Structural Analyses of Helicobacter Pylori FolC Conducting Glutamation in Folate Metabolism
Abstract
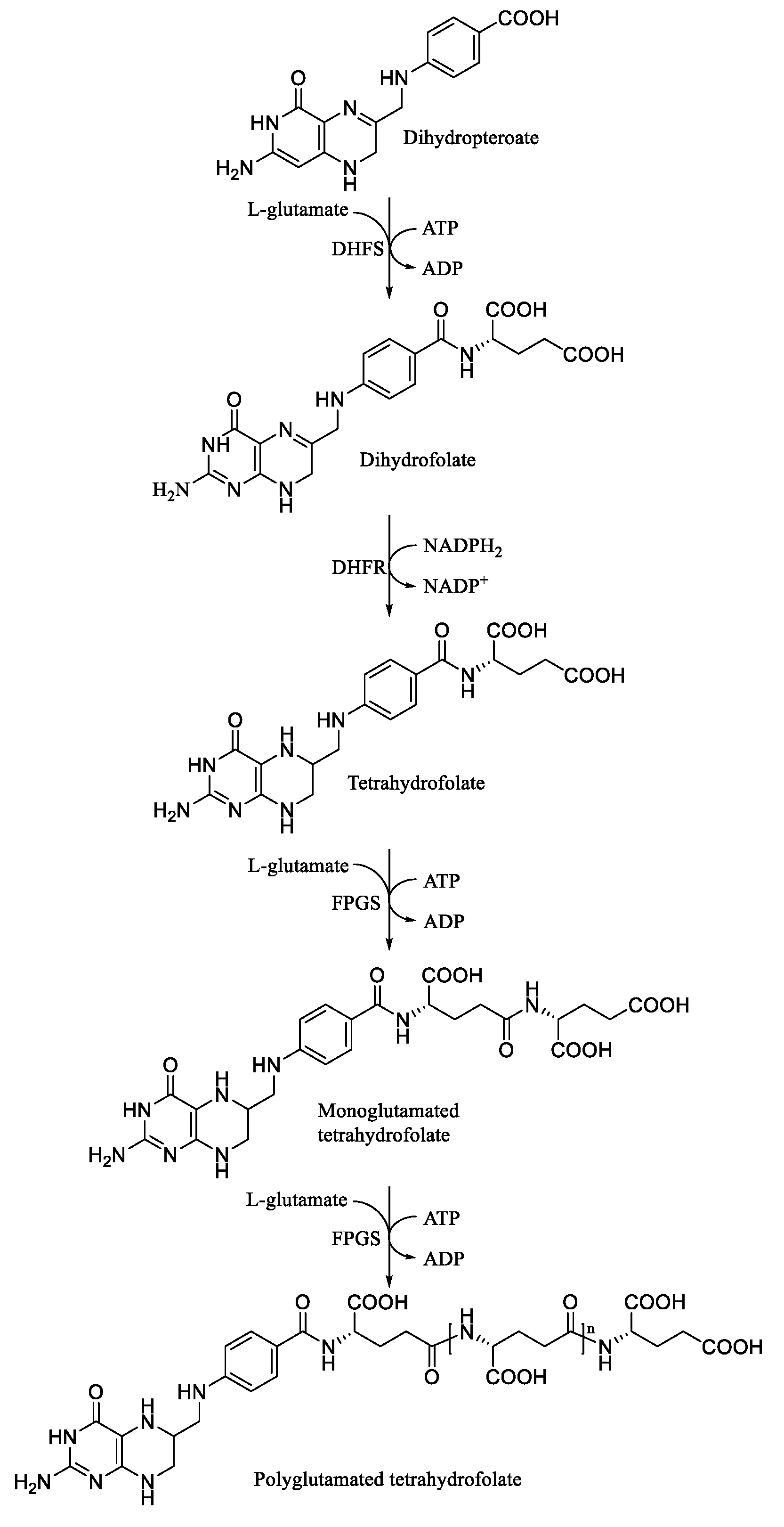
1. Introduction
2. Materials and Methods
2.1. Cloning, Protein Expression, and Purification
2.2. Crystallography
2.3. Data Availibility
3. Results and Discussions
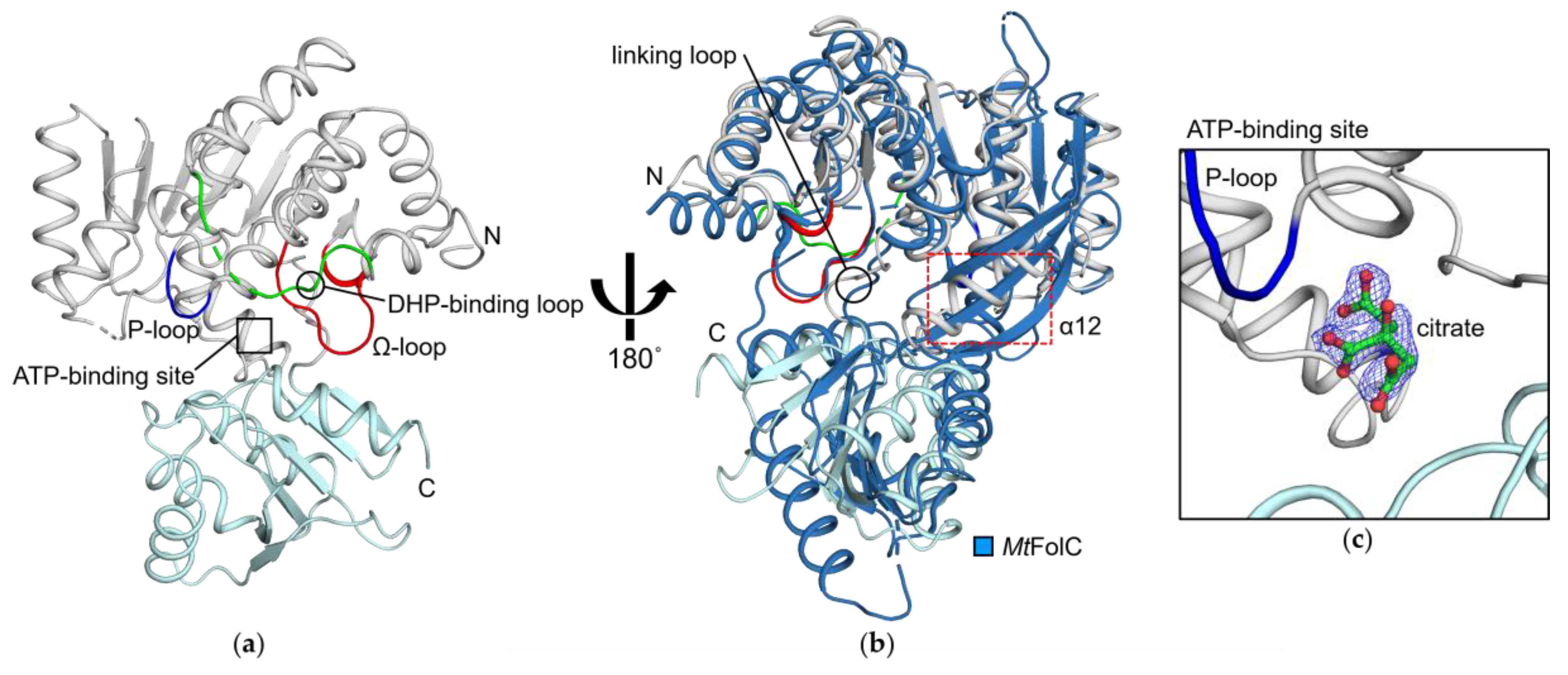
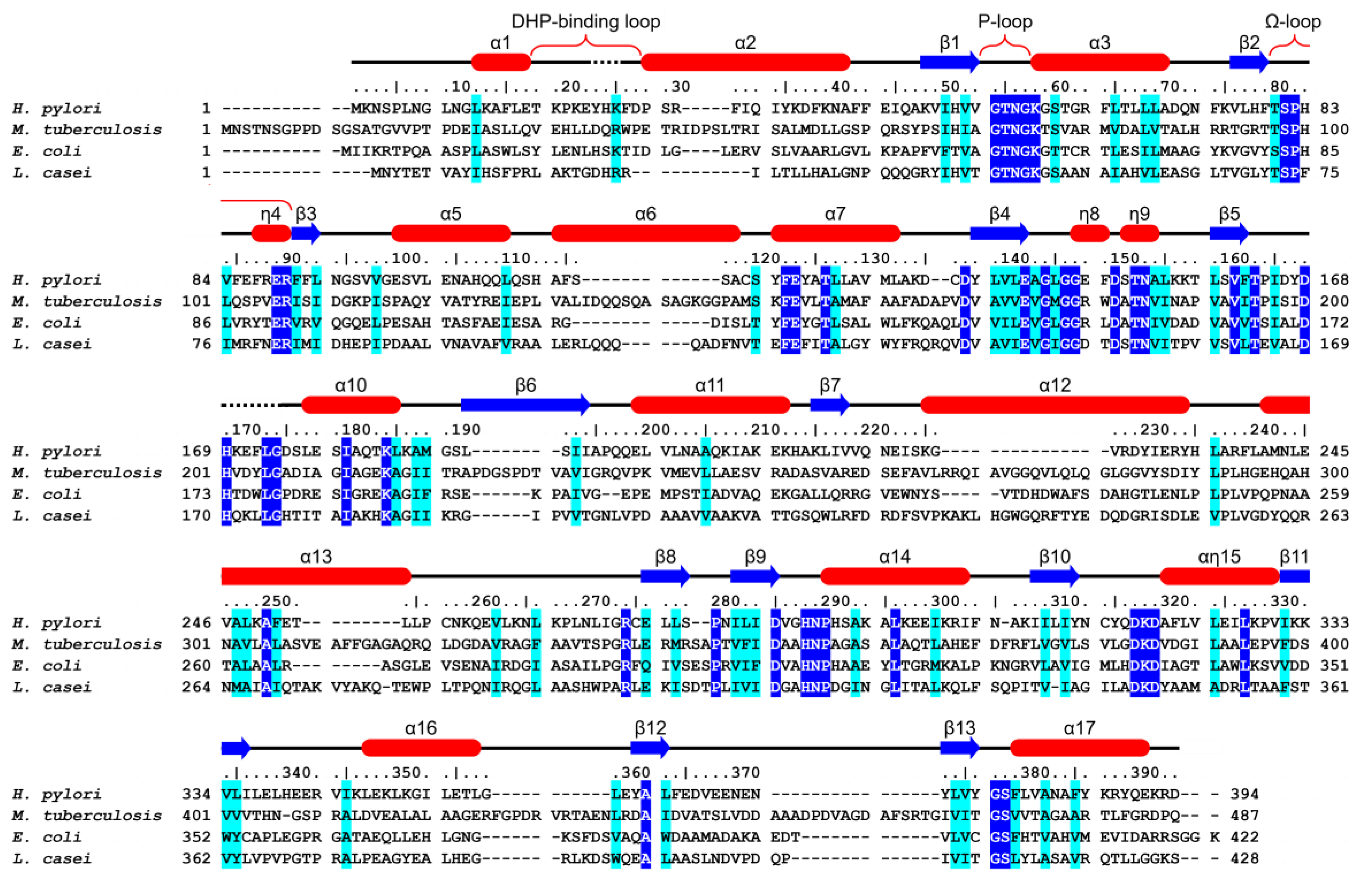
3.1. HpFolC Shares Structural Features of Other FolC/FPGSs
3.2. Binding Site of HpFolC for DHP in the Vicinity of the DHP-Binding Loop
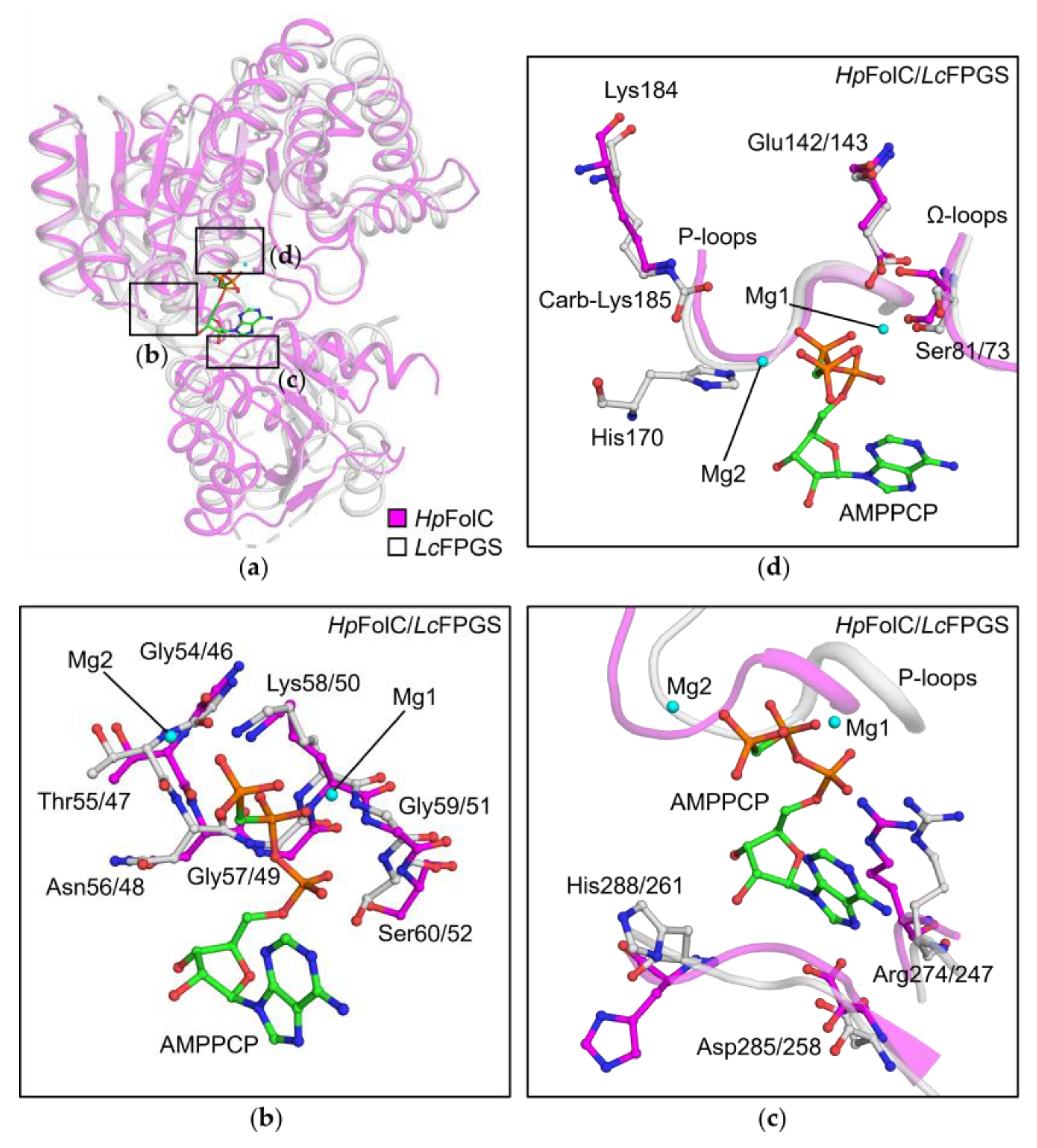
3.3. P-Loop, a Structural Motif of HpFolC for ATP Binding
3.4. Structural Motifs of HpFolC for ATP Binding via Two Magnesium Ions
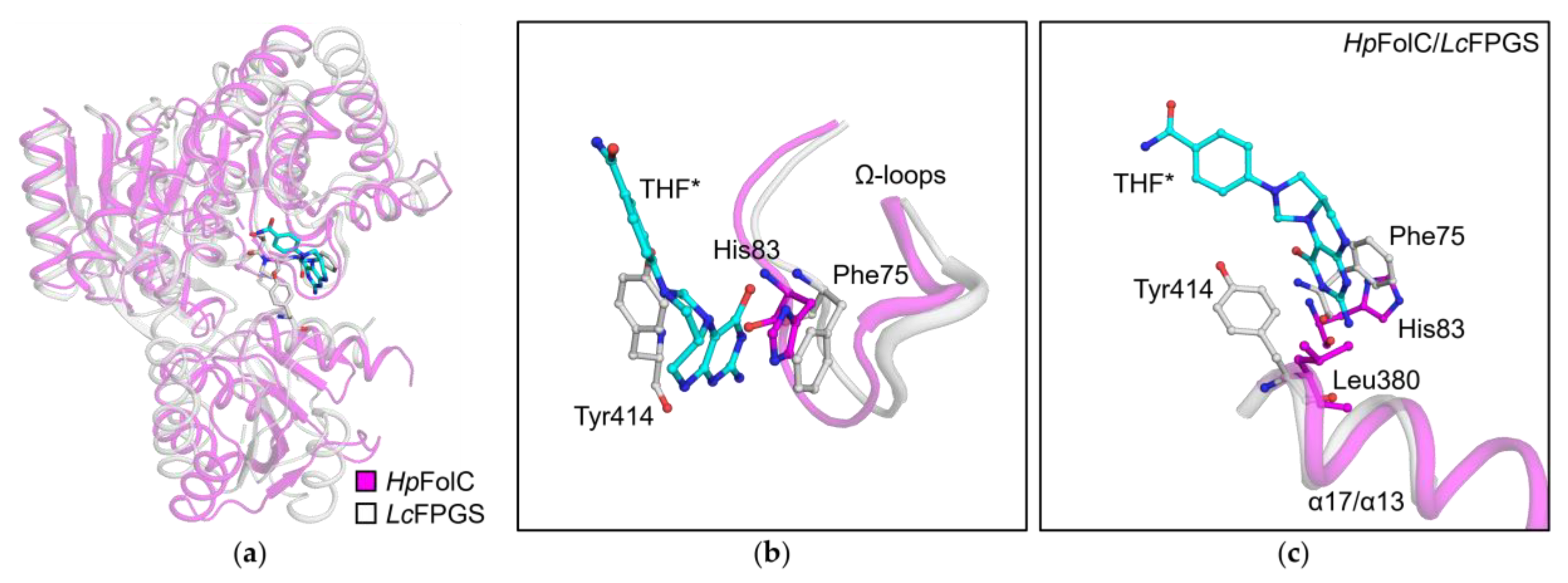
3.5. Analyses of Folate Binding of HpFolC, Focused on the Ω-Loop
3.6. l-Glu Binding Site of FolC/FPGSs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar] [PubMed]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- Harguindey, S.; Pedraz, J.L.; Garcia Canero, R.; Perez de Diego, J.; Cragoe, E.J., Jr. Hydrogen ion-dependent oncogenesis and parallel new avenues to cancer prevention and treatment using a H(+)-mediated unifying approach: pH-related and pH-unrelated mechanisms. Crit. Rev. Oncog. 