Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
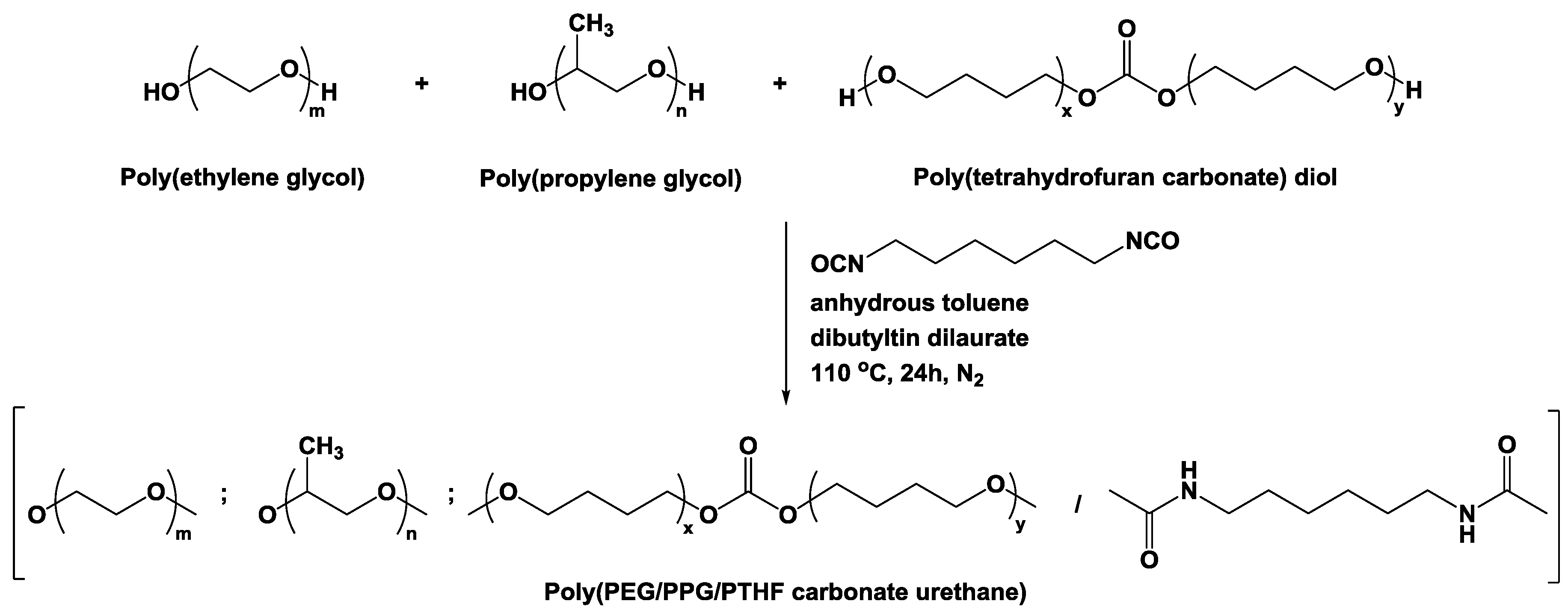
2.2. Synthesis of Poly(PEG/PPG/PTHF Carbonate Urethane)s
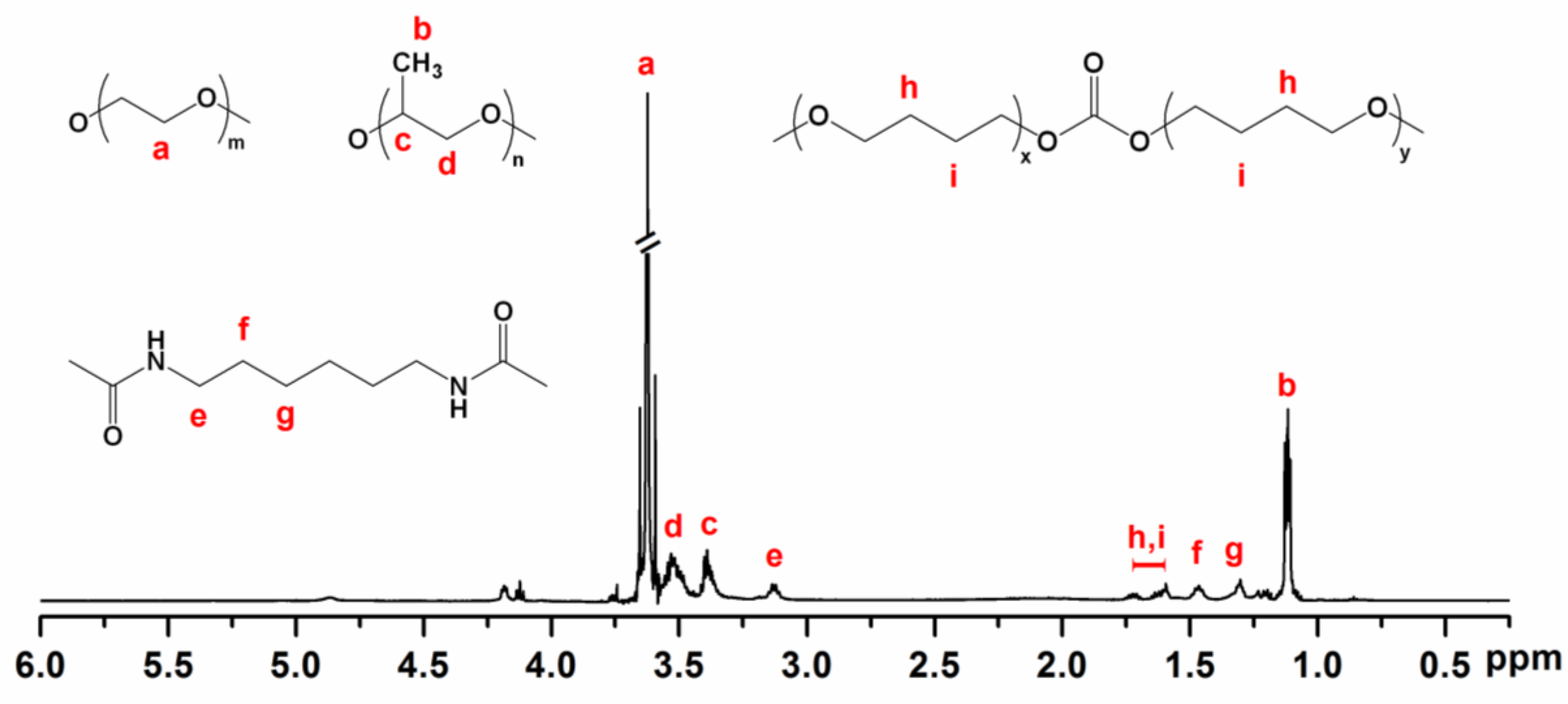
2.3. Molecular Characterisation
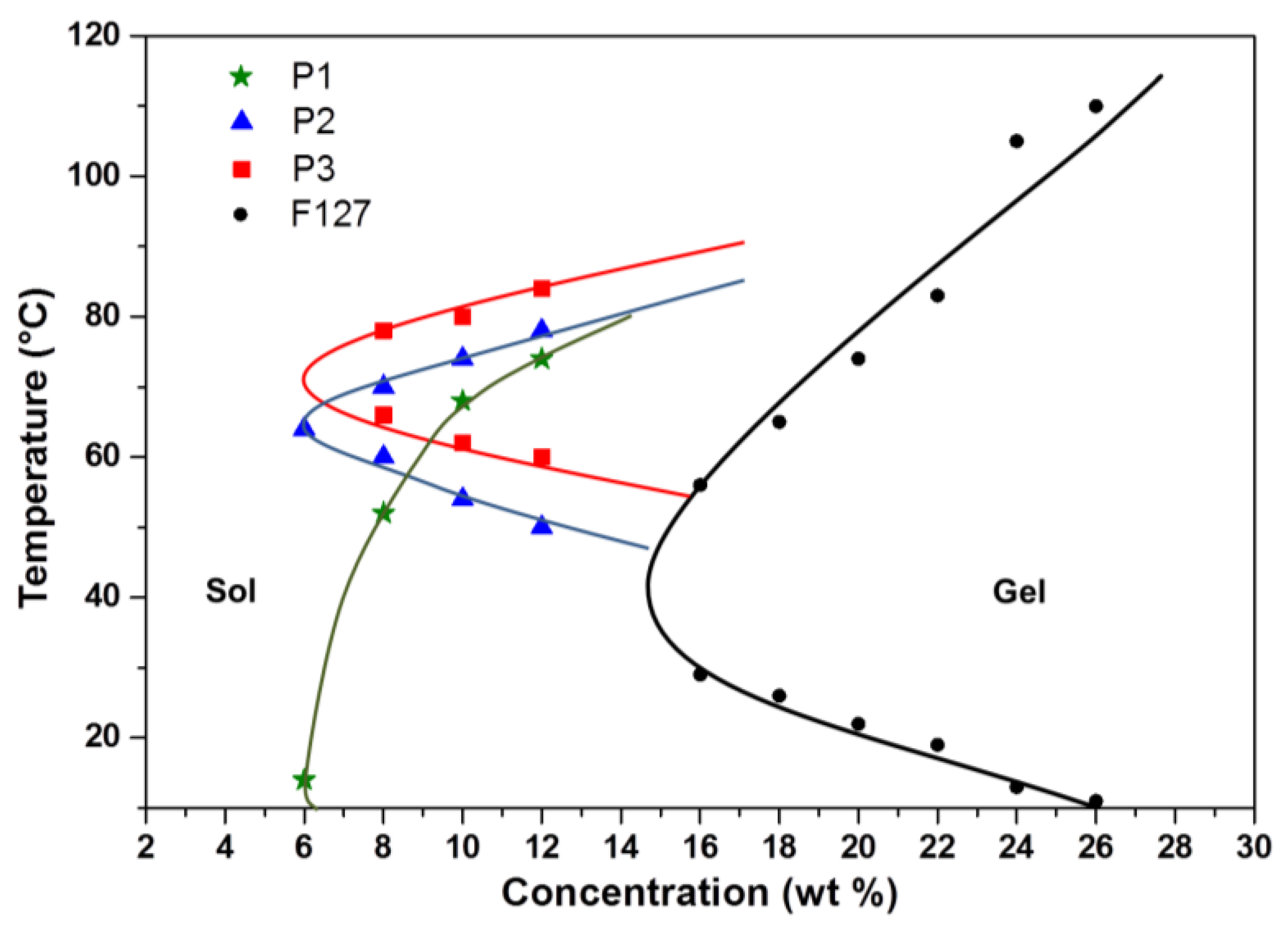
2.4. Determination of Sol-Gel Transition Temperatures
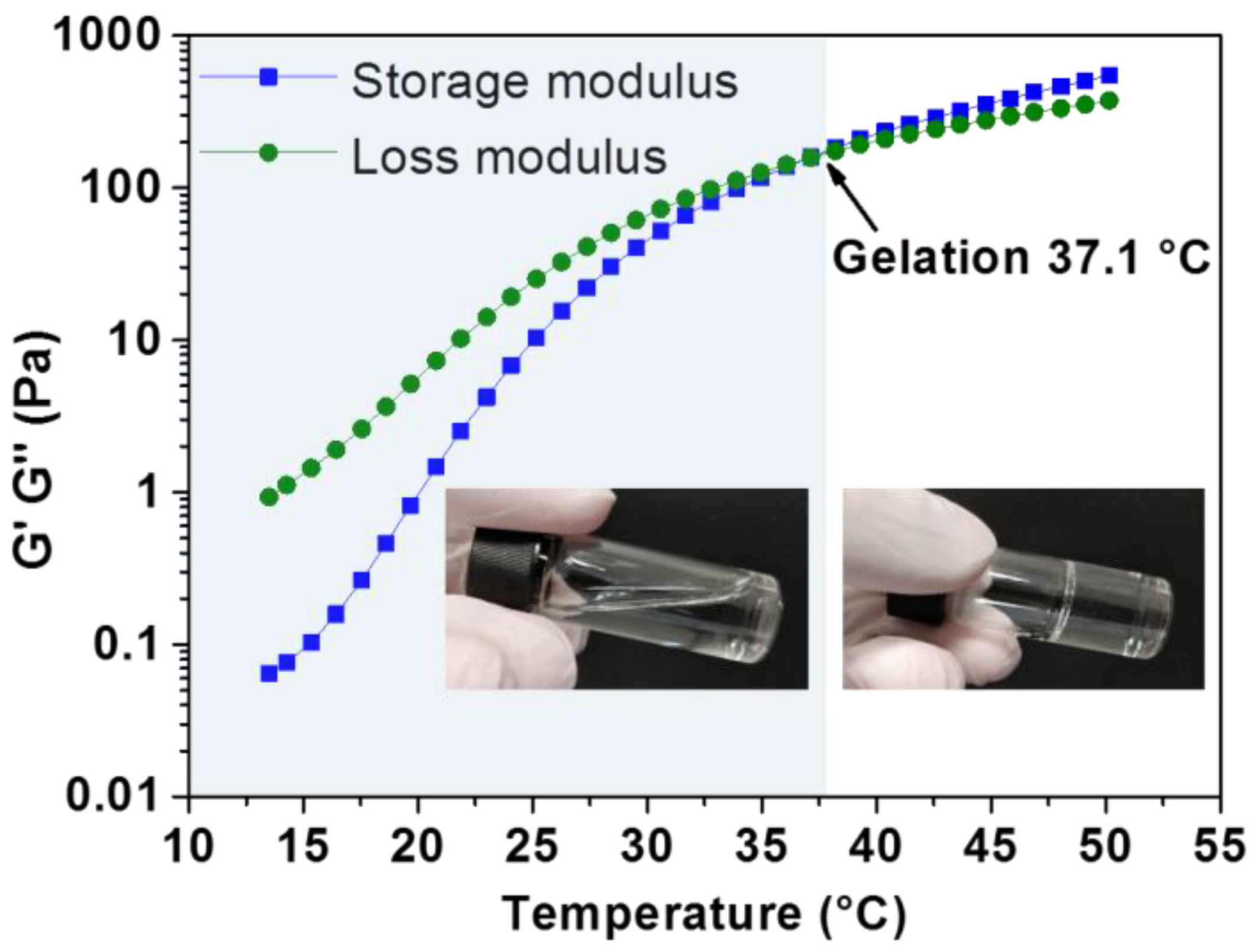
2.5. Rheological Characterisation
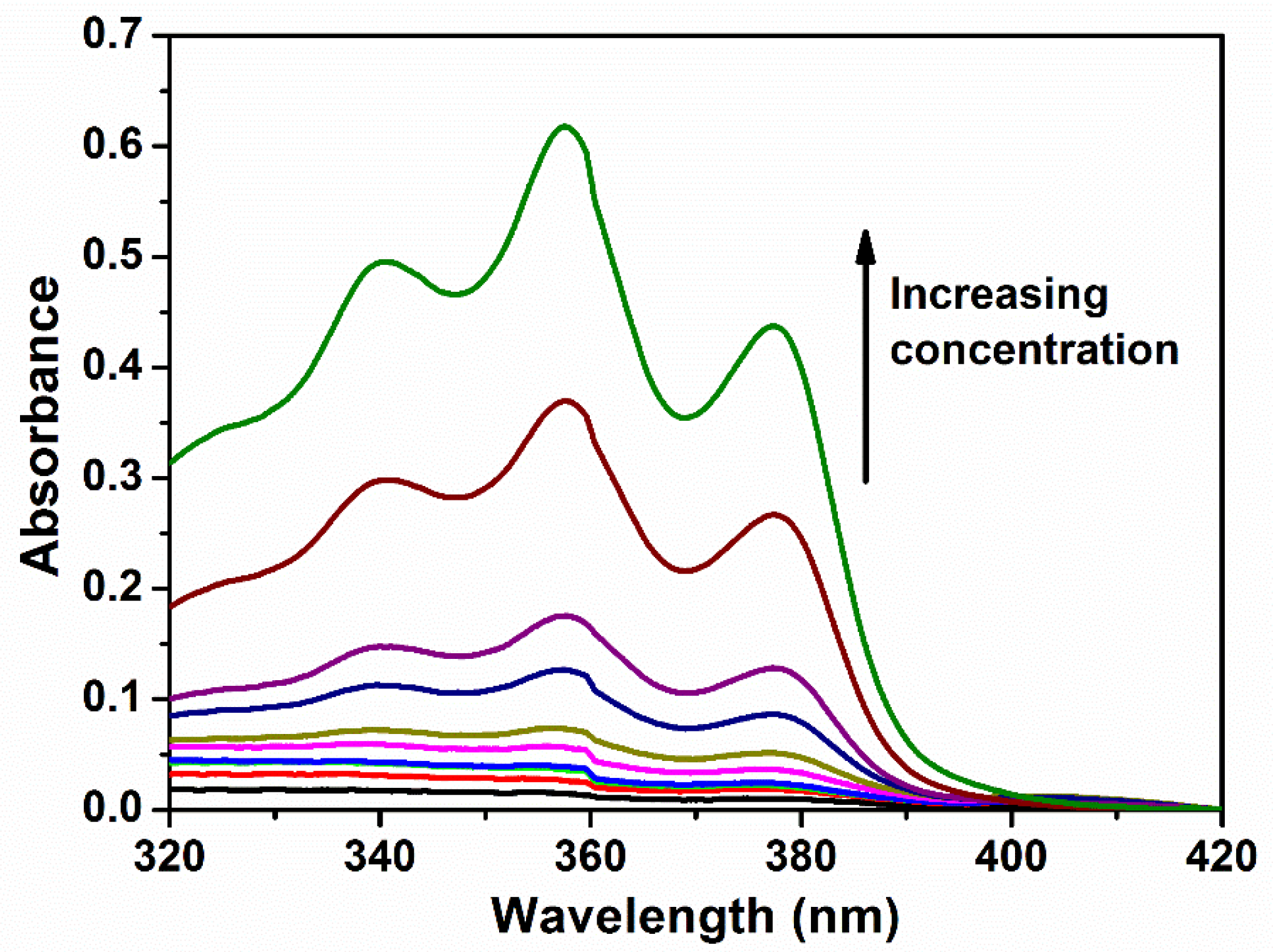
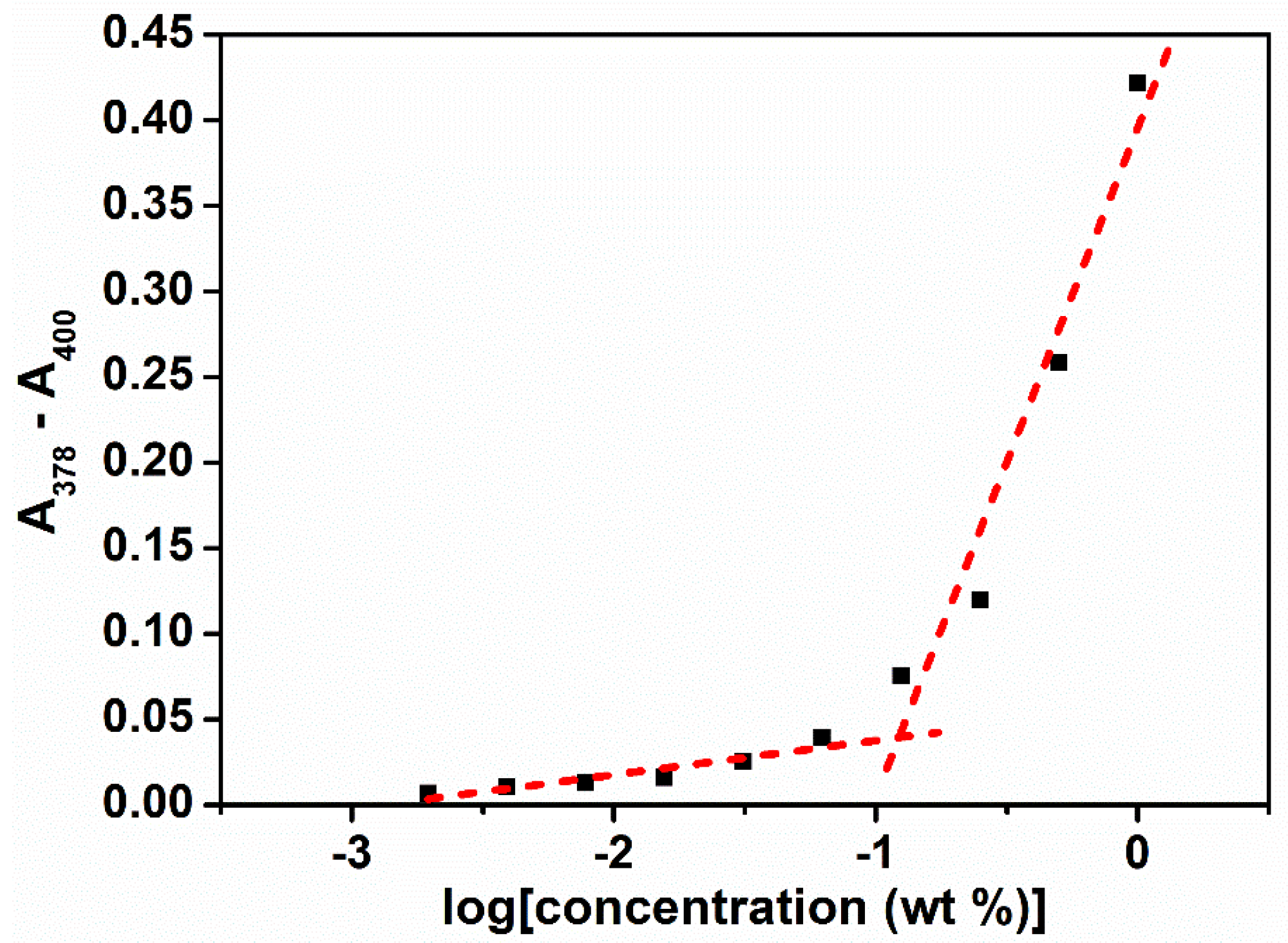
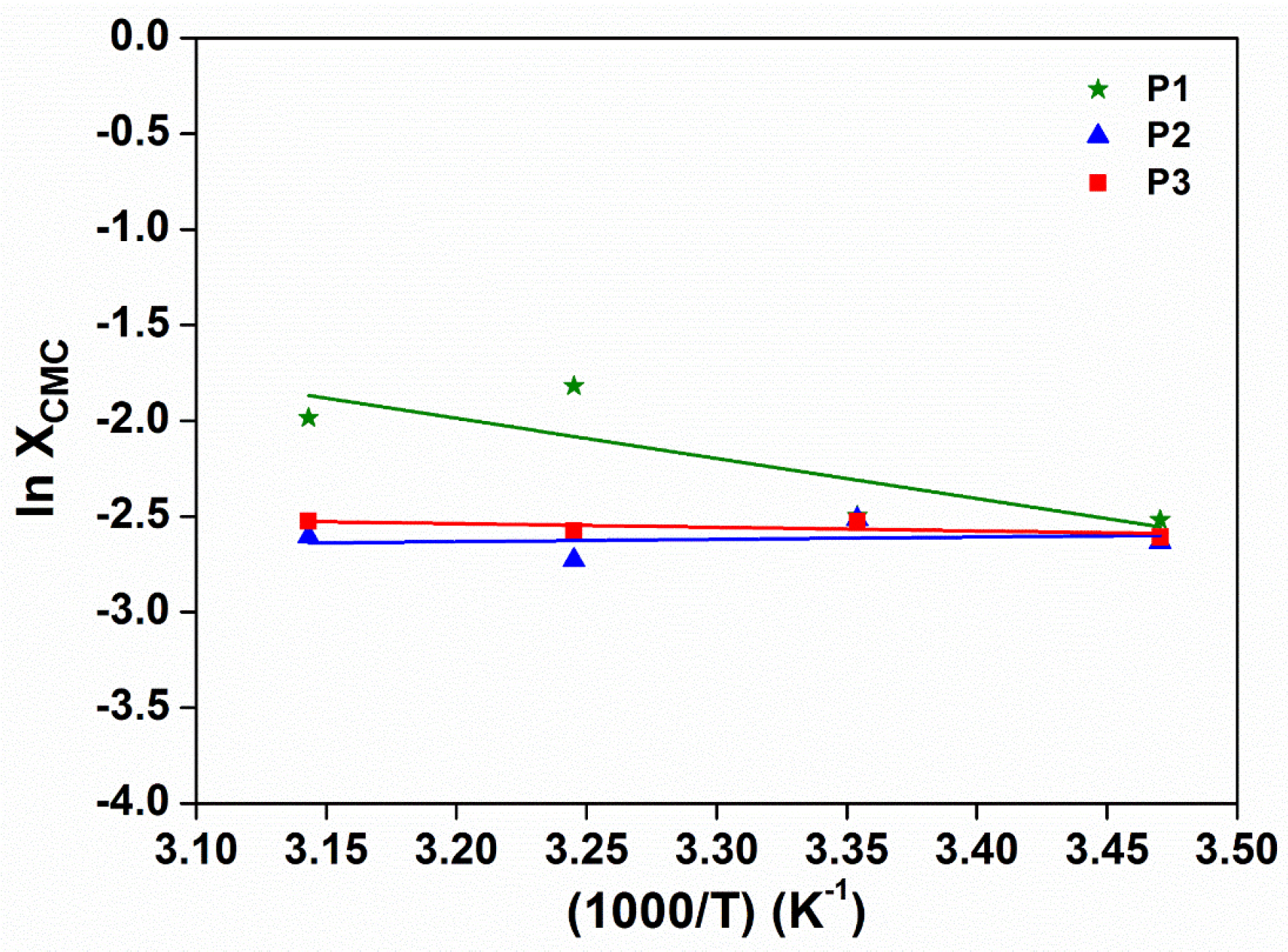
2.6. Determination of Critical Micelle Concentration (CMC)
2.7. Cell Culture
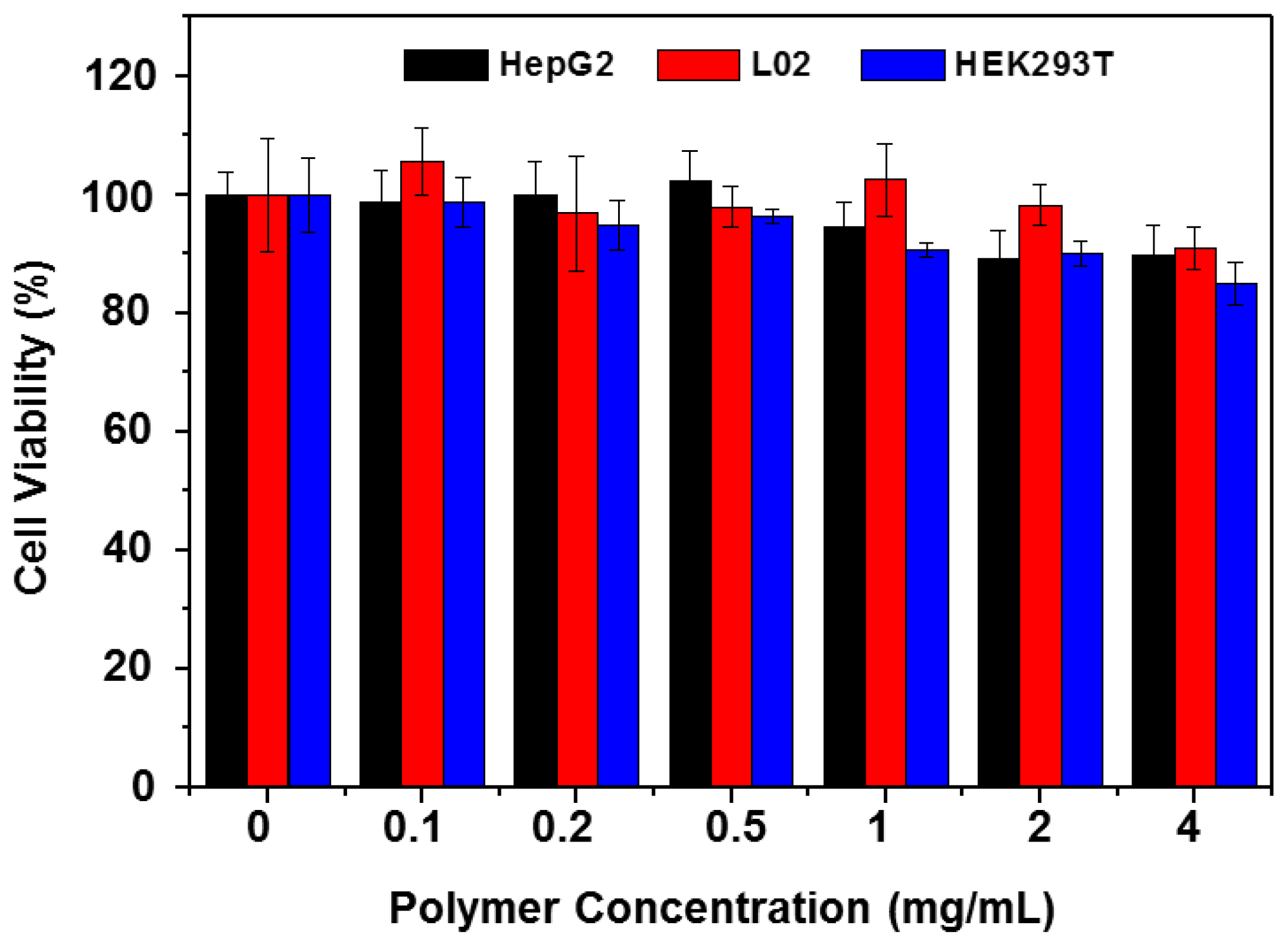
2.8. Cell Viability Assay
2.9. Preparation of Doxorubicin-Loaded Thermogel and In Vitro Drug Release Study
2.10. In Vivo Xenograft Tumor Assay
2.11. Histological Staining Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Molecular Characteristics of Poly(PEG/PPG/PTHF Carbonate Urethane)s
3.2. Thermo-Responsive Reversible Sol-Gel Transition
3.3. Micellar Properties of Poly(PEG/PPG/PTHF Carbonate Urethane)s in Aqueous Solutions
3.4. Cellular Toxicity of the Copolymer
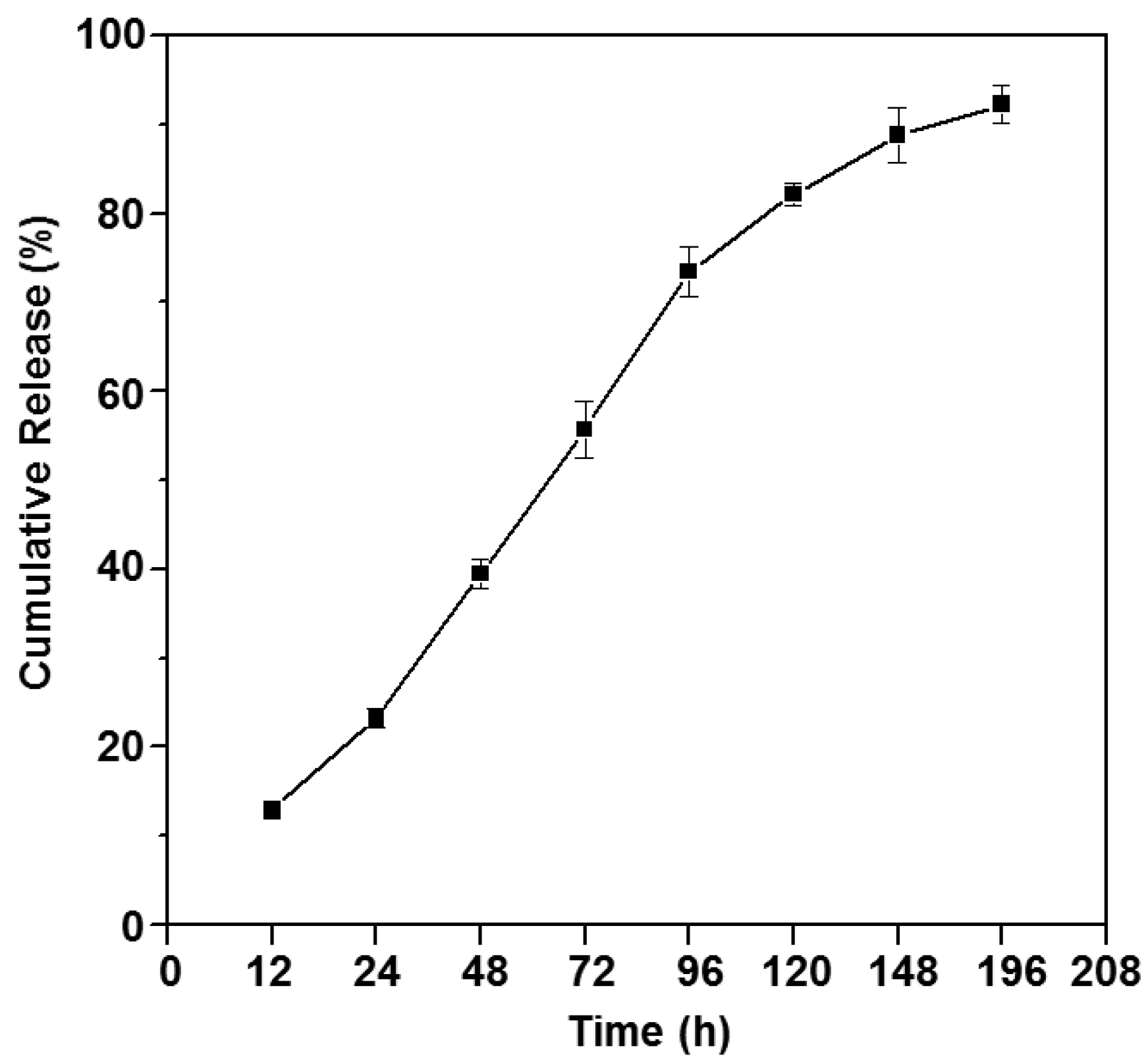
3.5. In Vitro Drug Release Study
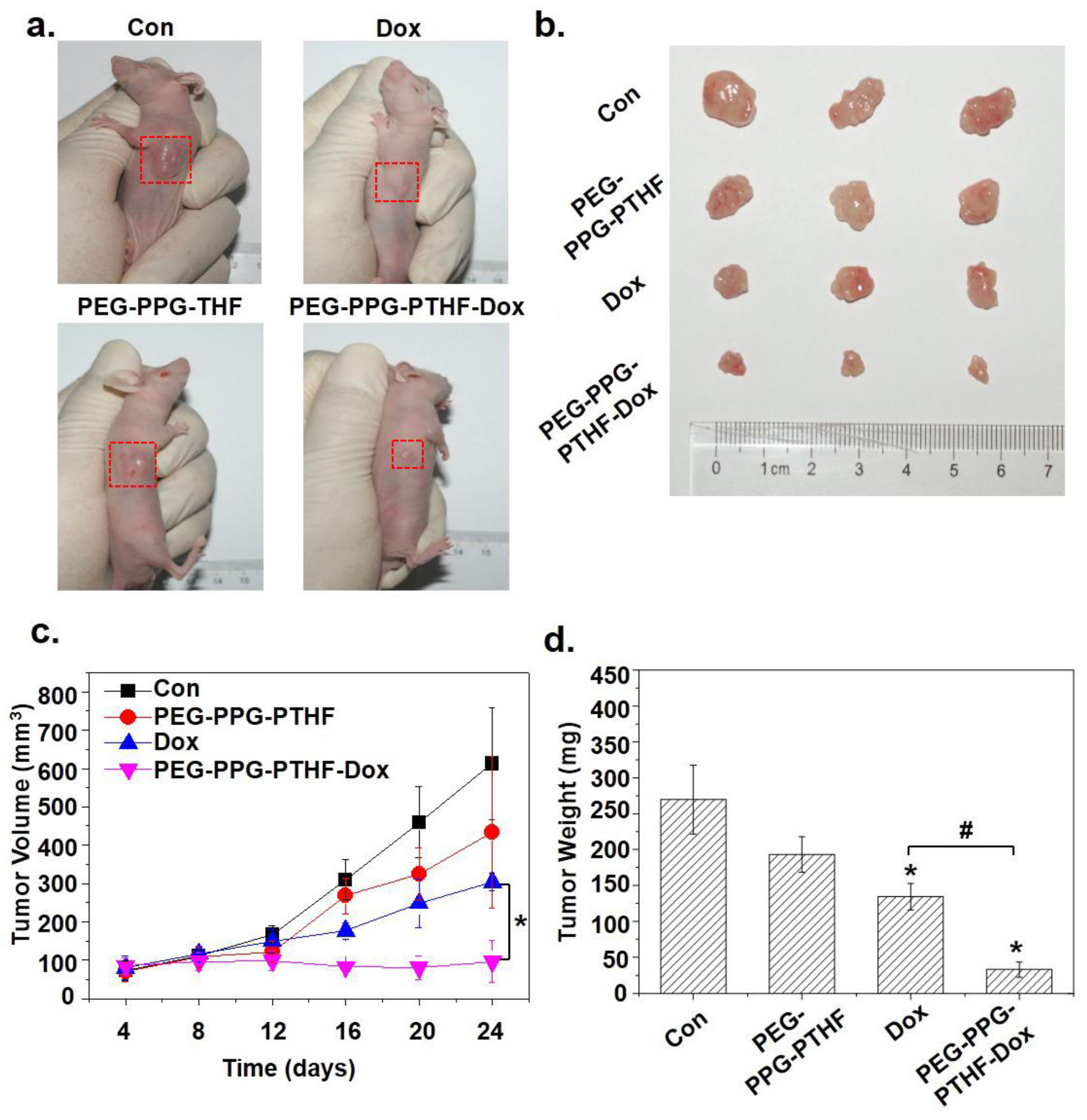
3.6. In Vivo Tumour Growth Inhibition
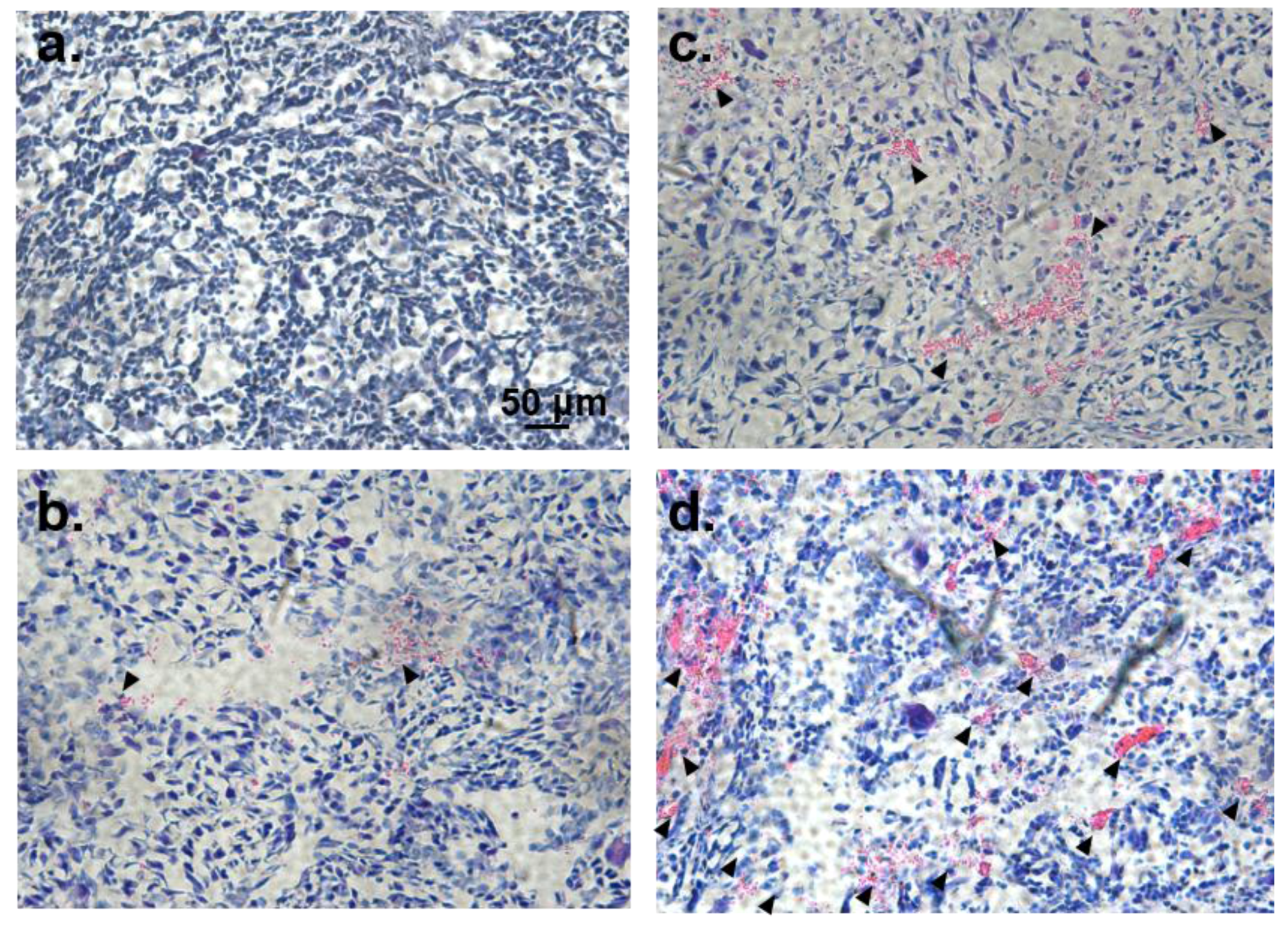
3.7. Histological Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Chua, M.X.; Li, Z.; Loh, X.J.; Wu, Y.-L. Injectable Supramolecular Hydrogels as Delivery Agents of BCL-2 Conversion Gene for the Effective Shrinkage of Therapeutic Resistance Tumors. