An Oligoimide Particle as a Pickering Emulsion Stabilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Characterization
2.4. Preparation of PMDA-ODA Particles in Water
2.5. Preparation of PMDA-ODA Particles in NMP
2.6. Preparation of Reference PMDA-ODA Sample for Degree of Imidization (DI) Measurement
2.7. DI Measurement
2.8. Preparation of Pickering Emulsions Stabilized by PMDA-ODA Particles Synthesized in Water
2.9. Conductivity Measurements
2.10. Drop Test
2.11. Percentage of Survived Emulsion Measurements
2.12. Microencapsulation
3. Results and Discussion
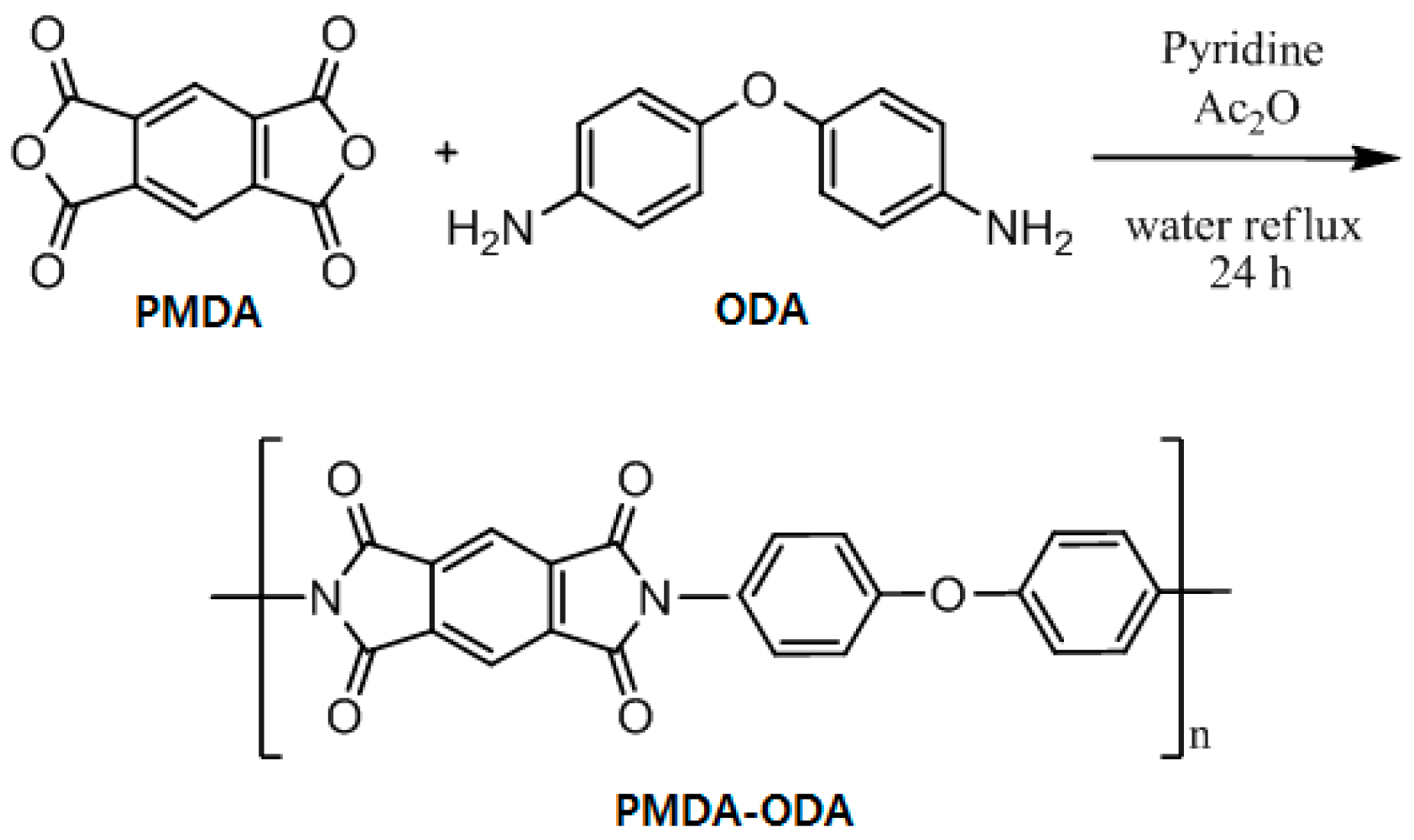
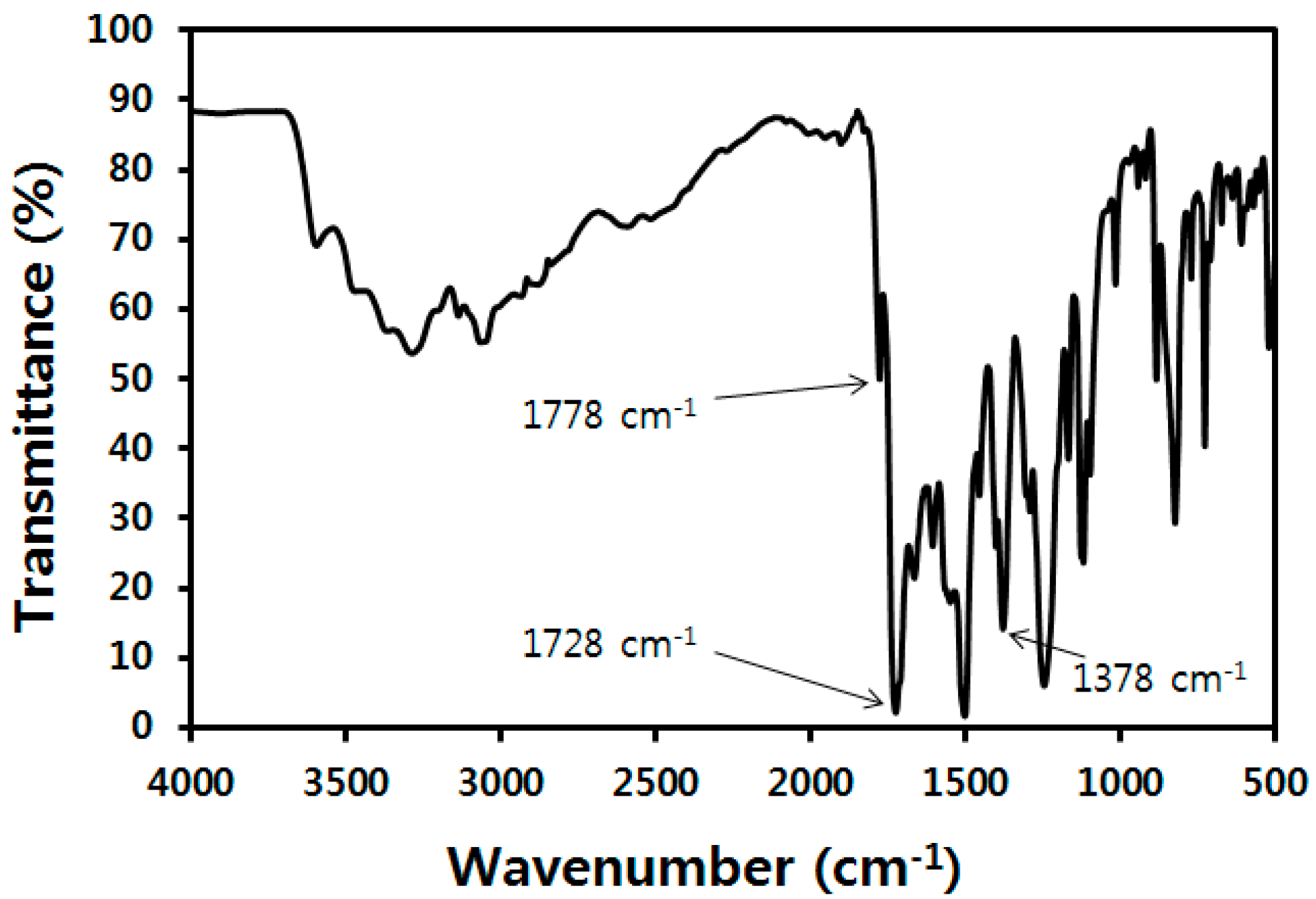
3.1. Synthesis and Properties of Oligoimides
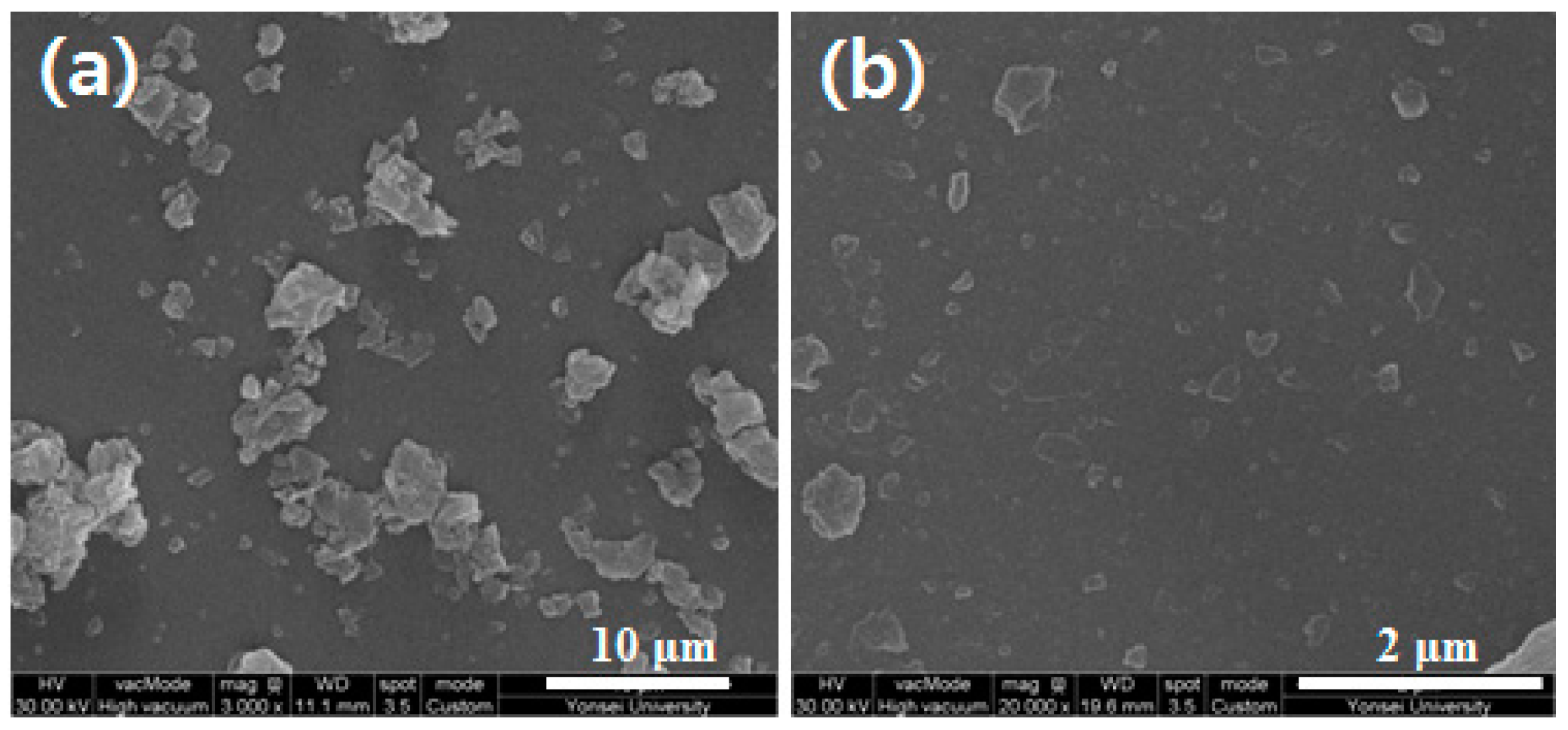
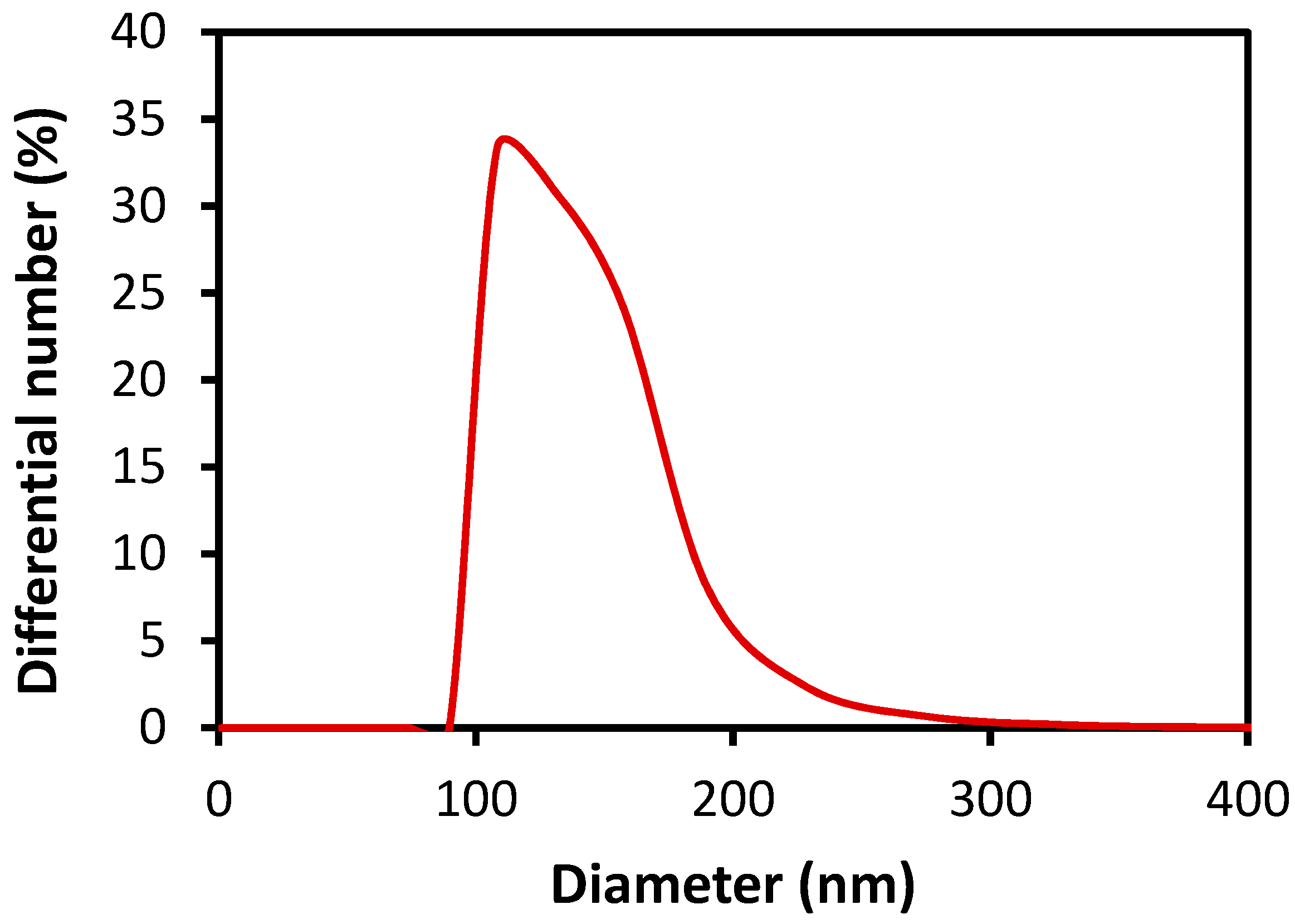
3.2. Characterization and Dispersion of Oligoimide Particles
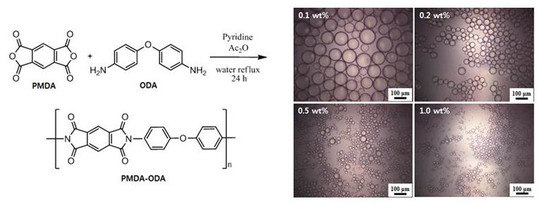
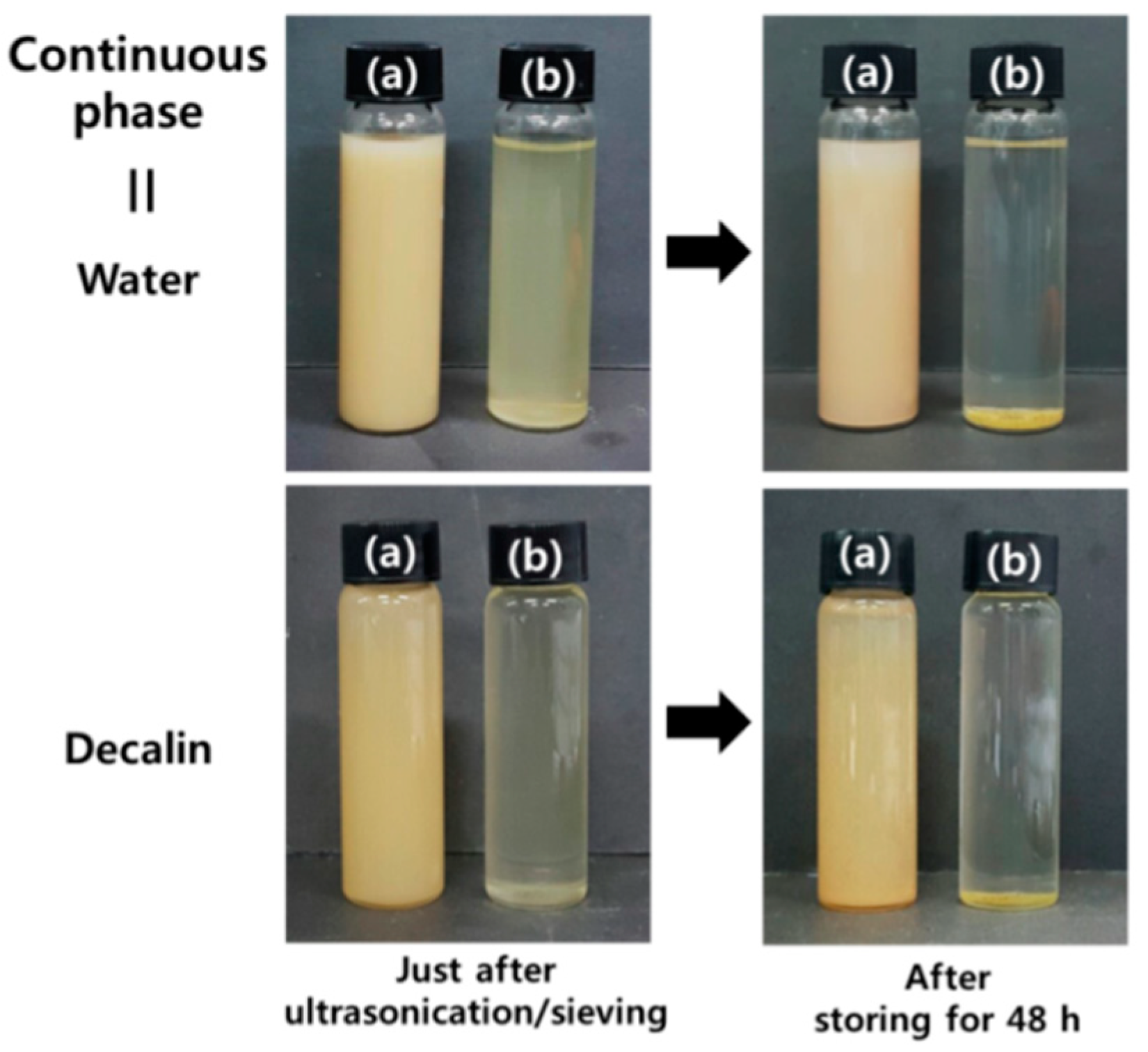
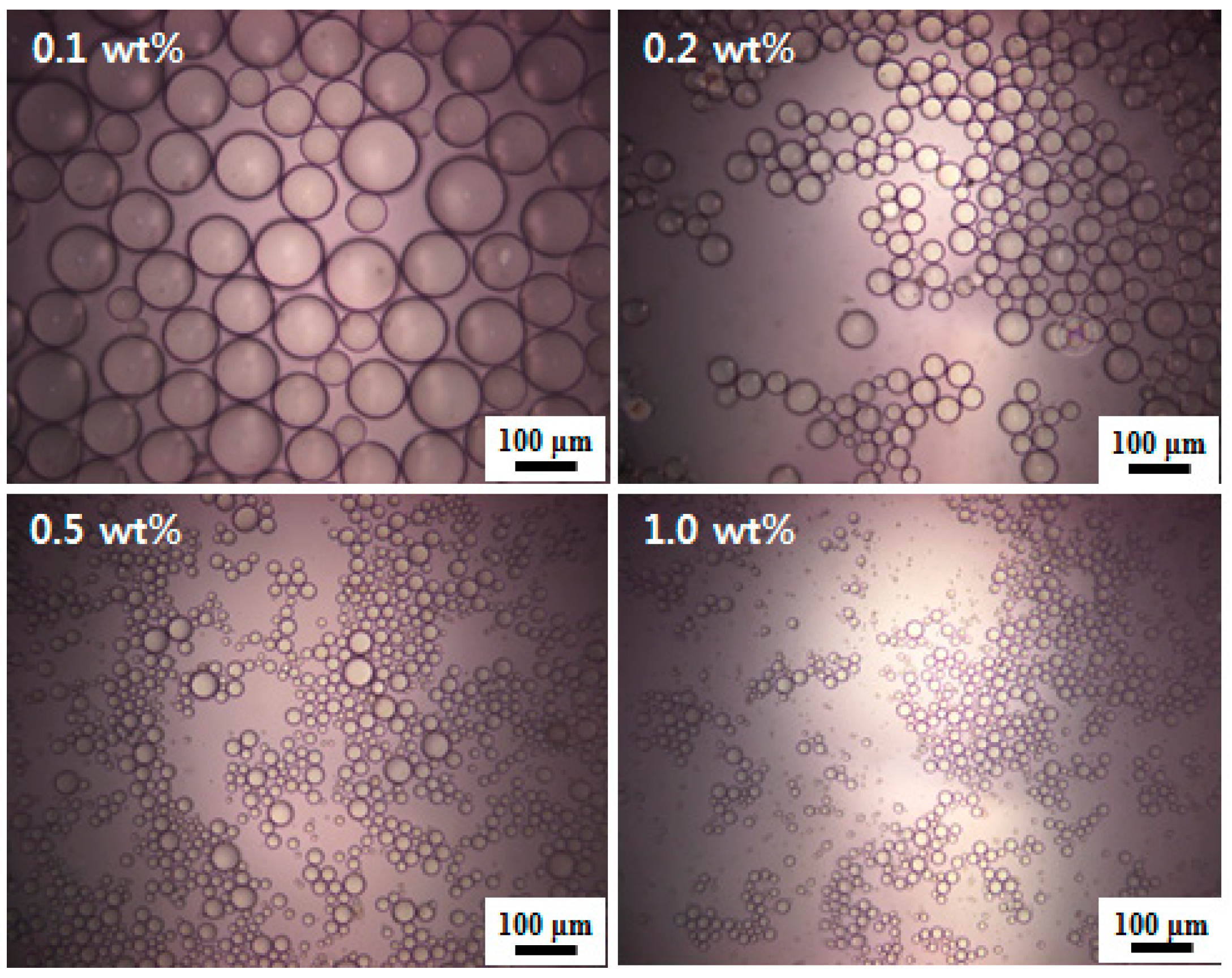
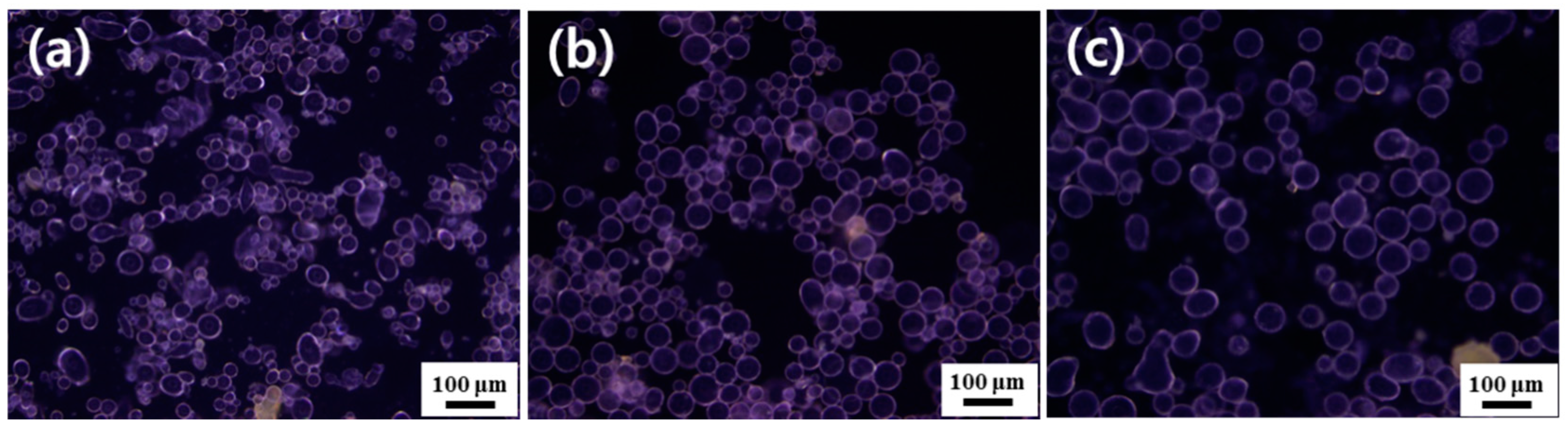
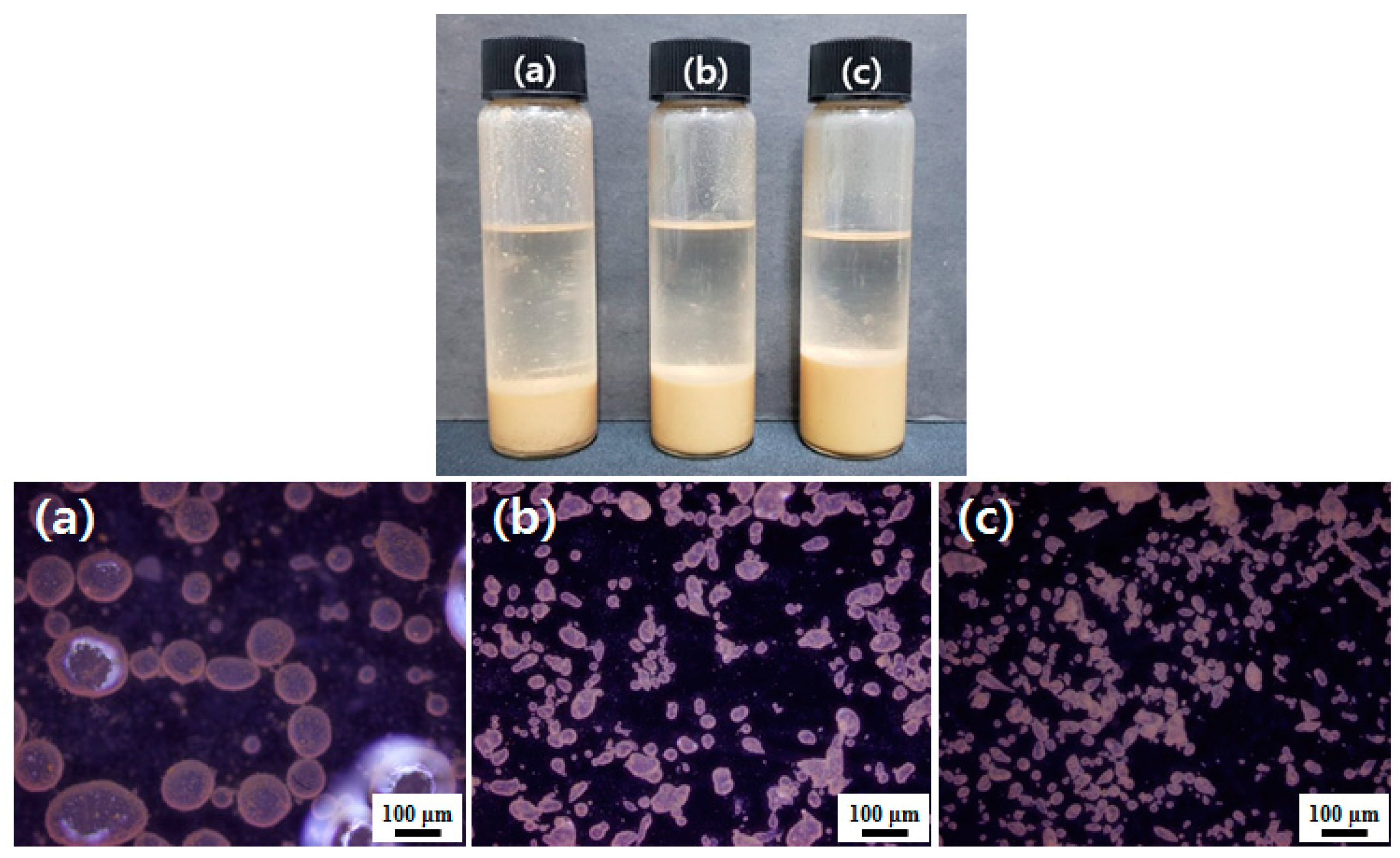
3.3. Oil-in-Water Pickering Emulsions
3.4. Water-in-Oil Pickering Emulsions
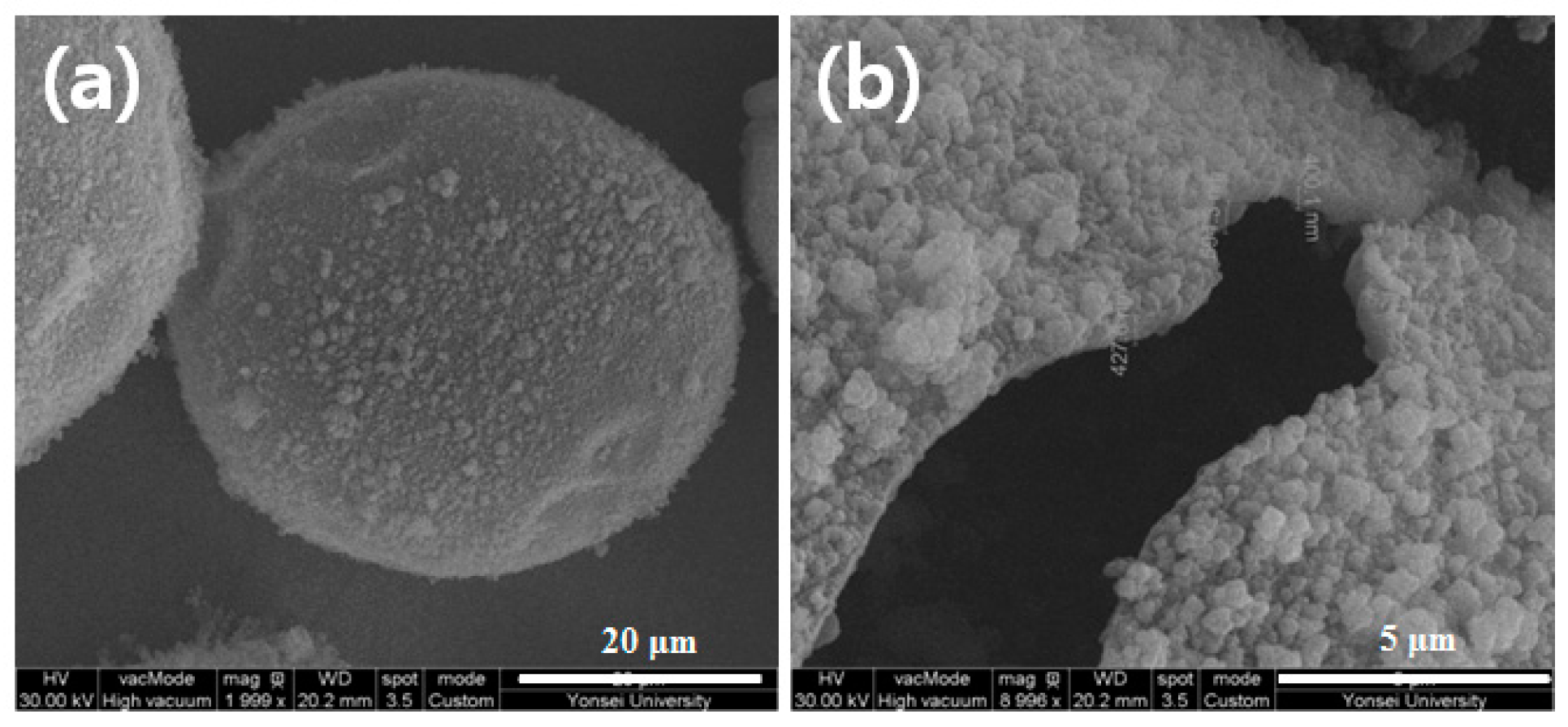
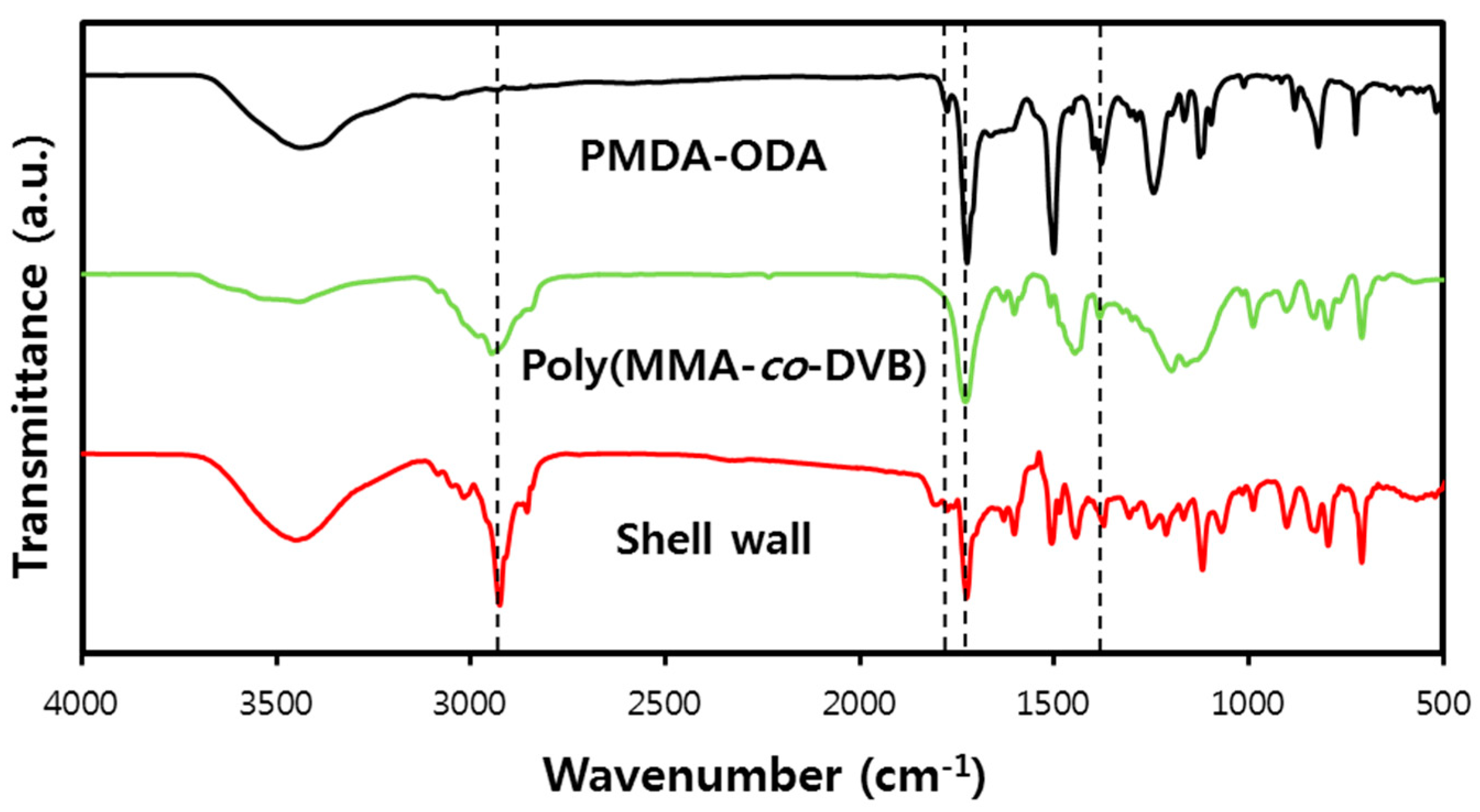
3.5. Microencapsulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Aspects 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Tuning amphiphilicity of particles for controllable Pickering emulsion. Materials 2016, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Daware, S.V.; Basavaraj, M.G. Emulsions stabilized by silica rods via arrested demixing. Langmuir 2015, 31, 6649–6654. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Ye, L.; Kong, M.; Yang, Q.; Li, G.; Huang, Y. Pickering emulsions stabilized by shape-controlled silica microrods. RSC Adv. 2016, 6, 24195–24202. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Xu, J.; Lan, Q.; Wei, F.; Sun, D. Pickering emulsions stabilized solely by layered double hydroxides particles: The effect of salt on emulsion formation and stability. J. Colloid Interface Sci. 2006, 302, 159–169. [Google Scholar] [CrossRef] [PubMed]
- De Folter, J.W.J.; Hutter, E.M.; Castillo, S.I.R.; Klop, K.E.; Philipse, A.P.; Kegel, W.K. Particle shape anisotropy in Pickering emulsions: Cubes and peanuts. Langmuir 2014, 30, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.J.; Jung, H.S.; Choi, H.J. Pickering emulsion polymerized smart magnetic poly(methyl methacrylate)/Fe2O3 composite particles and their stimulus-response. RSC Adv. 2015, 5, 23094–23100. [Google Scholar] [CrossRef]
- Creighton, M.A.; Zhu, W.; Van Krieken, F.; Petteruti, R.A.; Gao, H.; Hurt, R.H. Three-dimensional graphene-based microbarriers for controlling release and reactivity in colloidal liquid phases. ACS Nano 2016, 10, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Tasset, S.; Cathala, B.; Bizot, H.; Capron, I. Versatile cellular foams derived from CNC-stabilized Pickering emulsions. RSC Adv. 2014, 4, 893–898. [Google Scholar] [CrossRef]
