Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites
Abstract
:1. Introduction
2. Methods and Materials
2.1. Composites Preparation
2.2. Polarizing Microscopy (PLM) Characterization
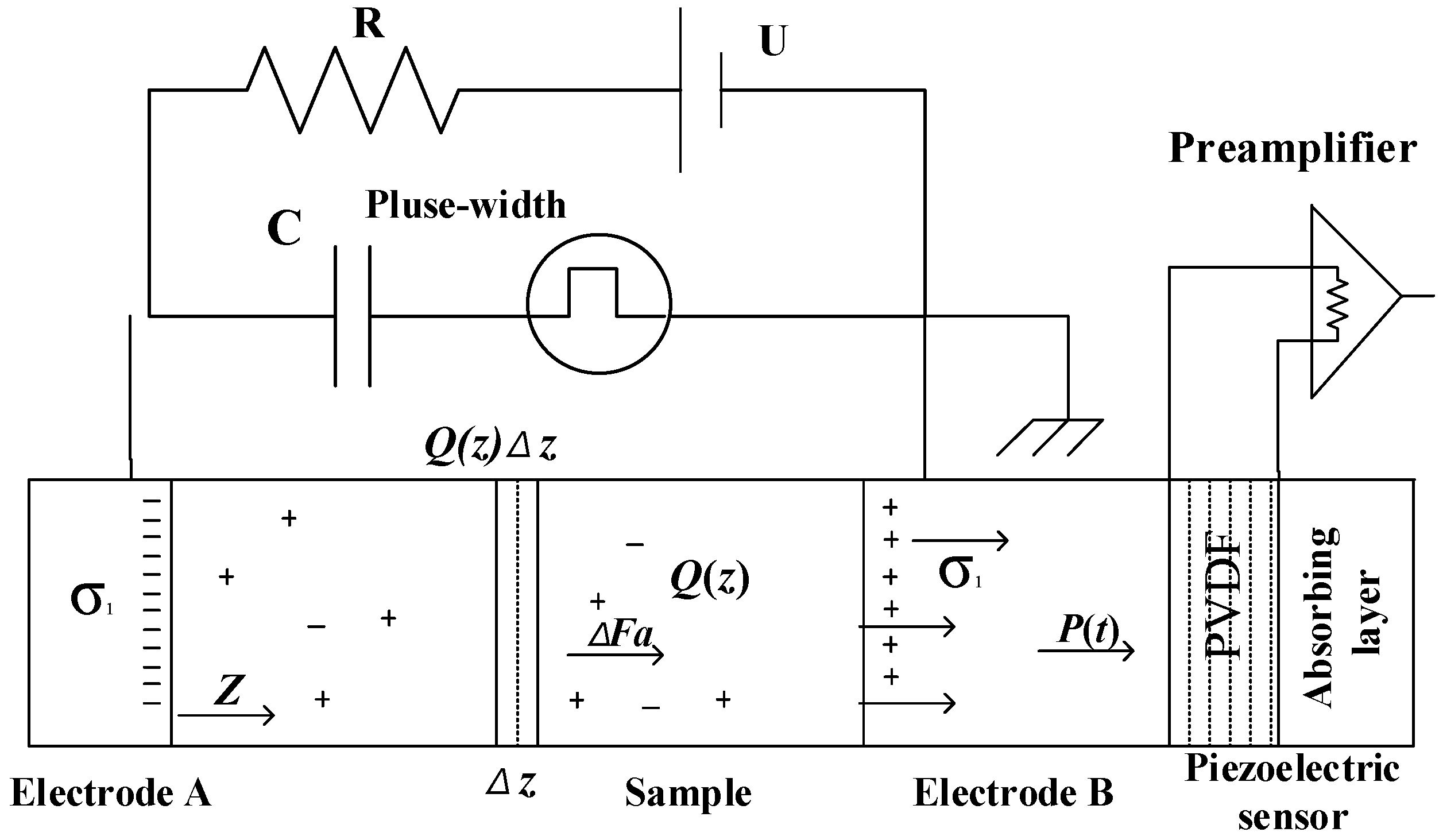
2.3. Space Charge Test
2.4. Breakdown Strength and Conductance Test
3. Results and Discussion
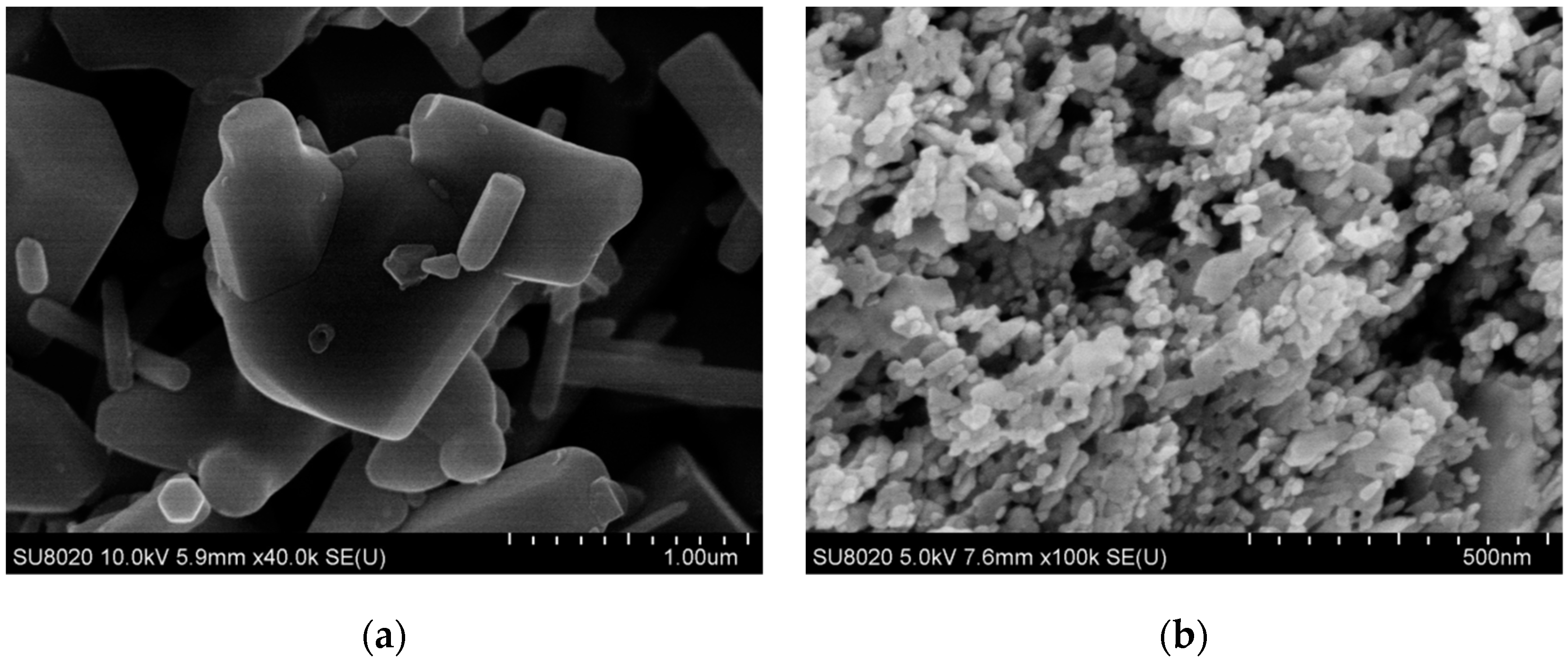
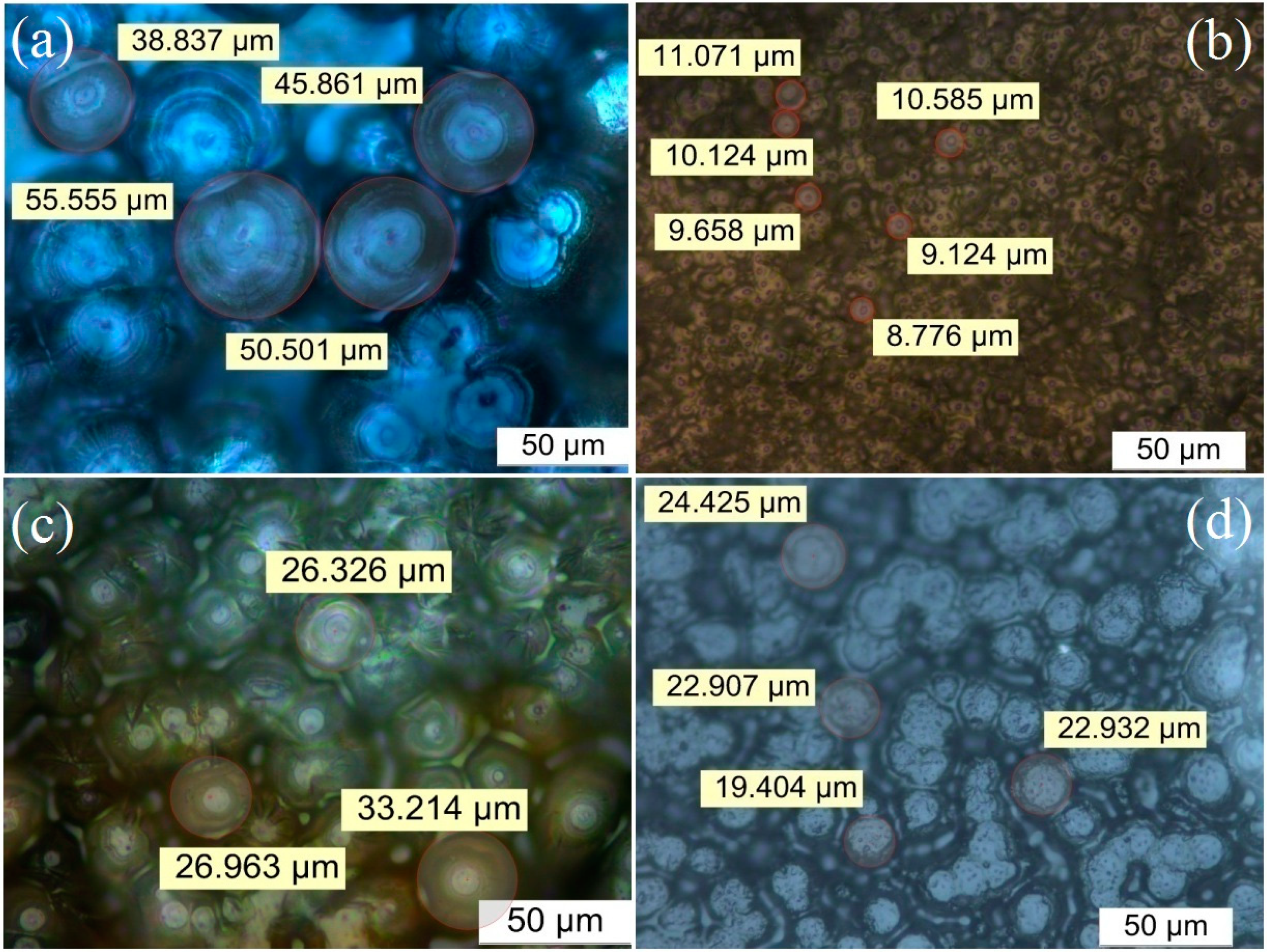
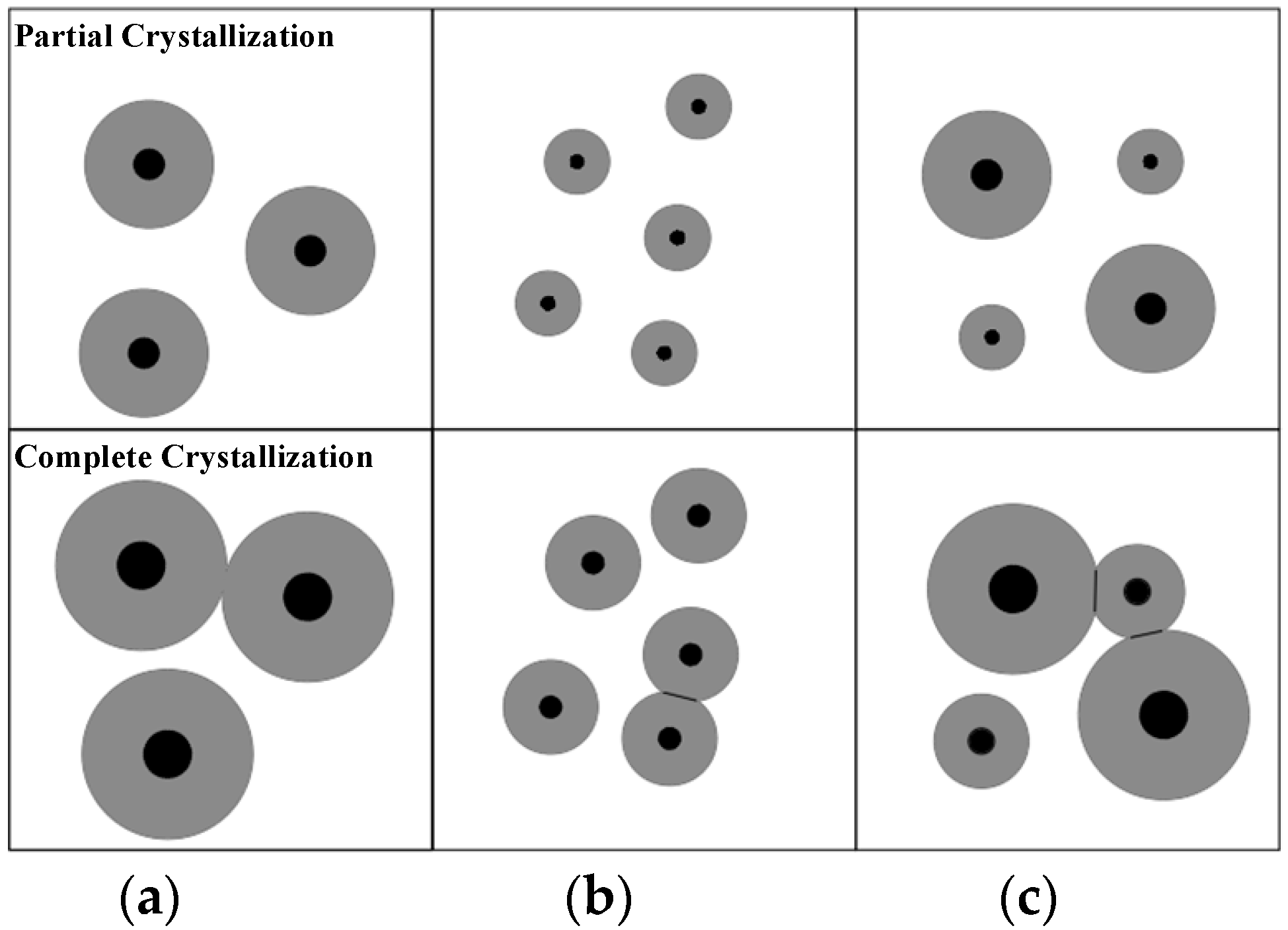
3.1. PLM Morphology
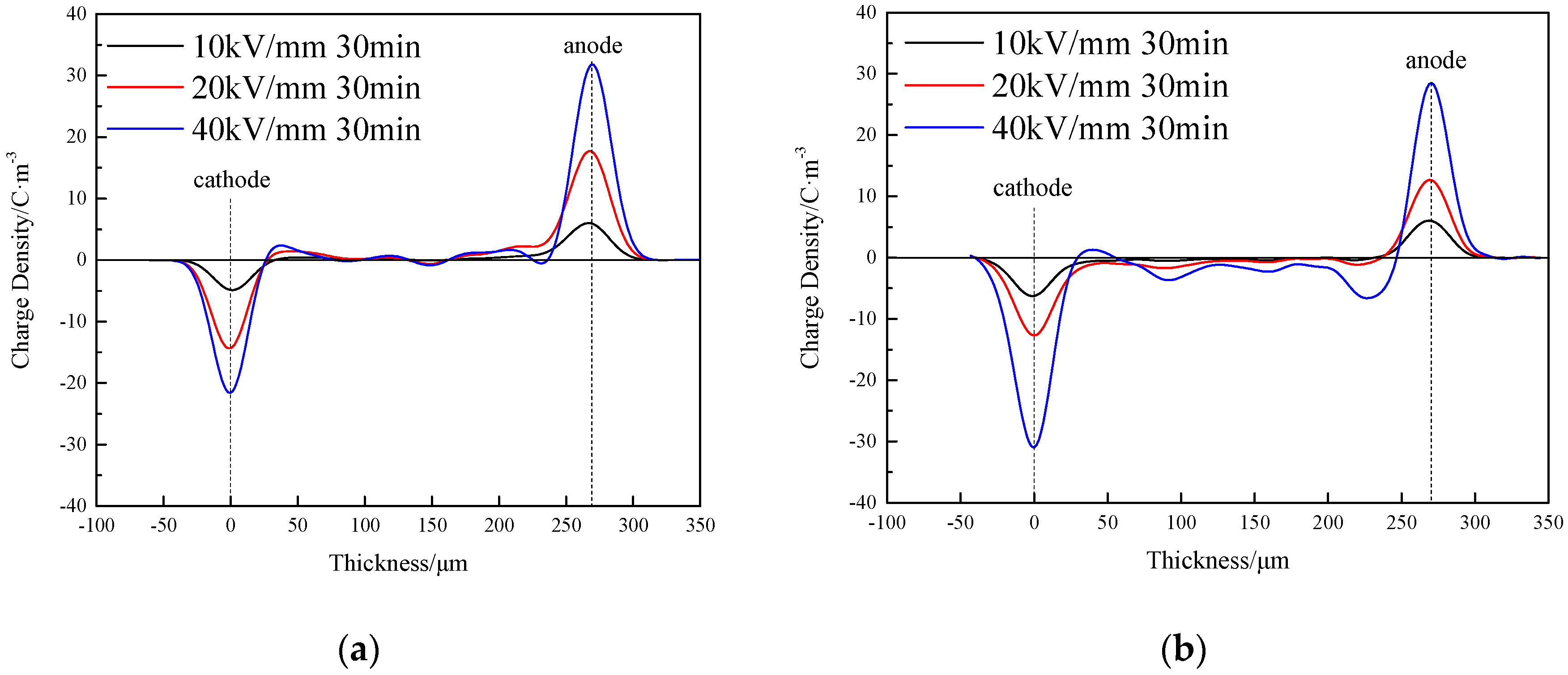
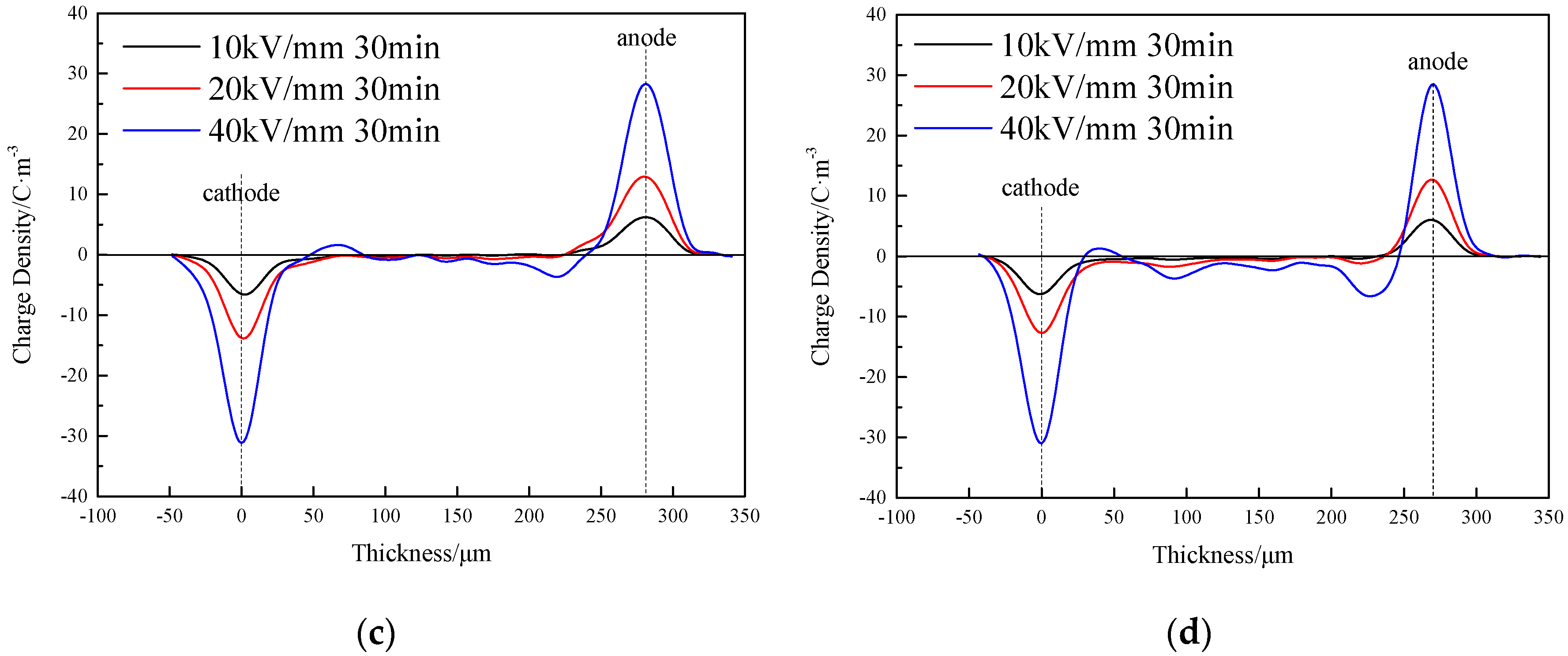
3.2. Space Charge Characteristics
3.3. DC Breakdown Strength
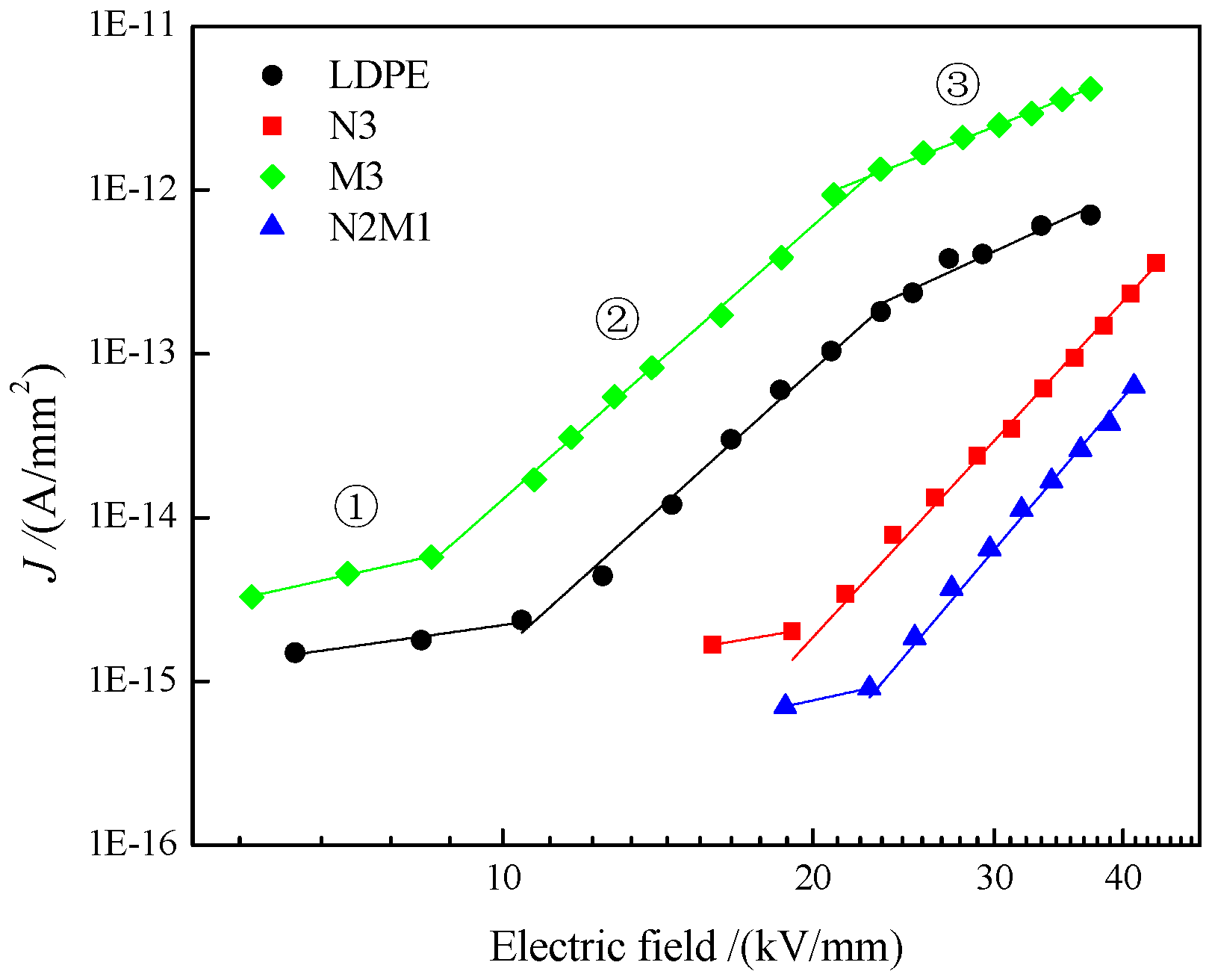
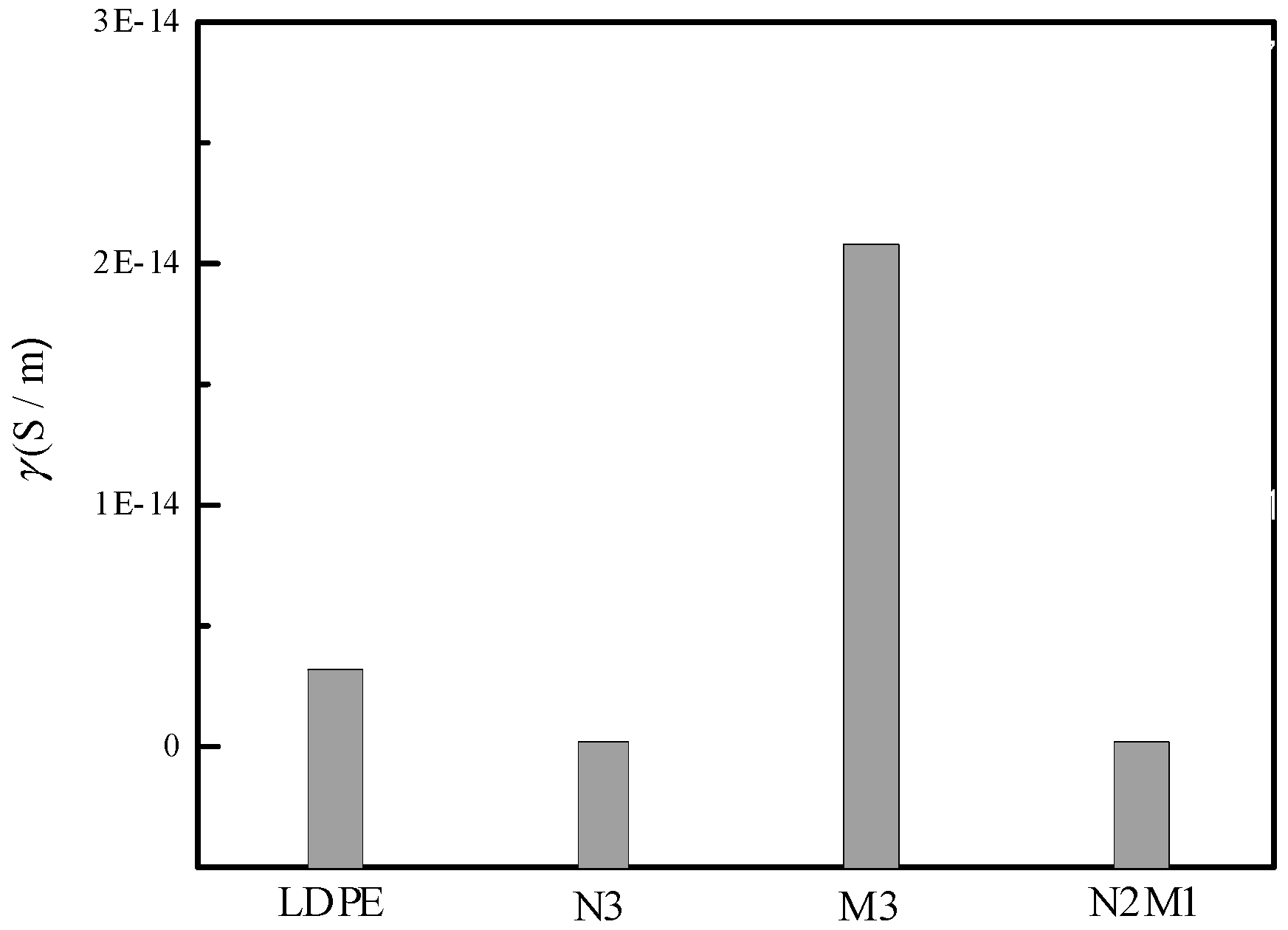
3.4. Electrical Conductance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A general review of polymeric insulation for use in HVDC cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G.; Hosier, I.L.; Holt, A.F.; Ching, K.Y. On the space charge and DC breakdown behavior of polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Wei, F.Q.; Wang, Z.N.; Xu, H.F.; Teng, C.M. Structures and properties of base resin for cross-linking low density polyethylene used as insulation materials. Mod. Plast. Process. Appl. 2015, 27, 32–34. [Google Scholar]