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Dou, Q.; Li, Z.; Loh, X.J. Emerging Supramolecular Therapeutic Carriers Based on Host-Guest Interactions. Chem. Asian J. 2016, 11, 1300–1321. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Oo, N.N.L.; Lee, J.P.; Li, Z.; Loh, X.J. Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov. Today 2017, 22, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.J.; Li, Z.; Wong, M.; Rajamani, L.; Lingam, G.; Loh, X.J. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules 2015, 16, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z. Ionic skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent Advances in Shape Memory Soft Materials for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J. Control of Hyperbranched Structure of Polycaprolactone/Poly(ethylene glycol) Polyurethane Block Copolymers by Glycerol and Their Hydrogels for Potential Cell Delivery. J. Phys. Chem. B 2013, 117, 14763–14774. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater.Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable Hyperbranched Amphiphilic Polyurethane Multiblock Copolymers Consisting of Poly(propylene glycol), Poly(ethylene glycol), and Polycaprolactone as in Situ Thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.Y.; Liow, S.S.; Li, Z.; Wu, Y.-L.; Loh, X.J. New Poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] (P3HB4HB)-Based Thermogels. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; Lakshminarayanan, R.; Loh, X.J. Recent Advances of Using Hybrid Nanocarriers in Remotely Controlled Therapeutic Delivery. Small 2016, 35, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chung, J.Y.; Lim, Y.X.; Li, Z.; Loh, X.J. A Review of Adaptive Programmable Materials and Their Bio-applications. ACS Appl. Mater. Interfaces 2016, 8, 33351–33370. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Loh, X.J. Water soluble polyhydroxyalkanoates: Future materials for therapeutic applications. Chem. Soc. Rev. 2015, 44, 2865–2879. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Oo, M.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Thixotropic supramolecular pectin-poly(ethylene glycol) methacrylate (pegma) hydrogels. Polymers 2016, 8, 404. [Google Scholar] [CrossRef]
- Loh, X.J.; Tan, K.K.; Li, X.; Li, J. The in vitro hydrolysis of poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol). Biomaterials 2006, 27, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Goh, S.H.; Li, J. New Biodegradable Thermogelling Copolymers Having very Low Gelation Concentrations. Biomacromolecules 2007, 8, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Sng, K.B.C.; Li, J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(ε-caprolactone), poly(ethylene glycol) and poly(propylene glycol). Biomaterials 2008, 29, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Zhang, Z.-X.; Wu, Y.-L.; Lee, T.S.; Li, J. Synthesis of Novel Biodegradable Thermoresponsive Triblock Copolymers Based on Poly[(R)-3-hydroxybutyrate] and Poly(N-isopropylacrylamide) and Their Formation of Thermoresponsive Micelles. Macromolecules 2009, 42, 194–202. [Google Scholar] [CrossRef]
- Loh, X.J.; Goh, S.H.; Li, J. Biodegradable Thermogelling Poly[(R)-3-hydroxybutyrate]-Based Block Copolymers: Micellization, Gelation, and Cytotoxicity and Cell Culture Studies. J. Phys. Chem. B 2009, 113, 11822–11830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Wang, H.; Qiu, Y.-K.; Liow, S.S.; Li, Z.; Loh, X.J. PHB-Based Gels as Delivery Agents of Chemotherapeutics for the Effective Shrinkage of Tumors. Adv. Healthc. Mater. 2016, 5, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Barouti, G.; Liow, S.S.; Dou, Q.; Ye, H.; Orione, C.; Guillaume, S.M.; Loh, X.J. New Linear and Star-Shaped Thermogelling Poly([R]-3-hydroxybutyrate) Copolymers. Chem. Eur. J. 2016, 22, 10501–10512. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, S.; Li, Z.; Xu, K.; Chen, G.-Q. Characterization, biodegradability and blood compatibility of poly[(R)-3-hydroxybutyrate] based poly(ester-urethane)s. J. Biomed. Mater. Res. A 2009, 90A, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater 2016, 8, e265. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, S.; Li, S.; Liu, Q.; Xu, K.; Chen, G.-Q. Novel amphiphilic poly(ester-urethane)s based on poly[(R)-3-hydroxyalkanoate]: Synthesis, biocompatibility and aggregation in aqueous solution. Polym. Int. 2008, 57, 887–894. [Google Scholar] [CrossRef]
- Chan, S.Y.; Chan, B.Q.Y.; Liu, Z.; Parikh, B.H.; Zhang, K.; Lin, Q.; Su, X.; Kai, D.; Choo, W.S.; Young, D.J.; et al. Electrospun pectin-polyhydroxybutyrate nanofibers for retinal tissue engineering. ACS Omega 2017, 2, 8959–8968. [Google Scholar] [CrossRef]