- Nishizawa, N.; Kawamura, A.; Kohri, M.; Nakamura, Y.; Fujii, S. Polydopamine particle as a particulate emulsifier. Polymers 2016, 8, 62. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Sun, G.; Nan, F.; Ngai, T.; Ma, G. Systematic studies of Pickering emulsions stabilized by uniform-sized PLGA particles: Preparation and stabilization mechanism. J. Mater. Chem. B 2014, 2, 7605–7611. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive Pickering emulsion stabilized by poly 2-(dimethylamino)ethyl methacrylate grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, Q.; Xu, Z. Surfactant-free switchable emulsions using CO2-responsive particles. ACS Appl. Mater. Interfaces 2014, 6, 6898–6904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, L.; Bing, W.; Zhang, Z.; Li, Z.; Ren, J.; Qu, X. Light controlled reversible inversion of nanophosphor-stabilized Pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 2014, 136, 7498–7504. [Google Scholar] [CrossRef] [PubMed]
- Santini, E.; Guzman, E.; Ferrari, M.; Liggieri, L. Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water-hexane interface. Colloids Surf. A 2014, 460, 333–341. [Google Scholar] [CrossRef]
- Sturzenegger, P.N.; Gonzenbach, U.T.; Koltzenburg, S.; Gauckler, L.J. Controlling the formation of particle-stabilized water-in-oil emulsions. Soft Matter 2012, 8, 7471–7479. [Google Scholar] [CrossRef]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic stabilization of emulsions and emulsion gels with water-soluble polymers and cellulose nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Temperature-induced inversion of nanoparticle-stabilized emulsions. Angew. Chem. Int. Ed. 2005, 44, 4795–4798. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ghosh, K.L.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Yudin, V.E.; Divoux, G.M.; Otaigbe, J.U.; Svetichnyi, V.M. Synthesis and rheological properties of oligoimide/montmorillonite nanocomposites. Polymer 2005, 46, 10866–10872. [Google Scholar] [CrossRef]
- Cammarata, V.; Kolaskie, C.J.; Miller, L.L.; Stallman, B.J. Rigid rod oligoimides form oriented Langmuir-Blodgett Films. J. Chem. Soc. Chem. Commun. 1990, 1290–1292. [Google Scholar] [CrossRef]
- Andre, S.; Guida-Pietrasanta, F.; Rousseau, A.; Boutevin, B.; Caporiccio, G. Synthesis, characterization, and thermal properties of anhydride terminated and allyl terminated oligoimides. J. Polym. Sci. A Polym. Chem. 2000, 38, 2993–3003. [Google Scholar] [CrossRef]
- Sun, H.; Huo, H.; Nie, H.; Yang, S.; Fan, L. Phenylethynyl terminated oligoimides derived from 3,3′,4,4′-diphenylsulfonetetracarboxylic dianhydride and their adhesive properties. Eur. Polym. J. 2009, 45, 1169–1178. [Google Scholar] [CrossRef]