- Dissado, L.A.; Mazzanti, G.; Montanari, G.C. The role of trapped space charges in the electrical aging of insulating materials. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 496–506. [Google Scholar] [CrossRef]
- Hayase, Y.; Aoyama, H.; Tanaka, Y.; Takada, T.; Murata, Y. Space Charge Formation in LDPE/MgO Nano-composite Thin Film under Ultra-high DC Electric Stress. In Proceedings of the IEEE 8th International Conference on Properties & Applications of Dielectric Materials (ICPADM), Bali, Indonesia, 26–30 June 2006; Volume 126, pp. 159–162. [Google Scholar]
- Wang, X.; He, H.Q.; Tu, D.M.; Lei, C.; Du, Q.G. Dielectric properties and crystalline morphology of low density polyethylene blended with metallocene catalyzed polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 319–326. [Google Scholar] [CrossRef]
- Wu, K.; Chen, X.; Wang, X.; Tu, D.M. A polyethylene nanocomposite insulating material for HVDC cable. Insul. Mater. 2010, 43, 1–3. [Google Scholar]
- Gong, B.; Zhang, Y.W.; Zheng, F.H.; Xiao, C.; Wu, C.S. Experimental research on influences of space charge distribution and trap levels in low density polyethylene doped with inorganic powders. Mater. Sci. Eng. 2006, 24, 109–113. [Google Scholar]
- Tanaka, Y.; Chen, G.; Zhao, Y.; Davies, A.E.; Vaughan, A.S.; Takada, T. Effect of additives on morphology and space charge accumulation in low density polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 148–154. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wu, K.; Peng, Z.R.; Cheng, Y.H.; Tu, D.M. Space charge measurement in LPDE films under temperature gradient and DC stress. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1796–1805. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wu, K.; Peng, Z.R.; Cheng, Y.H.; Tu, D.M. Space charge accumulation and stress distortion in polyethylene under high DC voltage and temperature gradient. Trans. China Electrotech. Soc. 2011, 26, 13–19. [Google Scholar]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Xu, M.Z.; Zhao, H.; Ji, C.; Yang, J.M.; Zhang, W.L. Preparation of MgO/LDPE nanocomposites and its space charge property. High Volt. Eng. 2012, 38, 684–690. [Google Scholar]