1995, 6, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Venerito, M.; Schulz, C. Helicobacter pylori Infection: New Facts in Clinical Management. Curr. Treat. Options Gastroenterol. 2018, 16, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Shane, B. Folylpolyglutamate synthesis and role in the regulation of one-carbon metabolism. Vitam. Horm. 1989, 45, 263–335. [Google Scholar] [PubMed]
- Lu, Y.Z.; Aiello, P.D.; Matthews, R.G. Studies on the polyglutamate specificity of thymidylate synthase from fetal pig liver. Biochemistry 1984, 23, 6870–6876. [Google Scholar] [CrossRef] [PubMed]
- Schirch, V.; Strong, W.B. Interaction of folylpolyglutamates with enzymes in one-carbon metabolism. Arch. Biochem. Biophys. 1989, 269, 371–380. [Google Scholar] [CrossRef]
- Lowe, K.E.; Osborne, C.B.; Lin, B.F.; Kim, J.S.; Hsu, J.C.; Shane, B. Regulation of folate and one-carbon metabolism in mammalian cells. II. Effect of folylpoly-gamma-glutamate synthetase substrate specificity and level on folate metabolism and folylpoly-gamma-glutamate specificity of metabolic cycles of one-carbon metabolism. J. Biol. Chem. 1993, 268, 21665–21673. [Google Scholar]
