthesis of chitosan/gamma-Fe2O3/fly-ash-cenospheres composites for the fast removal of bisphenol A and 2,4,6-trichlorophenol from aqueous solutions. J. Hazard. Mater. 2011, 190, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G.; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Marzougui, Z.; Chaabouni, A.; Elleuch, B.; Elaissari, A. Removal of bisphenol A and some heavy metal ions by polydivinylbenzene magnetic latex particles. Environ. Sci. Pollut. Res. 2016, 23, 15807–15819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Niu, H.; Zhang, X.; Meng, Z.; Cai, Y. Strong adsorption of chlorotetracycline on magnetite nanoparticles. J. Hazard. Mater. 2011, 192, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 2011, 46, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhou, L.; Li, X.; Yuan, J. β-Cyclodextrin-modified hybrid magnetic nanoparticles for catalysis and adsorption. J. Mater Chem. 2011, 21, 3704–3710. [Google Scholar] [CrossRef]
- Tao, W.; Li, A.; Long, C.; Qian, H.; Xu, D.; Chen, J. Adsorption of 5-sodiosulfoisophthalic acids from aqueous solution onto poly (2-vinylpyridine) resin. J. Hazard. Mater. 2010, 175, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Li, A.; Long, C.; Fan, Z.; Wang, W. Preparation, characterization and application of a copper (II)-bound polymeric ligand exchanger for selective removal of arsenate from water. J. Hazard. Mater. 2011, 193, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, S.; Wang, Z.; Zhang, X.; Jiang, M.; Chi, L.; Fuchs, H. Multilayer assemblies of copolymer PSOH and PVP on the basis of hydrogen bonding. Langmuir 2000, 16, 10490–10494. [Google Scholar] [CrossRef]
- Wang, P.; Shi, Q.; Shi, Y.; Clark, K.K.; Stucky, G.D.; Keller, A.A. Magnetic permanently confined micelle arrays for treating hydrophobic organic compound contamination. J. Am. Chem. Soc. 2008, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Akçay, M. The surface acidity and characterization of Fe-montmorillonite probed by in situ FT-IR spectroscopy of adsorbed pyridine. Appl. Catal. A Gen. 2005, 294, 156–160. [Google Scholar] [CrossRef]
- Huang, D.; Wang, X.; Deng, C.; Song, G.; Cheng, H.; Zhang, X. Facile preparation of raisin-bread sandwich-structured magnetic graphene/mesoporous silica composites with C18-modified pore-walls for efficient enrichment of phthalates in environmental water. J. Chromatogr. A 2014, 1325, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zheng, S.W.; Hong, R.Y.; Wang, Y.Q.; Feng, W.G. Preparation of magnetic poly(styrene-co-acrylic acid) microspheres with adsorption of protein. Colloids Surf. A 2014, 443, 425–431. [Google Scholar] [CrossRef]
- Orhan, T.; Hacaloglu, J. Thermal degradation of poly (2-vinylpyridine) copolymers. Polym. Degrad. Stabil. 2013, 98, 356–360. [Google Scholar] [CrossRef]
- Li, X.-G. High-resolution thermogravimetry of poly (4-vinylpyridine-co-divinylbenzene). React. Funct. Polym. 1999, 42, 53–58. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Figdore, P.E. Adsorption of surfactants on kaolinite: NaCl versus CaCl2, salt effects. J. Colloid Interface Sci. 1982, 87, 500–517. [Google Scholar] [CrossRef]
- Zhang, X.; Kah, M.; Jonker, M.T.; Hofmann, T. Dispersion state and humic acids concentration-dependent sorption of pyrene to carbon nanotubes. Environ. Sci. Technol. 2012, 46, 7166–7173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, T.; Karanfil, T. The effects of dissolved natural organic matter on the adsorption of synthetic organic chemicals by activated carbons and carbon nanotubes. Water Res. 2011, 45, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Chen, N.; Zhou, Y.; Li, B.; Gu, W.; Shi, X.; Xian, Y. Recyclable removal of bisphenol A from aqueous solution by reduced graphene oxide-magnetic nanoparticles: Adsorption and desorption. J. Colloid Interface Sci. 2014, 421, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Oikawa, K.; Kawata, K.; Ishihara, S.; Iyobe, T.; Yamada, A. Study of removal effect of bisphenol A and β-estradiol by porous carbon. J. Health Sci. 2004, 50, 588–593. [Google Scholar] [CrossRef]