- Li, J.; Feng, B.; Zhang, H.Z.; Yang, L.J.; Huang, Z.Y. Properties of water tree growing in low density polyethylene/montmorillonite Nano-composite. High Volt. Eng. 2012, 38, 2410–2415. [Google Scholar]
- Chen, X.; Wu, K.; Wang, X.; Cheng, Y.H.; Tu, D.M.; Qing, K. Modified low density polyethylene by Nano-fills as insulating material of DC cable(I). High Volt. Eng. 2012, 38, 2691–2697. [Google Scholar]
- Lv, Z.; Wang, X.; Wu, K.; Chen, X.; Cheng, Y.H.; Dissado, L.A. Dependence of charge accumulation on sample thickness in Nano-SiO2 doped LDPE. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 337–345. [Google Scholar]
- Tian, F.; Lei, Q.; Wang, X.; Wang, Y. Investigation of electrical properties of ZnO/LDPE nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 763–769. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, L.; Sha, Y.C.; Tian, J. Numerical analysis of space charge characteristics in low-density polyethylene nanocomposite under external DC electric field. High Volt. Eng. 2013, 39, 1813–1820. [Google Scholar]
- Murakami, Y.; Nemoto, M.; Okuzumi, S.; Masuda, S.; Nagao, M.; Hozumi, N.; Sekiguchi, Y.; Murata, Y. DC conduction and electrical breakdown of MgO/LDPE nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 33–39. [Google Scholar] [CrossRef]
- Yang, M.L.; Zhou, K.; Wu, K.; Tao, W.B.; Yang, D. A new rejuvenation technology based on formation of Nano-SiO2 composite fillers for water tree aged XLPE cables. Trans. China Electrotech. Soc. 2015, 30, 481–487. [Google Scholar]
- Takada, T.; Hayase, Y.; Tanaka, Y. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, S.Q.; Wang, X.; Tu, D.M. Effect of nano-ZnO on space charge distribution in polyethylene during corona aging. Insul. Mater. 2008, 41, 44–48. [Google Scholar]
- Zhang, T.X. Investigation on Dielectric Properties of Zno/Ldpe Composites; Harbin University of Science and Technoligy: Harbin, China, 2016. [Google Scholar]
- Wang, Q. Investigation of the Thermal Conductivity and Electrical Property of Epoxy Resin with Hand of Micro Alumina Fillers; School of Electronics and Electric Engineering Shanghai Jiao Tong University: Shanghai, China, 2013. [Google Scholar]