- Synold, T.W.; Willits, E.M.; Barredo, J.C. Role of folylpolygutamate synthetase (FPGS) in antifolate chemotherapy; a biochemical and clinical update. Leuk. Lymphoma 1996, 21, 9–15. [Google Scholar] [CrossRef]
- Shane, B. Pteroylpoly(gamma-glutamate) synthesis by Corynebacterium species. In vivo synthesis of folates. J. Biol. Chem. 1980, 255, 5649–5654. [Google Scholar] [PubMed]
- Bognar, A.L.; Osborne, C.; Shane, B.; Singer, S.C.; Ferone, R. Folylpoly-gamma-glutamate synthetase-dihydrofolate synthetase. Cloning and high expression of the Escherichia coli folC gene and purification and properties of the gene product. J Biol. Chem. 1985, 260, 5625–5630. [Google Scholar]
- Fussenegger, M.; Meyer, T.F. Cloning and characterization of the Neisseria gonorrhoeae MS11 folC gene. Mol. Gen. Genet. 1996, 250, 277–285. [Google Scholar] [PubMed]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Q.; Yang, Y.; Coward, J.K.; Nzila, A.; Sims, P.F.; Hyde, J.E. Characterisation of the bifunctional dihydrofolate synthase-folylpolyglutamate synthase from Plasmodium falciparum; a potential novel target for antimalarial antifolate inhibition. Mol. Biochem. Parasitol. 