- Shirata, T.; Kon, T.; Sasaki, K.; Oishi, Y.; Shibasaki, Y. Preparation of polyimide-cellulose composite using oligoimide with ethynyl terminals. Chem. Lett. 2014, 43, 787–789. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Aspects 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Yu, H.-C.; Jung, J.-W.; Choi, J.-Y.; Chung, C.-M. Kinetic study of low-temperature imidization of poly(amic acid)s and preparation of colorless, transparent polyimide films. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Li, Z.; Xu, Q.; Zhang, Q. Polyimide fibers prepared by dry-spinning process: Imidization degree and mechanical properties. J. Mater. Sci. 2013, 48, 7863–7868. [Google Scholar] [CrossRef]
- Oishi, Y.; Kakimoto, M.; Imai, Y. Synthesis of aromatic polyimides from N,N′-Bis(trimethylsily1)-substituted aromatic diamines and aromatic tetracarboxylic dianhydrides. Macromolecules 1991, 24, 3475–3480. [Google Scholar] [CrossRef]
- Fujii, S.; Aichi, A.; Muraoka, M.; Kishimoto, N.; Iwahori, K.; Nakamura, Y.; Yamashita, I. Ferritin as a bionano-particulate emulsifier. J. Colloid Interface Sci. 2009, 338, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kotera, M.; Nishino, T.; Nakamae, K. Imidization processes of aromatic polyimide by temperature modulated DSC. Polymer 2000, 41, 3615–3619. [Google Scholar] [CrossRef]
- Mushtaq, N.; Chen, G.; Sidra, L.R.; Fang, X. Organosoluble and high Tg polyimides from asymmetric diamines containing N-amino and N-aminophenyl naphthalimide moieties. RSC Adv. 2016, 6, 25302–25310. [Google Scholar] [CrossRef]