- Loh, X.J.; Goh, S.H.; Li, J. Hydrolytic degradation and protein release studies of thermogelling polyurethane copolymers consisting of poly[(R)-3-hydroxybutyrate], poly(ethylene glycol), and poly(propylene glycol). Biomaterials 2007, 28, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Wang, H.; Qiu, Y.-K.; Loh, X.J. Pla-based thermogel for the sustained delivery of chemotherapeutics in a mouse model of hepatocellular carcinoma. RSC Adv. 2016, 6, 44506–44513. [Google Scholar] [CrossRef]
- Loh, X.J.; Tan, Y.X.; Li, Z.; Teo, L.S.; Goh, S.H.; Li, J. Biodegradable thermogelling poly(ester urethane)s consisting of poly(lactic acid)—Thermodynamics of micellization and hydrolytic degradation. Biomaterials 2008, 29, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Li, Z.; Sugiarto, S.; Yu, C.Y.Y.; Kwok, R.T.K.; Chen, X.; Wu, Y.-L.; Ong, S.T.; et al. Long-Term Real-Time in vivo Drug Release Monitoring with AIE Thermogelling Polymer. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, K.; Peng, D.; Liu, S.; Wu, H. Preparation of poly(butylene-co-ε-caprolactone carbonate) and their use as drug carriers for a controlled delivery system. J. Polym. Sci. A 2007, 45, 2152–2160. [Google Scholar] [CrossRef]
- Wang, X.L.; Du, F.G.; Jiao, J.; Meng, Y.Z.; Li, R.K.Y. Preparation and properties of biodegradable polymeric blends from poly(propylene carbonate) and poly(ethylene-co-vinyl alcohol). J. Biomed. Mater. Res. B 2007, 83B, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, E.; Bracco, P.; Kurtz, S.M.; Costa, L.; Zanetti, M. In-vivo degradation of poly(carbonate-urethane) based spine implants. Polym. Degrad. Stab. 2013, 98, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing hydrogels for on-demand therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Devadasu, V.R.; Bhardwaj, V.; Kumar, M.N.V.R. Can Controversial Nanotechnology Promise Drug Delivery? Chem. Rev. 2013, 113, 1686–1735. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kajimoto, K.; Hatakeyama, H.; Harashima, H. Advances in an active and passive targeting to tumor and adipose tissues. Expert Opin. Drug Deliv. 2015, 12, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Liow, S.S.; Loh, X.J. Organic-inorganic shape memory thermoplastic polyurethane based on polycaprolactone and polydimethylsiloxane. RSC Adv. 2016, 6, 34946–34954. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Chan, B.Q.Y.; Liow, S.S.; Ramakrishna, S.; Xu, F.; Loh, X.J. Elastic poly(ε-caprolactone)-polydimethylsiloxane copolymer fibers with shape memory effect for bone tissue engineering. Biomed. Mater. 2016, 11, 015007. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Tan, M.J.; Prabhakaran, M.P.; Chan, B.Q.Y.; Liow, S.S.; Ramakrishna, S.; Loh, X.J. Biocompatible Electrically Conductive Nanofibers from Inorganic-Organic Shape Memory Polymers. Colloids Surf. B 2016, 148, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Q.Y.; Heng, S.J.W.; Liow, S.S.; Zhang, K.; Loh, X.J. Dual-responsive hybrid thermoplastic shape memory polyurethane. Mater. Chem. Front. 2017, 1, 767–779. [Google Scholar] [CrossRef]
- Ang, J.Y.; Chan, B.Q.Y.; Kai, D.; Loh, X.J. Engineering Porous Water-Responsive Poly(PEG/PCL/PDMS Urethane) Shape Memory Polymers. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Loh, X.J.; Gan, H.X.; Wang, H.; Tan, S.J.E.; Neoh, K.Y.; Jean Tan, S.S.; Diong, H.F.; Kim, J.J.; Sharon Lee, W.L.; Fang, X.; et al. New thermogelling poly(ether carbonate urethane)s based on pluronics F127 and poly(polytetrahydrofuran carbonate). J. Appl. Polym. Sci. 2014, 131, 39924. [Google Scholar] [CrossRef]
- Nguyen, V.P.N.; Kuo, N.; Loh, X.J. New biocompatible thermogelling copolymers containing ethylene-butylene segments exhibiting very low gelation concentrations. Soft Matter 2011, 7, 2150–2159. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, Y.; Bae, K.H.; Ahn, C.-H.; Park, T.G. Temperature/pH-Sensitive Hydrogels Prepared from Pluronic Copolymers End-Capped with Carboxylic Acid Groups via an Oligolactide Spacer. Macromol. Rapid Commun. 2007, 28, 1172–1176. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Bae, S.J.; Suh, J.M.; Sohn, Y.S.; Bae, Y.H.; Kim, S.W.; Jeong, B. Thermogelling Poly(caprolactone-b-ethylene glycol-b-caprolactone) Aqueous Solutions. Macromolecules 2005, 38, 5260–5265. [Google Scholar] [CrossRef]
- Hwang, M.J.; Suh, J.M.; Bae, Y.H.; Kim, S.W.; Jeong, B. Caprolactonic Poloxamer Analog: PEG-PCL-PEG. Biomacromolecules 2005, 6, 885–890. [Google Scholar] [CrossRef] [PubMed]
| Copolymer | Copolymer composition a (wt %) | Copolymer characteristics | ||||
|---|---|---|---|---|---|---|
| PEG | PPG | PTHF carbonate | Mn b (×103) | ĐM b (Mw/Mn) | CMC c (×10−4 g·mL−1) | |
| P1 | 65.8 | 21.7 | 12.6 | 22.7 | 1.34 | 13.8 |
| P2 | 68.5 | 23.2 | 8.34 | 14.6 | 1.39 | 12.3 |
| P3 | 72.2 | 23.2 | 4.60 | 17.6 | 1.32 | 11.9 |