2010, 172, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Bourne, C.R. Utility of the Biosynthetic Folate Pathway for Targets in Antimicrobial Discovery. Antibiotics (Basel) 2014, 3, 1–28. [Google Scholar] [CrossRef]
- Mathieu, M.; Debousker, G.; Vincent, S.; Viviani, F.; Bamas-Jacques, N.; Mikol, V. Escherichia coli FolC structure reveals an unexpected dihydrofolate binding site providing an attractive target for anti-microbial therapy. J. Biol. Chem. 2005, 280, 18916–18922. [Google Scholar] [CrossRef]
- Sun, X.; Bognar, A.L.; Baker, E.N.; Smith, C.A. Structural homologies with ATP- and folate-binding enzymes in the crystal structure of folylpolyglutamate synthetase. Proc. Natl. Acad. Sci. USA 1998, 95, 6647–6652. [Google Scholar] [CrossRef]
- Sun, X.; Cross, J.A.; Bognar, A.L.; Baker, E.N.; Smith, C.A. Folate-binding triggers the activation of folylpolyglutamate synthetase. J. Mol. Biol. 2001, 310, 1067–1078. [Google Scholar] [CrossRef]
- Smith, C.A.; Cross, J.A.; Bognar, A.L.; Sun, X. Mutation of Gly51 to serine in the P-loop of Lactobacillus casei folylpolyglutamate synthetase abolishes activity by altering the conformation of two adjacent loops. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Young, P.G.; Smith, C.A.; Metcalf, P.; Baker, E.N. Structures of Mycobacterium tuberculosis folylpolyglutamate synthase complexed with ADP and AMPPCP. Acta Crystallogr. D Biol. Crystallogr. 2008, D64, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Terwilliger, T.C. SOLVE and RESOLVE: Automated structure solution and density modification. Methods Enzymol. 2003, 374, 22–37. [Google Scholar] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.A.; Auger, G.; Fanchon, E.; Martin, L.; Blanot, D.; van Heijenoort, J.; Dideberg, O. Crystal structure of UDP-N-acetylmuramoyl-L-alanine:D-glutamate ligase from Escherichia coli. EMBO J. 1997, 16, 3416–3425. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Auger, G.; Martin, L.; Fanchon, E.; Blanot, D.; Le Beller, D.; van Heijenoort, J.; Dideberg, O. Determination of the MurD mechanism through crystallographic analysis of enzyme complexes. J. Mol. Biol. 1999, 289, 579–590. [Google Scholar] [CrossRef] [PubMed]