- Vora, R.H. Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; Mittal, K.L., Ed.; VSP: Leiden, The Netherlands, 2007. [Google Scholar]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Wang, J.; Yang, F.; Tan, J.; Liu, G.; Xu, J.; Sun, D. Pickering emulsions stabilized by a lipophilic surfactant and hydrophilic platelike particles. Langmuir 2010, 26, 5397–5404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, L.; Wang, J.; Xu, J.; Sun, D. Phase Inversion of Emulsions Containing a Lipophilic Surfactant Induced by Clay Concentration. Langmuir 2013, 29, 3889–3894. [Google Scholar] [CrossRef] [PubMed]
- Abend, S.; Bonnke, N.; Gutschner, U.; Lagaly, G. Stabilization of emulsions by heterocoagulation of clay minerals and layered double hydroxides. Colloid Polym. Sci. 1998, 276, 730–737. [Google Scholar] [CrossRef]
- Abend, S.; Lagaly, G. Bentonite and double hydroxides as emulsifying agents. Clay Miner. 2001, 36, 557–570. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Lotya, M.; Rakovich, A.; Donegan, J.F.; Coleman, J.N. Measuring the lateral size of liquid-exfoliated nanosheets with dynamic light scattering. Nanotechnology 2013, 24, 265703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Xu, K.; Liu, C.; Li, Y.; Lu, C.; Wang, P. Fabrication of starch-based nanospheres to stabilize Pickering emulsion. Carbohydr. Polym. 2012, 88, 1358–1363. [Google Scholar] [CrossRef]