- Mallakpour, S.; Behranvand, V. Nanocomposites based on biosafe nano ZnO and different polymeric matrixes for antibacterial, optical, thermal and mechanical applications. Eur. Polym. J. 2016, 84, 377–403. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. The effect of the coupling agents KH550 and KH570 on the nanostructure and interfacial interaction of zinc oxide/chiral poly (amideimide) nanocomposites containing L-leucine amino acid moieties. J. Mater. Sci. 2014, 49, 5112–5118. [Google Scholar] [CrossRef]
- Li, S.T.; Yin, G.L.; Bai, S.N.; Li, J.Y. A new potential barrier model in epoxy resin nanodielectrics. Trans. Dielectr. Electr. Insul. 2011, 18, 1535–1543. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhao, H.; Zhang, W.L.; Xu, M.Z. Influence of moisture absorption on the DC conduction and space charge property of MgO/LDPE nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1957–1964. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Meng, G.D.; Dong, C.Y. Review on the breakdown characteristics and discharge behaviors at the micro and nano scale. Trans. China Electrotech. Soc. 2017, 32, 13–23. [Google Scholar]
- Zhang, L.; Zhou, Y.X.; Cui, X.Y.; Sha, Y.C.; Le, T.H.; Ye, Q.; Tian, J.H. Effect of nanoparticle surface modification on breakdown and space charge behavior of XLPE/SiO2 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1554–1564. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, J.; Li, Z.; Xiao, D.M. High field conduction of the composites of low-density polyethylene/nano-SiOx. Trans. China Electrotech. Soc. 2006, 21, 22–26. [Google Scholar]
- Ma, T.X. Research on DC Dielectric Properties of LDPE/Micro-Nano ZnO Composites; Harbin University of Science and Technology: Harbin, China, 2017. [Google Scholar]
- Beyer, J.; Morshuis, P.H.F.; Smit, J.J. Conduction current measurements on polycarbonates subjected to electrical and thermal stress. Electr. Insul. Dielectr. Phenom. 2000, 2, 617–621. [Google Scholar]
- Yan, S.Q.; Liao, R.J.; Yang, L.J.; Zhao, X.T.; Yuan, Y.; He, L.H. Influence of nano-Al2O3 on electrical properties of insulation paper under thermal aging. Trans. China Electrotech Soc. 2017, 32, 225–232. [Google Scholar]



| Samples | Micron-ZnO Filling Rate/wt% | Nano-ZnO Filling Rate/wt% |
|---|---|---|
| LDPE | 0 | 0 |
| M3 | 3 | 0 |
| N3 | 0 | 3 |
| N2M1 | 1 | 2 |
| Samples | Scale Parameter Eb/(kV·mm−1) | Shape Parameter β |