| HpFolC | SeMet-substituted HpFolC | |
|---|---|---|
| PDB code | 6K8C | |
| Data collection | ||
| Beam line | PLS-5C | |
| Space group | C2 | P41212 |
| Wavelength (Å) | 0.9795 | 0.9798 (Peak) |
| Unit cell parameter | ||
| a, b, c (Å) | 131.45, 61.64, 69.74 | 117.90, 117.90, 69.18 |
| α, β, γ (°) | 90, 115.96, 90 | 90, 90, 90 |
| Resolution range (Å) | 50.00–1.95 (1.98–1.95)1 | 50.00–2.50 (2.54–2.50)1 |
| No. of unique reflections | 36178 (3591)1 | 17401 (1701)1 |
| I/σ(I) | 26.5 (4.2)1 | 69.2 (15.9)1 |
| Wilson B-factor (Å2) | 33.9 | 28.4 |
| Rmerge (%) | 0.059 (0.431)1 | 0.148 (0.635)1 |
| Redundancy | 7.4 (7.5)1 | 38.1 (39.9)1 |
| Completeness (%) | 98.9 (98.9)1 | 100.0 (100.0)1 |
| Refinement | ||
| Resolution | 30.00–1.95 (2.02–1.95)1 | |
| Rwork/Rfree2 | 0.184/0.219 | |
| R.m.s. deviations | ||
| Bonds (Å) | 0.004 | |
| Angles (°) | 0.640 | |
| No. of non-H atoms | 3362 | |
| Protein | 3029 | |
| Ligand | 193 | |
| Water | 314 | |
| Average B-factors (Å2) | 41.22 | |
| Protein | 40.42 | |
| Ligand | 62.68 | |
| Water | 47.59 | |