- Ma, C.; Bi, X.; Ngai, T.; Zhang, G. Polyurethane-based nanoparticles as stabilizers for oil-in-water or water-in-oil Pickering emulsions. J. Mater. Chem. A 2013, 1, 5353–5360. [Google Scholar] [CrossRef]
- Fujii, S.; Read, E.S.; Binks, B.P.; Armes, S.P. Stimulus-responsive emulsifiers based on nanocomposite microgel particles. Adv. Mater. 2005, 17, 1014–1018. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles. Langmuir 2006, 22, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Salari, J.W.O. Pickering Emulsions, Colloidosomes & Micro-Encapsulation; Technische Universiteit: Eindhoven, The Netherlands, 2011. [Google Scholar]
- Teo, G.H.; Ng, Y.H.; Zetterlund, P.B.; Thickett, S.C. Factors influencing the preparation of hollow polymer-graphene oxide microcapsules via Pickering miniemulsion polymerization. Polymer 2015, 63, 1–9. [Google Scholar] [CrossRef]


| Yield (%) | Inherent Viscosity a (dL/g) | DI b (%) | Thermal Properties (°C) | ||
|---|---|---|---|---|---|
| T5 c | T10 d | Tg e | |||
| 97 | 0.15 | 95 | 476 | 513 | 352 |
| Particle Concentration (wt %) | pH of Dispersion | Conductivity of Dispersion (μS cm−1) | Conductivity of Emulsion (μS cm−1) | Type of Emulsion Formed a | Emulsion Remaining for 48 h b (%) | Mean Droplet Diameter c (μm) |
|---|---|---|---|---|---|---|
| 0.1 | 4.88 | 60 | 44 | Oil-in-water | ~100 | ~118 |