| Copolymer | T (°C) | CMC (×10−4 g·mL−1) | ΧCMC (×10−7) | ∆G (kJ·mol−1) | ∆H (kJ·mol−1) | ∆S (kJ·mol−1) |
|---|---|---|---|---|---|---|
| P1 | 15 | 19.1 | 15.1 | −32.1 | +27.7 | 0.207 |
| 25 | 13.8 | 10.9 | −34.0 | 0.207 | ||
| 34 | 10.7 | 8.45 | −35.8 | 0.206 | ||
| 45 | 6.16 | 4.88 | −38.4 | 0.208 | ||
| P2 | 15 | 12.4 | 15.3 | −32.1 | +10.0 | 0.146 |
| 25 | 12.3 | 15.1 | −33.2 | 0.145 | ||
| 35 | 10.0 | 12.3 | −34.8 | 0.146 | ||
| 45 | 8.57 | 10.5 | −36.4 | 0.146 | ||
| P3 | 15 | 12.7 | 12.9 | −32.4 | +7.35 | 0.138 |
| 25 | 11.9 | 12.1 | −33.7 | 0.138 | ||
| 35 | 10.9 | 11.2 | −35.1 | 0.138 | ||
| 45 | 9.42 | 9.62 | −36.6 | 0.138 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, B.Q.Y.; Cheng, H.; Liow, S.S.; Dou, Q.; Wu, Y.-L.; Loh, X.J.; Li, Z. Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications. Polymers 2018, 10, 89. https://doi.org/10.3390/polym10010089
Chan BQY, Cheng H, Liow SS, Dou Q, Wu Y-L, Loh XJ, Li Z. Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications. Polymers. 2018; 10(1):89. https://doi.org/10.3390/polym10010089
Chicago/Turabian StyleChan, Benjamin Qi Yu, Hongwei Cheng, Sing Shy Liow, Qingqing Dou, Yun-Long Wu, Xian Jun Loh, and Zibiao Li. 2018. "Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications" Polymers 10, no. 1: 89. https://doi.org/10.3390/polym10010089
APA StyleChan, B. Q. Y., Cheng, H., Liow, S. S., Dou, Q., Wu, Y.-L., Loh, X. J., & Li, Z. (2018). Poly(carbonate urethane)-Based Thermogels with Enhanced Drug Release Efficacy for Chemotherapeutic Applications. Polymers, 10(1), 89. https://doi.org/10.3390/polym10010089