| Ramachandran favored/outlier (%) | 98.4/0.04 | |
| Rotamer outlier (%) | 0.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Kim, H.S.; Park, S.H.; Park, M.S.; Kang, S.-M.; Kim, H.-J.; Han, B.W. Structural Analyses of Helicobacter Pylori FolC Conducting Glutamation in Folate Metabolism. Crystals 2019, 9, 429. https://doi.org/10.3390/cryst9080429
Park JS, Kim HS, Park SH, Park MS, Kang S-M, Kim H-J, Han BW. Structural Analyses of Helicobacter Pylori FolC Conducting Glutamation in Folate Metabolism. Crystals. 2019; 9(8):429. https://doi.org/10.3390/cryst9080429
Chicago/Turabian StylePark, Joon Sung, Hyoun Sook Kim, Sang Ho Park, Mi Seul Park, Sung-Min Kang, Hyun-Jung Kim, and Byung Woo Han. 2019. "Structural Analyses of Helicobacter Pylori FolC Conducting Glutamation in Folate Metabolism" Crystals 9, no. 8: 429. https://doi.org/10.3390/cryst9080429
APA StylePark, J. S., Kim, H. S., Park, S. H., Park, M. S., Kang, S.-M., Kim, H.-J., & Han, B. W. (2019). Structural Analyses of Helicobacter Pylori FolC Conducting Glutamation in Folate Metabolism. Crystals, 9(8), 429. https://doi.org/10.3390/cryst9080429