| 0.2 | 4.47 | 111 | 98 | Oil-in-water | ~100 | ~45 |
| 0.5 | 4.10 | 243 | 201 | Oil-in-water | ~100 | ~19 |
| 1.0 | 4.02 | 287 | 239 | Oil-in-water | ~100 | ~11 |
| Oil Used | pH of Dispersion | Conductivity of Dispersion (μS cm−1) | Conductivity of Emulsion (μS cm−1) | Type of Emulsion Formed a | Emulsion Remaining for 48 h b (%) | Mean Droplet Diameter c (μm) |
|---|---|---|---|---|---|---|
| n-Hexadecane | 4.10 | 243 | 201 | Oil-in-water | ~100 | ~19 |
| Linseed oil | 4.10 | 243 | 202 | Oil-in-water | ~100 | ~30 |
| Olive oil | 4.10 | 243 | 200 | Oil-in-water | ~100 | ~41 |
| Phenyl acetate | 4.10 | 243 | 203 | Oil-in-water | ~100 | ~59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-J.; Kim, D.-M.; Song, I.-H.; Choi, J.-Y.; Jin, S.-W.; Kim, B.-J.; Jeong, J.-W.; Jang, C.-E.; Chu, K.; Chung, C.-M. An Oligoimide Particle as a Pickering Emulsion Stabilizer. Polymers 2018, 10, 1071. https://doi.org/10.3390/polym10101071
Cho Y-J, Kim D-M, Song I-H, Choi J-Y, Jin S-W, Kim B-J, Jeong J-W, Jang C-E, Chu K, Chung C-M. An Oligoimide Particle as a Pickering Emulsion Stabilizer. Polymers. 2018; 10(10):1071. https://doi.org/10.3390/polym10101071
Chicago/Turabian StyleCho, Yu-Jin, Dong-Min Kim, In-Ho Song, Ju-Young Choi, Seung-Won Jin, Beom-Jun Kim, Jin-Won Jeong, Chae-Eun Jang, Kunmo Chu, and Chan-Moon Chung. 2018. "An Oligoimide Particle as a Pickering Emulsion Stabilizer" Polymers 10, no. 10: 1071. https://doi.org/10.3390/polym10101071