|---|---|---|
| LDPE | 299.0 | 15.3 |
| N3 | 340.4 | 10.1 |
| M3 | 257.8 | 11.1 |
| N2M1 | 309.3 | 9.4 |
| Samples | Slope | Eth/(kV·mm−1) | |||
|---|---|---|---|---|---|
| ① | ② | ③ | E1 | E2 | |
| LDPE | 0.9 | 5.6 | 2.6 | 10.4 | 23.3 |
| N3 | 1.1 | 6.8 | - | 19.1 | - |
| M3 | 1.4 | 5.6 | 2.5 | 8.5 | 23.3 |
| N2M1 | 1.4 | 6.9 | - | 22.7 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-G.; Li, X.; Yang, W.-H.; Zhang, X.-H. Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites. Crystals 2019, 9, 481. https://doi.org/10.3390/cryst9090481
Gao J-G, Li X, Yang W-H, Zhang X-H. Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites. Crystals. 2019; 9(9):481. https://doi.org/10.3390/cryst9090481
Chicago/Turabian StyleGao, Jun-Guo, Xia Li, Wen-Hua Yang, and Xiao-Hong Zhang. 2019. "Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites" Crystals 9, no. 9: 481. https://doi.org/10.3390/cryst9090481
APA StyleGao, J.-G., Li, X., Yang, W.-H., & Zhang, X.-H. (2019). Space Charge Characteristics and Electrical Properties of Micro-Nano ZnO/LDPE Composites. Crystals, 9(9), 481. https://doi.org/10.3390/cryst9090481