- Kuo, C.-Y. Comparison with as-grown and microwave modified carbon nanotubes to removal aqueous bisphenol, A. Desalination 2009, 249, 976–982. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, J.; Xu, T.; Yang, L.; Jiang, X.; Li, Q. High efficiency removal and recovery of an endocrine disrupting compound-bisphenol AF from wastewaters. Sep. Purif. Technol. 2013, 116, 145–153. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Wu, X.; Qin, Y.; Cheng, J.; Chen, Y.; Tan, P.; Li, H. A novel magnesium ascorbyl phosphate graphene-based monolith and its superior adsorption capability for bisphenol A. Chem. Eng. J. 2018, 334, 948–956. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Zhang, W.; Xian, R. Shell-free three-dimensional graphene-based monoliths for the aqueous adsorption of organic pollutants. Chem. Eng. J. 2017, 316, 24–32. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.F. Elimination of bisphenol A from water via graphene oxide adsorption. Acta Phys. Chim. Sin. 2013, 29, 829–836. [Google Scholar]
- Acosta, R.; Nabarlatz, D.; Sánchez-Sánchez, A.; Jagiello, J.; Gadonneix, P.; Celzard, A.; Fierro, V. Adsorption of bisphenol A on KOH-activated tyre pyrolysis char. J. Environ. Chem. Eng. 2018, 6, 823–833. [Google Scholar] [CrossRef]

| Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Adsorbents | KL | Qm | R2 | KF | n | R2 |
| Mag-PVP | 0.09 | 115.87 | 0.94 | 16.20 | 2.09 | 0.93 |
| Fe3O4 nanospheres | 0.22 | 42.05 | 0.94 | 11.27 | 2.67 | 0.74 |
| Water Samples | Deionized Water (D) | Tap Water (T) | Surface Water (S) | Effluent Water (E) |
|---|---|---|---|---|
| Temperature (°C) | 19.8 | 19.8 | 19.9 | 19.9 |
| pH | 7.1 | 8.3 | 8.4 | 8.6 |
| Conductivity (μS/cm) | 1.2 | 241.4 | 348.3 | 1120.3 |
| Total organic carbon (mg/L) | 0 | 0.2 | 2.6 | 10.1 |
| Name of Sorbent | pH | Temp. (°C) | Qm (mg/g) | Reference |
|---|---|---|---|---|
| Mag-PVP | 6–7 | 20 | 115.9 | Our study |
| Porous carbon produced at 1000 from Moso bamboo | - | 23 | 41.8 | [41] |
| AC purchased from Wako | - | 23 | 56.5 | [41] |
| Modified CNTs | 6.0 | 7 | 70.0 | [42] |
| Magnetic chitosan fly-ash-cenospheres | 7.0 | 20 | 31.9 | [18] |
| Magnetic carbon nanotube | 6.2 | 25 | 45.3 | [17] |
| Multi-walled carbon nanotube | 5.2 | 25 | 88.27 | [43] |
| Magnetic particles MPDVB | 8.0 | 20 | 90.9 | [20] |
| MAP-GBM | 7.0 | 20 | 324.0 | [44] |
| Vc-GBM | 7.0 | 25 | 133.1 | [45] |
| Graphene oxide | 6.0 | 25 | 87.8 | [46] |
| KOH-activated tyre pyrolysis char | 6.5–7.5 | 25 | 123.0 | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Pan, F.; Li, W.; Li, D.; Xu, H.; Xia, D.; Li, A. Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles. Polymers 2018, 10, 1136. https://doi.org/10.3390/polym10101136
Li Q, Pan F, Li W, Li D, Xu H, Xia D, Li A. Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles. Polymers. 2018; 10(10):1136. https://doi.org/10.3390/polym10101136
Chicago/Turabian StyleLi, Qiang, Fei Pan, Wentao Li, Dongya Li, Haiming Xu, Dongsheng Xia, and Aimin Li. 2018. "Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles" Polymers 10, no. 10: 1136. https://doi.org/10.3390/polym10101136
APA StyleLi, Q., Pan, F., Li, W., Li, D., Xu, H., Xia, D., & Li, A. (2018). Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles. Polymers, 10(10), 1136. https://doi.org/10.3390/polym10101136







